Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3178
Peer-review started: July 12, 2021
First decision: September 28, 2021
Revised: October 10, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: April 6, 2022
Processing time: 259 Days and 21 Hours
Hemophagocytic lymphohistiocytosis (HLH) is a rare disorder with rapid progression and high mortality. HLH occurs mostly due to infection, malignant tumors, and immune disorders. Among infections that cause HLH, viral infections, especially Epstein-Barr virus infections, are common, whereas tuberculosis is rare. Tuberculosis-associated HLH has a wide range of serological and clinical manifestations that are similar to those of systemic lupus erythematosus (SLE).
This study describes a case of tuberculosis-associated HLH misdiagnosed as SLE because of antinuclear antibody (ANA), Smith (Sm) antibody and lupus anticoagulant positivity; leukopenia; thrombocytopenia; pleural effusion; decreased C3, quantitatively increased 24 h urinary protein and fever. The patient was initially treated with glucocorticoids, which resulted in peripheral blood cytopenia and symptom recurrence. Then, caseating granulomas and hemophagocytosis were observed in her bone marrow. She was successfully treated with conventional category 1 antituberculous drugs. In addition, we reviewed the literature on tuberculosis-associated HLH documented in PubMed, including all full-text articles published in English from December 2009 to December 2019, and summarized the key points, including the epidemiology, clinical manifestations, diagnosis, and treatment of tuberculosis-associated HLH and the differences of the present case from previous reports.
Tuberculosis should be considered in patients with fever or respiratory symptoms. Antituberculous drugs are important for treating tuberculosis-associated HLH.
Core Tip: Misdiagnosis often occurs in tuberculosis patients with hemophagocytic lymphohistiocytosis (HLH). This manuscript reports a case of tuberculosis-associated HLH misdiagnosed as systemic lupus erythematosus (SLE) and presents a literature review. This report is intended to increase the understanding of tuberculosis-associated HLH and emphasize that for the diagnosis of SLE. Tuberculosis should be considered in patients with fever or respiratory symptoms. Antituberculous drugs are important for treating tuberculosis-associated HLH.
- Citation: Chen WT, Liu ZC, Li MS, Zhou Y, Liang SJ, Yang Y. Tuberculosis-associated hemophagocytic lymphohistiocytosis misdiagnosed as systemic lupus erythematosus: A case report. World J Clin Cases 2022; 10(10): 3178-3187
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3178.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3178
Hemophagocytic lymphohistiocytosis (HLH) is a rare disorder characterized by uncontrolled proliferation of macrophages[1]. HLH is divided into primary HLH and secondary HLH; the former has a genetic predisposition, and the latter is related to various nongenetic causes. Tuberculosis, a rare but deadly cause of secondary HLH, mainly manifests as fever and fatigue but lacks specific presenting symptoms. Systemic lupus erythematosus (SLE) is a common autoimmune disease with diverse symptoms, and definitive diagnostic tests that rely on classification criteria are used for SLE diagnosis[2]. These factors can cause misdiagnosis and even lead to fatality. Here, we report a case of tuberculosis-associated HLH misdiagnosed as SLE and perform a literature review of tuberculosis-associated HLH to increase the understanding of this unusual infection.
A 47-year-old woman was admitted to our hospital with a 1 mo history of sore throat, irregular fever and malaise, with temperatures up to 39.7°C.
A high-resolution chest computed tomography (CT) scan revealed scattered inflammatory lesions and a large pleural effusion in both lungs. Due to the bilateral scattered inflammatory lung lesions, leukopenia and thrombocytopenia (white blood cell (WBC) count 1.28 x 109/L, platelet (PLT) count 64 x 109/L), she was treated with antibiotics and granulocyte colony-stimulating factor (G-CSF) and underwent PLT transfusion many times at local hospitals before presenting to our hospital. However, clinical deterioration was observed, and the patient developed chest tightness and tachypnea.
The patient denied a previous history of tuberculosis or contact with tuberculosis patients.
The patient had a free personal and family history.
After admission, a physical examination showed paleness, weakness and weight loss. A pulmonary examination indicated a reduction in bilateral respiratory sounds. No lymphadenopathy, jaundice or hepatosplenomegaly was detected.
