Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3156
Peer-review started: July 13, 2021
First decision: October 3, 2021
Revised: October 8, 2022
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: April 6, 2022
Processing time: 259 Days and 7.8 Hours
Malignant peritoneal mesothelioma (MPM) is a rare malignancy arising in mesothelial cells in the peritoneum. It can be mistaken for many other diseases, such as peritoneal carcinomatosis and tuberculous peritonitis (TBP), because its clinical manifestations are often nonspecific. Therefore, the diagnosis of MPM is often challenging and delayed.
A 42-year-old man was referred to our hospital with lower abdominal pain for 1 wk and ascites observed under abdominal sonography. His laboratory findings revealed an isolated elevated tumor marker of carcinoma antigen 125 (167.4 U/mL; normal, < 35 U/mL), and contrast enhanced computed tomography showed peritoneal thickening. Thus, differential diagnoses of TBP, carcinomatosis of an unknown nature, and primary peritoneal malignancy were considered. After both esophagogastroduodenoscopy and colonoscopy produced negative findings, laparoscopic intervention was performed. The histopathological results revealed mesothelioma invasion into soft tissue composed of a papillary, tubular, single-cell arrangement of epithelioid cells. In addition, immunohistochemical staining was positive for mesothelioma markers and negative for adenocarcinoma markers. Based on the above findings, TBP was excluded, and the patient was diagnosed with MPM.
It is important to distinguish MPM from TBP because they have similar symptoms and blood test findings.
Core Tip: Malignant peritoneal mesothelioma (MPM) is an uncommon malignant neoplasm that arises from peritoneum. We present a case of malignant peritoneal mesothelioma mimicking tuberculous peritonitis (TBP). This case highlights the difficulty to distinguish MPM from TBP only based on unspecific clinical manifestations, laboratory tests and images especially in high prevalence area of TB, and therefore sheds light on the importance of laparoscopy which finally helps us confirm the diagnosis in this patient with unexplained ascites.
- Citation: Lin LC, Kuan WY, Shiu BH, Wang YT, Chao WR, Wang CC. Primary malignant peritoneal mesothelioma mimicking tuberculous peritonitis: A case report. World J Clin Cases 2022; 10(10): 3156-3163
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3156.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3156
Malignant peritoneal mesothelioma (MPM) is a rare disease arising in the mesothelial cells in the peritoneum[1]. While it is essential to accurately detect this disease due to its high mortality and poor prognosis with late diagnosis[2], the clinical presentation of MPM is unspecific, causing difficulty with early diagnosis. Clinicians should always consider differential diagnoses, such as peritoneal carcinomatosis, tuberculous peritonitis (TBP), and primary peritoneal neoplasms, in patients with unexplained ascites. To improve our understanding of the disease, avoid misdiagnosis, and provide evidence for clinical treatment and prognosis, we report on this case of MPM mimicking TBP.
This 42-year-old man was admitted to our hospital with lower abdominal pain for 1 wk.
This patient was a Canadian who had lived in Taiwan for 5–6 years. He had suffered from intermittent lower abdominal pain for one week prior to admission. He had visited the clinic, where abdominal ultrasonography showed some ascites. Thus, he was referred to our outpatient department (OPD) for further survey. His accompanying symptoms included intermittent diarrhea, with some “white wire” noted in the stool and tenesmus. He denied fever, cough, dyspnea, weight loss, or urinary symptoms.
The patient had no comorbidity or operation history.
The patient drinks beer sometimes without addiction. He does not smoke or chew betel nuts. There was no remarkable family medical history.
The height and weight of the patient at admission were 174 cm and 74 kg, respectively. His consciousness was clear, and his vital signs were stable. His physical examination showed lower abdomen tenderness with rebounding pain over the suprapubic area. The remainder of the physical examination was normal.