Upon admission, laboratory tests showed anemia (red blood cell (RBC) count 3.11 x 1012/L, hemoglobin (HGB) 89 g/L, WBC count 2.59 x 109/L, neutrophil (NEU) count 2.13 x 109/L, PLT count 276 x 109/L), and routine urine tests demonstrated protein (++) and proteinuria (1.15 g/24 h). In addition, liver enzyme levels were elevated (total bilirubin (TBIL) 29.4 μmol/L, direct bilirubin (DBIL) 11.4 μmol/L, indirect bilirubin (IBIL) 18.0 μmol/L, alanine aminotransferase (ALT) 58.2 U/L, aspartate aminotransferase (AST) 99.9 U/L, lactate dehydrogenase (LDH) 390.5 U/L), C-reactive protein (CRP) level was 25.4 mg/L, and the erythrocyte sedimentation rate (ESR) was 10 mm/h. The ferritin level was significantly elevated (679.93 ng/mL), and hypofibrinogenemia was detected (fibrinogen (FIB) 1.26 g/L). Tests for antinuclear antibodies (ANAs) (1:100), Smith (Sm) antibodies and lupus anticoagulant were positive; complement 3 (C3) level was decreased (0.50 g/L); and complement 4 (C4) level was normal (0.29 g/L). The 24-h urinary protein level was 1.19 g/24 h. Flow cytometry revealed that the absolute CD3+ T cell count was 0.12 x 109/L (0.47 x 109/L-3.26 x 109/L) and that the CD3+CD4+ T cell count and CD3+CD8+ T cell count were 0.09 x 109/L (0.20 x 109/L-1.82 x 109/L) and 0.02 x 109/L (0.13 x 109/L-1.35 x 109/L), respectively. The CD3-CD19+ B cell count and CD3-16-56 natural killer (NK) cell count were 0.03 x 109/L (0.05 x 109/L-0.67 x 109/L) and 0.01 x 109/L (0.04 x 109/L-0.99 x 109/L), respectively. Tests for hepatitis virus markers, human cytomegalovirus, herpes simplex virus, rubella virus, Epstein-Barr (EB) virus, human immunodeficiency virus serology, and anti-Treponema pallidum antibodies were negative, as were tuberculin skin, Coombs’, and parasitic ovum tests and blood and urine cultures.
At the same time, a high-resolution chest CT scan revealed localized emphysema in the inferior lobe of the right lung and an anomalous density in the upper lobe of the left lung.
Due to the patient’s cough without phlegm, a sputum smear test was not performed. After excluding a variety of specific infections and considering the ANA, Sm antibody and lupus anticoagulant positivity; leukopenia; thrombocytopenia; pleural effusion; decreased C3, quantitatively increased 24 h urinary protein and fever, SLE was suspected per the 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria[3] (Table 1).
| Domain | Item | Our case | Relative weights |
| Constitutional | Fever | 39.7°C | 2 |
| Hematological | Leukopenia | 1.28 x 109/L | 3 |
| Thrombocytopenia | 64 x 109/L | 4 | |
| Autoimmune hemolysis | N | 4 | |
| Neuropsychiatric | Delirium | N | 2 |
| Psychosis | N | 3 | |
| Seizure | N | 5 | |
| Mucocutaneous | Alopecia | N | 2 |
| Oral ulcers | N | 2 | |
| Subacute cutaneous or discoid lupus | N | 4 | |
| Acute cutaneous lupus | N | 6 | |
| Serosal | Effusion | CT scan revealed pleural effusion | 5 |
| Acute pericarditis | N | 6 | |
| Musculoskeletal | Joint involvement | N | 6 |
| Renal | Proteinuria | Proteinuria (1.15 g/24 h) | 4 |
| Class II/V | N | 8 | |
| Class III/IV | N | 10 | |
| APL antibodies | Anti-phospholipid antibodies | APL antibodies were positive | 2 |
| Complements | C3 or C4 low | C3 level was reduced (0.50 g/L) | 3 |
| C3 and C4 low | N | 4 | |
| SLE-specific antibodies | Anti-Sm | Sm antibodies were positive | 6 |
| Anti-dsDNA | N | 6 |
Methylprednisolone (24 mg QD po) and hydroxychloroquine sulfate (200 mg BID po) were started. After 2 days, the patient felt much better, her fever subsided, and her appetite improved. At that time, repeat routine blood tests demonstrated the following: RBC count 2.65 x 1012/L, HGB 75 g/L, WBC count 2.07 x 109/L, NEU count 1.60 x 109/L, and PLT count 264 x 109/L. We adjusted the treatment plan to intravenous methylprednisolone (40 mg/day QD) for 1 d. However, repeat routine blood tests showed further reductions in blood cell counts (RBC count 2.30 x 1012/L, HGB 64 g/L, WBC count 1.71 x 109/L, NEU count 1.01 x 109/L, and PLT count 199 x 109/L). We increased the dosage of methylprednisolone (80 mg/d QD) again and suggested performing histopathological examination of the bone marrow to determine the reason for the observed peripheral blood cytopenia. The patient received methylprednisolone and hydroxychloroquine sulfate, which are conventional category 1 drugs for SLE, but the patient's routine blood tests showed further reductions in the blood count, which is rarely observed in patients with SLE. According to the 2019 EULAR/ACR classification criteria, a diagnosis of SLE can be made after excluding other diseases[3]. If the patient’s symptoms can be explained by another disease, SLE should not be considered first. We suspected that the diagnosis of SLE was incorrect. Unfortunately, the patient refused further examinations and requested to be discharged.
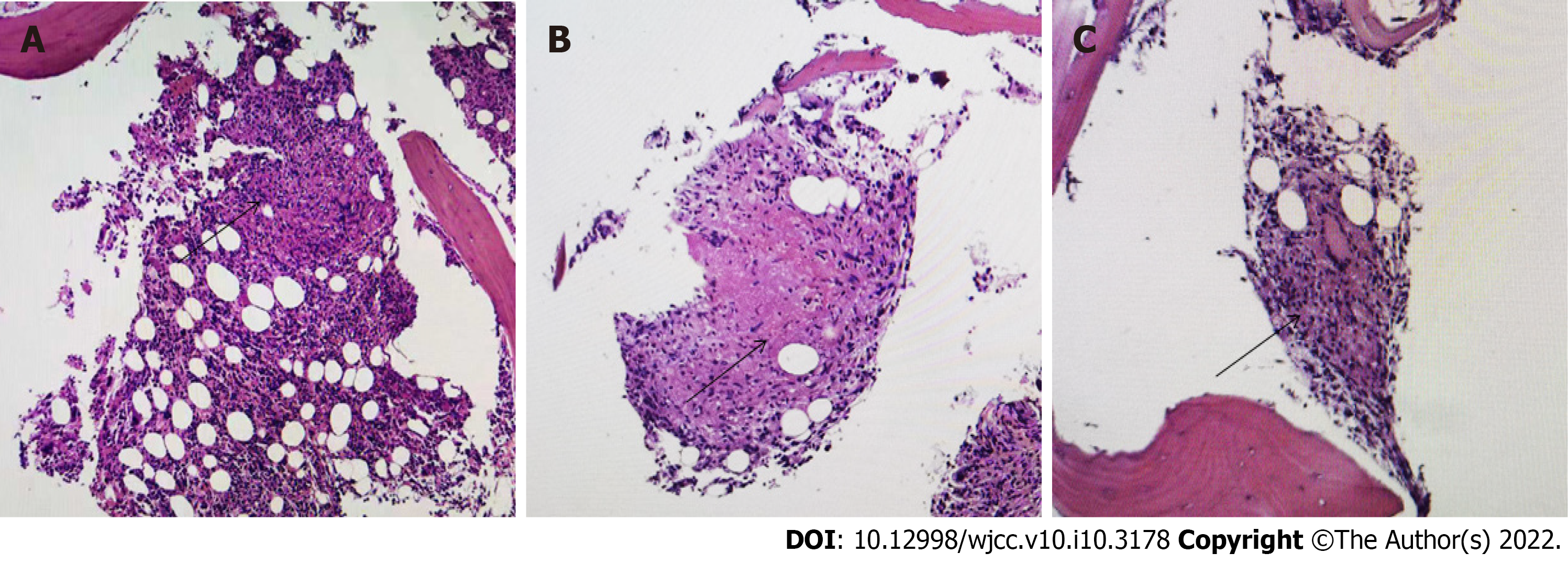
Fifteen days after discharge, the patient was readmitted with fever. At this time, routine blood tests demonstrated peripheral pancytopenia (RBC count 1.92 x 1012/L, HGB 53 g/L, WBC count 4.31 x 109/L, NEU count 4.03 x 109/L, and PLT count 293 x 109/L) and an increase in the triglyceride (TG) level to 2.6 mmol/L. We conducted positron emission tomography (PET), which revealed strong uptake of fluorodeoxyglucose (FDG) in the bone marrow, both lungs, right subscapularis muscle and left kidney. Bone marrow aspiration indicated hemophagocytosis (Figure 1). According to the HLH-2004 criteria, given the presence of cytopenia (HGB < 90 g/L; PLT count < 100 × 109/L; 2 out of 3 Lineages), fever, elevated ferritin, hypofibrinogenemia and hemophagocytosis, a diagnosis of HLH was made (Table 2). As it refers to the most updated recommendations for the management of hemophagocytic lymphohistiocytosisHLH in adults, the HScore may be a helpful diagnostic tool. Our patient had an HScore of 201 (temperature > 39.4°C: 49, cytopenia involving 2 Lineages: 24, triglycerideTG level of 2.6 mmol/L6 mmol/L: 44, fibrinogen FIB level of 1.26 g/L: 30, aspartate aminotransferase AST level of 99 U/L: 19, hemophagocytosis on bone marrow aspirate: 35), which is much higher than the threshold score of 169[4] (Table 3). At this time, caseating granulomas were found in the bone marrow (Figure 2), and Mycobacterium tuberculosis was detected by PCR of the bone marrow. Then, the patient developed purulent yellow sputum. A sputum smear revealed acid-fast stain positivity. Given the overall clinical picture, marrow granulomas and sputum, these findings were thought to be most consistent with tuberculosis-associated HLH. Antituberculous and antiinfection treatment (isoniazid, rifampicin, pyrazinamide, ethambutol, moxifloxacin and linezolid) was initiated. After more than ten days, the patient’s laboratory indexes improved, and her clinical symptoms were relieved. The patient was discharged in good health and continued antitubercular therapy. She was followed up as an outpatient and showed no signs of recurrence, and antitubercular therapy was continued for 7 mo. The patient was then not followed up to outpatient. Telephone follow-up with no signs of recurrence was noted after treatment for more than a year. The treatment line is shown in Figure 3.
| A diagnosis of HLH can be made if either 1 or 2 is met | Our case |
| 1. Molecular diagnosis consistent with HLH (e.g., pathologic mutations of PRF1, UNC13D or STX11) | N |
| 2. Clinical and laboratory criteria (at least 5/8 should be fulfilled) | Y (5/8) |
| Fever ≥ 38.5°C | Y (39.7°C) |
| Splenomegaly | N |
| Cytopenia ≥ 2-3 cell lines in peripheral blood (hemoglobin < 90 g/L, platelets < 100 x 109/L, neutrophils < 1.0 x 109/L) | Y (hemoglobin 53 g/L, platelets 64 x 109/L) |
| Hypertriglyceridemia (fasting triglycerides > 265 mg/dL) and/or hypofibrinogenemia (fibrinogen < 150 mg/dL) | Y (fibrinogen 1.26 g/L) |
| Reduced or absent NK cell activity | N |
| Hemophagocytosis in bone marrow, spleen, CSF or lymph nodes | Y (hemophagocytosis in bone marrow) |
| Ferritin ≥ 500 mg/L | Y (ferritin 679.93 ng/mL) |
| Elevated soluble CD 25 | N |
| Parameter | No. of points (criteria forscoring) | Our case |
| Known underlyingImmunosuppression1 | 0 (no) or 18 (yes) | Yes (steroid therapy): 18 |
| Temperature (°C) | 0 (< 38.4), 33 (38.4–39.4), or 49 (> 39.4) | Temperature > 39.4°C: 49 |
| Organomegaly | 0 (no), 23 (hepatomegaly or splenomegaly), or 38 (hepatomegaly and splenomegaly) | No: 0 |