Laboratory testing showed an elevated C-reactive protein (2.6 mg/dL), normal procalcitonin level, and no leukocytosis. Tumor markers, such as carcinoembryonic antigen, carcinoma antigen 199, and tissue polypeptide antigen, were within normal limits, but he had an elevated carcinoma antigen 125 (CA-125) of 167.4 U/mL. Screening for autoimmune titers, HIV, amebiasis antibody test, blood culture, and stool all showed negative results. The laboratory results are shown in Table 1.
| Parameter | Reference range | On admission (5/21) | Day 6 (5/26) |
| Hemoglobin (g/L) | 13-17 | 13.9 | |
| Leucocytes (/µL) | 4000-11000 | 5670 | 6190 |
| Platelets (/µL) | 150000-200000 | 287000 | |
| Procalcitonin (ng/mL) | 0.5 | < 0.05 | |
| HS C.R.P (mg/dL) | 0.748 | 2.640↑ | 3.824↑ |
| Albumin (g/dL) | 3.5-5.7 | 4.2 | |
| Total bilirubin (mg/dL) | 0.3-1.2 | 0.7 | |
| ESR (mm/h) | < 15 | 30↑ | |
| ANA | Negative | Negative (< 1:80X) | |
| ANCA | Negative | Negative (< 1:40X) | |
| Anti-HCV | Nonreactive | ||
| HBsAg | 0.05 | Nonreactive | |
| Anti-HIV | Nonreactive | ||
| CA-125 (U/mL) | 0-35 | 167.4↑ | |
| CA19-9 (U/mL) | 0-35 | 2.0 | |