| No. of cell lines involved in cytopenia2 | 0 (1 lineage), 24 (2 lineages), or 34 (3 lineages) | 2 lineages involved in cytopenia (HGB <90 g/L; platelet <100 × 109/L): 24 |
| Ferritin (μg/L) | 0 (< 2000), 35 (2000-6000), or 50 (> 6000) | Ferritin 679.93 ng/mL: 0 |
| Triglyceride (mmol/L) | 0 (< 1.5), 44 (1.5-4), or 64 (> 4) | Triglyceride 2.6 mmol/L: 44 |
| Fibrinogen (g/L) | 0 (> 2.5) or 30 (≤ 2.5) | Fibrinogen 1.26 g/L: 30 |
| Aspartate aminotransferase (U/L) | 0 (< 30) or 19 (≥ 30) | Aspartate aminotransferase 99 U/L: 19 |
| Hemophagocytosis on bone marrow aspirate | 0 (no) or 35 (yes) | Yes (hemophagocytosis on bone marrow aspirate): 35 |
We searched the PubMed database from December 2009 to December 2019 for full-text English publications using the combined terms “tuberculosis”, “hemophagocytic lymphohistiocytosis”, “hemophagocytic lymphohistiocytosis”, and “hemophagocytic syndrome” in titles/abstracts. This search identified 68 articles, of which 42 were excluded because they were not published in English, did not pertain to case reports, or were not full-text publications. In total, 29 cases from 26 publications (28 cases) and our own case (1 case) were considered here. (Supplementary Material).
In our review, 16 (55%) subjects were male, and 13 (45%) subjects were female. The age ranged from 3 wk to 80 years, and the median age was 34 years. The most common symptom was fever, which occurred in all cases. Approximately half of all patients with tuberculosis-related HLH had respiratory symptoms; this rate is higher than that described in reviews of HLH[5,6]. This finding may be related to the involvement of tuberculosis, which mostly involves the pulmonary system. Tuberculosis-related HLH may be an important consideration in patients who are diagnosed with HLH and have respiratory symptoms.
Most of the patients had blood cytopenia (n = 27, 93%). Alterations in HGB and PLT counts were present more often than alterations in WBC, NEU and RBC counts; the reasons remain unclear. Elevated ferritin levels (n = 26, 90%) and hemophagocytosis (n = 23, 79%) were detected in the majority of patients. HLH was considered the diagnosis after hemophagocytosis was found by bone marrow aspirate or biopsy. It is important to perform bone marrow aspiration or biopsy as early as possible for the diagnosis of HLH. Some laboratory test results were reported for approximately half of all cases, which included findings of hypertriglyceridemia (n = 16, 55%) and hypofibrinogenemia (n = 13, 45%). Among these patients, only five (17%) had elevated soluble CD25 Levels, and two (7%) had decreased NK cell activity. According to the diagnostic criteria for HLH, elevated soluble CD25 Levels and decreased NK cell activity are characteristic laboratory indications of HLH. Interestingly, these two tests were not performed in most cases, including our case (as these two tests were not available in our hospital). The lack of routine performance or availability in many hospitals may explain why these two tests were performed in only a few cases.
HLH is an immune system disorder and hyperinflammatory condition characterized by excessive proliferation and activation of macrophages, which engulf blood cells, resulting in pancytopenia[5,6]. HLH classified as primary or secondary HLH. Primary HLH involves an inherited disease, such as X-linked lymphoproliferative syndrome (XLP) or familial HLH (FHL)[7]. The onset of secondary HLH is mainly caused by infection, malignancy or autoimmune diseases and can present at any age. Most patients are immune-compromised due to AIDS, chronic dialysis or cancer chemotherapy[8]. In our review, 9 (31%) patients were immune-compromised due to conditions such as hypertension, lymphoma or leukemia. In our case, the patient did not mention immunodeficiency; however, her absolute CD3+ T, CD3+CD4+ T, CD3+CD8+ T, CD3-CD19+ B and CD3-16+56+ NK cell counts were reduced. These reduced cell counts suggested that our patient may have had concomitant immunocompromised conditions. Unfortunately, in our review, the CD3+CD4+/CD3+CD8+ ratio and CD3-CD16+CD56+ activity (NK cell activity) were reported in only one case and found to be reduced and normal, respectively. Our patient was also an immunocompetent adult, and her CD3+CD4+/ CD3+CD8+ ratio was reduced, which suggest that she may have had underlying immunosuppressive conditions. Immunodeficiency investigations are important to clearly identify possible concomitant immune-compromised conditions, which may help with early diagnosis and treatment.
In the cases in our review, diagnosis was mainly performed via biopsy (n = 14, 48%), culture (n = 13, 45%), imaging (n = 9, 31%), and PCR (n = 16, 55%). In the early stage of infection, Mycobacterium tuberculosis loads are low, and diagnosis via biopsy and imaging examination is difficult. Moreover, culture requires several weeks for diagnosis. One study revealed that PCR was effective for the diagnosis of tuberculosis, exhibiting high specificity (100%), sensitivity (97.2%) and positive predictive values (100%)[9]. In 2000, a PCR test was proposed by the Centers for Disease Control and Prevention[10]. PCR should be carried out in suspected cases of tuberculosis. In our patient, tuberculosis was demonstrated by bone marrow aspiration, and PET revealed the specific locations of tuberculosis lesions. Hence, extrapulmonary tuberculosis detection is important for the diagnosis of tuberculosis, and PET plays a vital role in the detection of lesion sites.
In our reviewed cases, most patients received antituberculous treatment (n = 28, 97%), and isoniazid, rifampicin, pyrazinamide, and ethambutol were the drugs most frequently used. Corticosteroids (n = 20, 69%), antibiotics (n = 16, 55%) and supportive care (n = 18, 62%) were also common. Interestingly, the overall mortality in recent reports was 21%, whereas that in previous reports was approximately 50%[11,12]; the diagnosis and treatment of tuberculosis-associated HLH has improved. Among fatal cases, delayed medical treatment or diagnosis, comorbidities, poor physical condition and lack of effective antituberculous drug administration at the early stage occurred in most cases[12-16]. Thus, prompt antituberculous treatment is crucial for patient survival. Immunocompetent patients are increasingly exhibiting tuberculosis-associated HLH, which has been ignored in past years. However, more positive treatments have been developed recently for all patients, regardless of whether they have underlying immunosuppressive conditions. This may be the key reason why the death rate has decreased in recent years according to our review. In addition, we observed that supportive care has been used in many patients in recent years, which may facilitate recovery.
As a deadly infection, tuberculosis kills more than 1 million people every year[17]. Tuberculosis infection is an important problem in developed countries, and it has the second highest incidence among infectious diseases worldwide[18]. Pulmonary tuberculosis is familiar to clinicians and easily diagnosed. However, tuberculosis can occur at other sites. Due to its rarity, lack of typical symptoms and inaccessible sites, the diagnosis of extrapulmonary tuberculosis is often delayed or the disease is misdiagnosed[10,19,20].
SLE is a heterogeneous disease with a complex clinical presentation, and its diagnosis is based on the threshold scores of the classification criteria[3]. It has overlapping features and complex interactions with tuberculosis. Studies have reported that tuberculosis is frequent among SLE patients[21-23]. In addition, tuberculosis may be a risk factor for SLE[24]. However, tuberculosis infections mimicking SLE symptoms are rare. Tuberculosis can induce the generation of diverse serum autoantibodies, which may be related to antibodies produced in response to tuberculosis cross-reacting with DNA[25].