| CEA (ng/mL) | 5 | 0.6 | |
| TPA (U/L) | 75 | 18.49 | |
| IHA for amebiasis | 1:32 negative | 1:32 negative |
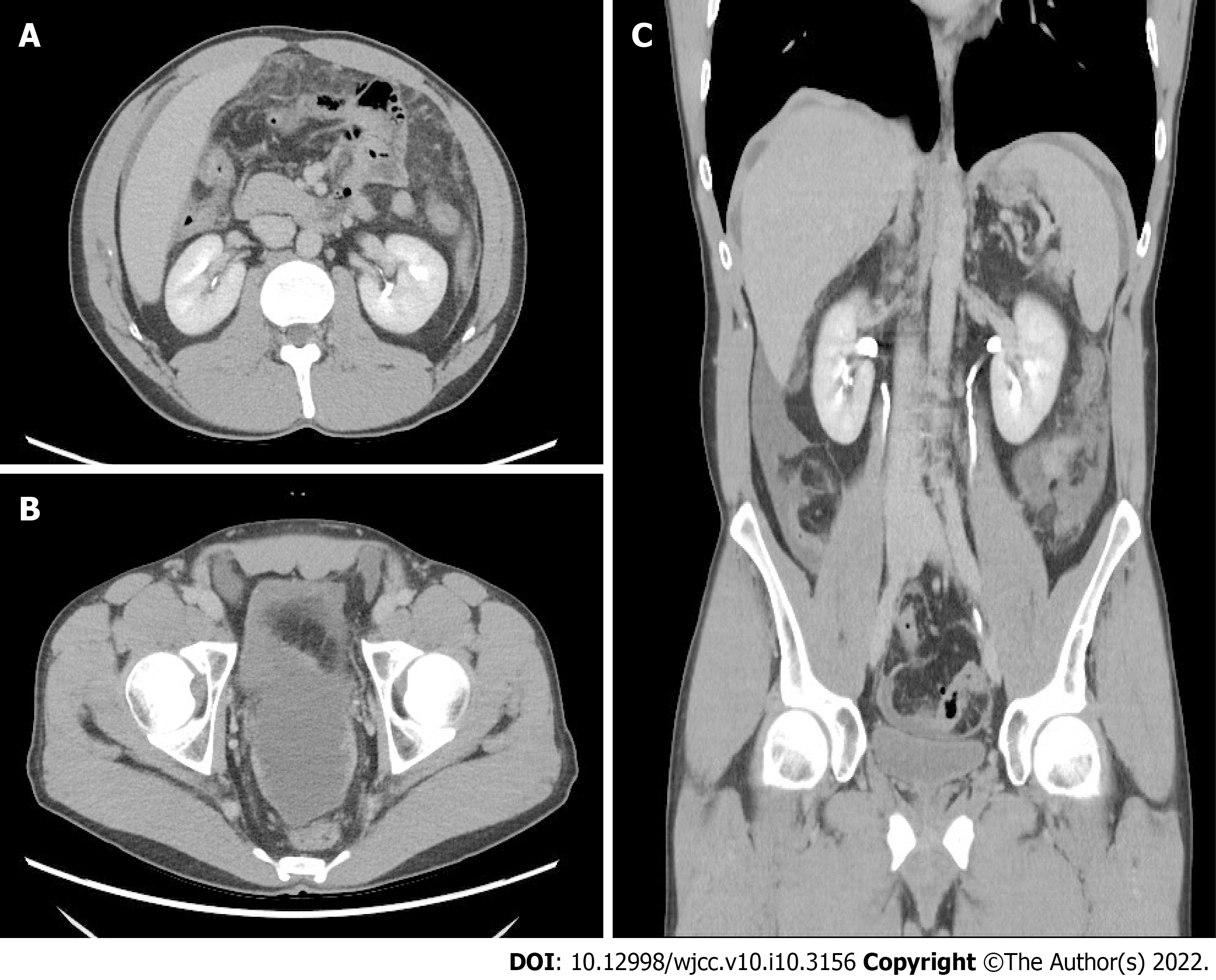
Abdominal sonography showed minimal ascites at the cul-de-sac and negative signs of cirrhosis. Abdominal contrast-enhanced computed tomography (CT) showed irregular peritoneal thickening over the perihepatic and pelvic peritoneum, fat stranding infiltration within the greater omentum, and some ascites in the bilateral subphrenic spaces, paracoclic gutter, and pelvic cavity (Figure 1). Thus, differential diagnoses of TBP and carcinomatosis of an unknown nature were considered.
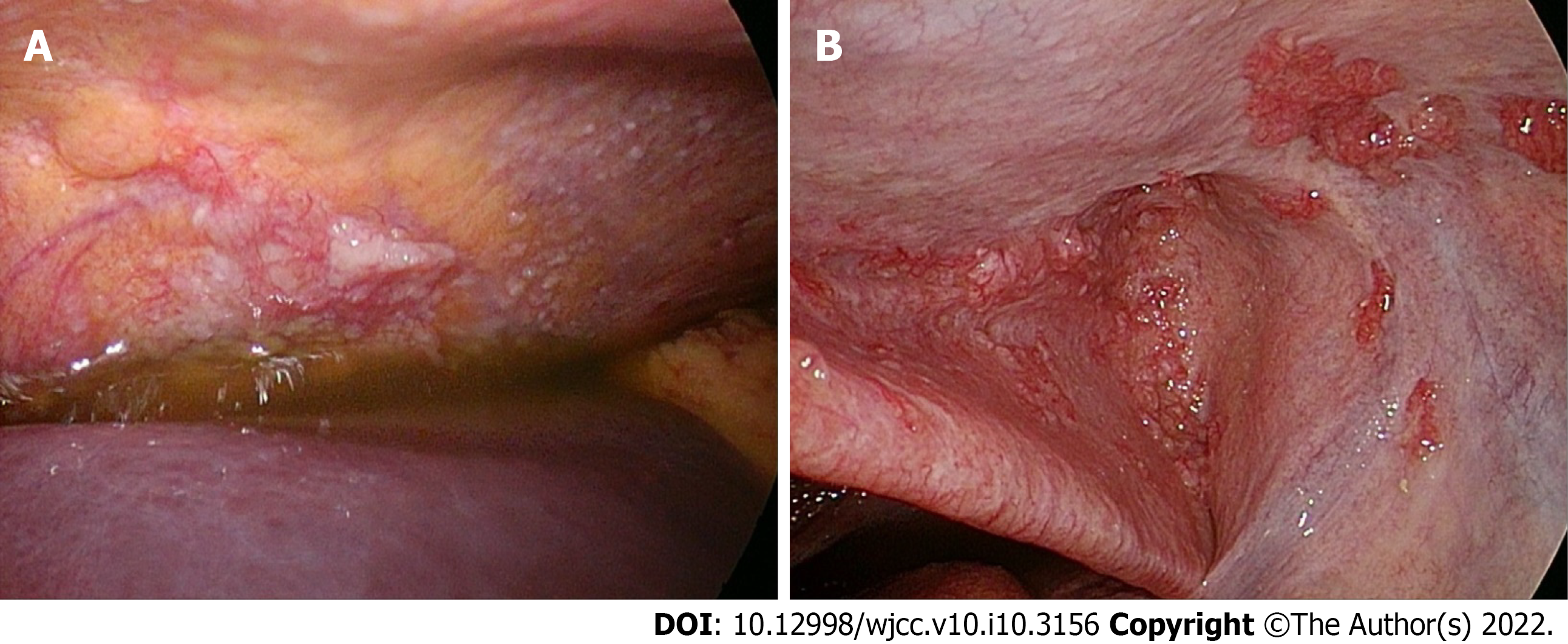
Esophagogastroduodenoscopy (EGD) and colonoscopy revealed no malignant lesion. Because there were not enough ascites under sonography for fine needle aspiration, laparoscopy was performed (Figure 2). In total, 1000 mL of yellow ascites were collected for cytology and other analysis. Multiple whitish nodules were noted over the entire abdominal cavity, and the peritoneal cancer index was over 10. A local resection of dark red papillary lesions on the peritoneum was performed.