In our case, the patient had a low ANA titer, in contrast to most SLE patients, who have high titers. This result highlights that the diagnosis of SLE may be faulty. However, the patient’s SLE classification score according to the 2019 EULAR/ACR classification criteria was 26, which is far higher than the threshold score. Nevertheless, according to the 2019 EULAR/ACR classification criteria, a diagnosis of SLE can be made only after excluding other diseases. If there is a more likely explanation than SLE, the diagnosis of SLE should not be made. Our patient received methylprednisolone and hydroxychloroquine sulfate at admission, which are conventional category 1 drugs for SLE, but the patient's routine blood tests showed further reductions in the blood counts, which is rarely observed in patients with SLE. Many studies have shown positive associations between nonadherence and risk of flares, morbidity, hospitalization, renal failure and death[26-28]. However, our patient received only antitubercular therapy, receiving no treatment for SLE, and attended telephone follow-up visits for more than a year. No signs of recurrence have been noted thus far. The diagnosis of SLE was incorrect. Because a sputum smear test and histopathological examination of the bone marrow were not performed in a timely manner, the patient was originally misdiagnosed. This result emphasizes that extrapulmonary histopathological examination plays a key role in the diagnosis of tuberculosis. If a patient has a cough, a sputum smear test should be performed immediately. Lack of exclusion of other diseases and diagnosis based on only classification criteria may result in misdiagnosis and inappropriate treatment. Therefore, it is necessary to distinguish tuberculosis from SLE.
Extrapulmonary tuberculosis patients with HLH are difficult to diagnose. Extrapulmonary findings play an important role in the diagnosis of tuberculosis, and the bone marrow is the crucial region involved. Tuberculosis should be considered in patients with fever or respiratory symptoms. Furthermore, HLH is a dangerous disease; however, the survival rate of tuberculosis-associated HLH can be increased by an aggressive workup and early treatment, especially treatment with category 1 antituberculous drugs. In the diagnosis of SLE, other diseases need to be excluded, even in cases where the SLE classification criteria score may be much higher than the threshold score.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Wang W S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Shi W, Jiao Y. Nontuberculous Mycobacterium infection complicated with Haemophagocytic syndrome: a case report and literature review. BMC Infect Dis. 2019;19:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res Ther. 2012;14 Suppl 4:S4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, Diamond B, Gladman DD, Hahn B, Hiepe F, Jacobsen S, Khanna D, Lerstrøm K, Massarotti E, McCune J, Ruiz-Irastorza G, Sanchez-Guerrero J, Schneider M, Urowitz M, Bertsias G, Hoyer BF, Leuchten N, Tani C, Tedeschi SK, Touma Z, Schmajuk G, Anic B, Assan F, Chan TM, Clarke AE, Crow MK, Czirják L, Doria A, Graninger W, Halda-Kiss B, Hasni S, Izmirly PM, Jung M, Kumánovics G, Mariette X, Padjen I, Pego-Reigosa JM, Romero-Diaz J, Rúa-Figueroa Fernández Í, Seror R, Stummvoll GH, Tanaka Y, Tektonidou MG, Vasconcelos C, Vital EM, Wallace DJ, Yavuz S, Meroni PL, Fritzler MJ, Naden R, Dörner T, Johnson SR. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 890] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 4. | La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 649] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 5. | Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Altook R, Ruzieh M, Singh A, Alamoudi W, Moussa Z, Alim H, Safi F, Duggan J. Hemophagocytic Lymphohistiocytosis in the Elderly. Am J Med Sci. 2019;357:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 859] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 8. | Shaw PH, Brown D, Shulman ST. Tuberculosis-associated hemophagocytic syndrome in an infant. Pediatr Infect Dis J. 2000;19:475-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Michos AG, Daikos GL, Tzanetou K, Theodoridou M, Moschovi M, Nicolaidou P, Petrikkos G, Syriopoulos T, Kanavaki S, Syriopoulou VP. Detection of Mycobacterium tuberculosis DNA in respiratory and nonrespiratory specimens by the Amplicor MTB PCR. Diagn Microbiol Infect Dis. 2006;54:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1084] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 11. | Brastianos PK, Swanson JW, Torbenson M, Sperati J, Karakousis PC. Tuberculosis-associated haemophagocytic syndrome. Lancet Infect Dis. 2006;6:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Shea YF, Chan JF, Kwok WC, Hwang YY, Chan TC, Ni MY, Li IW, Chiu PK, Luk JK, Chu LW. Haemophagocytic lymphohistiocytosis: an uncommon clinical presentation of tuberculosis. Hong Kong Med J. 2012;18:517-525. [PubMed] |
| 13. | Chen L, Weng H, Li H, Huang J, Pan J, Huang Y, Ma C. Potential killer in the ICU-severe tuberculosis combined with hemophagocytic syndrome: A case series and literature review. Medicine (Baltimore). 2017;96:e9142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Rodríguez-Medina B, Blanes M, Vinaixa C, Aguilera V, Rubín A, Prieto M, Berenguer M. Haemophagocytic syndrome in a liver transplant patient during treatment with Telaprevir. Ann Hepatol. 2013;12:974-978. [PubMed] [DOI] [Full Text] |
| 15. | Naha K, Dasari S, Vivek G, Prabhu M. Disseminated tuberculosis presenting with secondary haemophagocytic lymphohistiocytosis and Poncet's disease in an immunocompetent individual. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Hashmi HRT, Mishra R, Niazi M, Venkatram S, Diaz-Fuentes G. An Unusual Triad of Hemophagocytic Syndrome, Lymphoma and Tuberculosis in a Non-HIV Patient. Am J Case Rep. 2017;18:739-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9582] [Article Influence: 737.1] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Liang G, Qin H, Li Y, Zeng X. Tuberculosis-associated hemophagocytic lymphohistiocytosis with initial presentation of fever of unknown origin in a general hospital: An analysis of 8 clinical cases. Medicine (Baltimore). 2017;96:e6575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Asaji M, Tobino K, Murakami K, Goto Y, Sueyasu T, Nishizawa S, Yoshimine K, Munechika M, Ko Y, Yoshimatsu Y, Tsuruno K, Ide H, Miyajima H, Ebi N. Miliary Tuberculosis in a Young Woman with Hemophagocytic Syndrome: A Case Report and Literature Review. Intern Med. 2017;56:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Yang WF, Han F, Zhang XH, Zhang P, Chen JH. Extra-pulmonary tuberculosis infection in the dialysis patients with end stage renal diseases: case reports and literature review. J Zhejiang Univ Sci B. 2013;14:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Yang Y, Thumboo J, Tan BH, Tan TT, Fong CHJ, Ng HS, Fong KY. The risk of tuberculosis in SLE patients from an Asian tertiary hospital. Rheumatol Int. 2017;37:1027-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Ribeiro FM, Szyper-Kravitz M, Klumb EM, Lannes G, Ribeiro FR, Albuquerque EM, Shoenfeld Y. Can lupus flares be associated with tuberculosis infection? Clin Rev Allergy Immunol. 2010;38:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | HILL HM, KIRSHBAUM JD. Military tuberculosis developing during prolonged cortisone therapy of systemic lupus erythematosus. Ann Intern Med. 1956;44:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Balbi GGM, Machado-Ribeiro F, Marques CDL, Signorelli F, Levy RA. The interplay between tuberculosis and systemic lupus erythematosus. Curr Opin Rheumatol. 2018;30:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Ting LY, Shrestha B, Lu YL, Ping F. Post-delivery mycobacterium tuberculosis infection misdiagnosed as systemic lupus erythematosus. J Infect Dev Ctries. 2016;10:1352-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Arnaud L, Tektonidou MG. Long-term outcomes in systemic lupus erythematosus: trends over time and major contributors. Rheumatology (Oxford). 2020;59:v29-v38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 27. | Costedoat-Chalumeau N, Pouchot J, Guettrot-Imbert G, Le Guern V, Leroux G, Marra D, Morel N, Piette JC. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol. 2013;27:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Bruce IN, Gladman DD, Urowitz MB. Factors associated with refractory renal disease in patients with systemic lupus erythematosus: the role of patient nonadherence. Arthritis Care Res. 2000;13:406-408. [PubMed] |