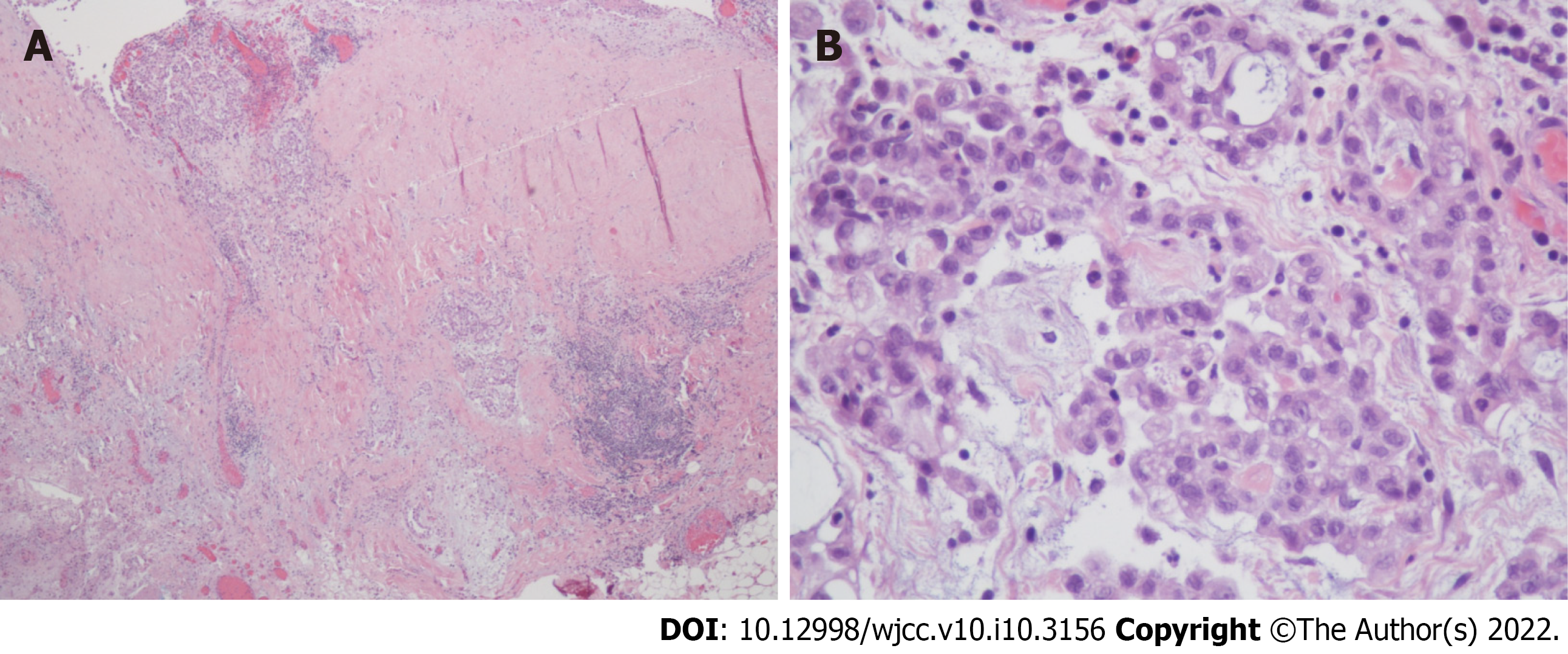
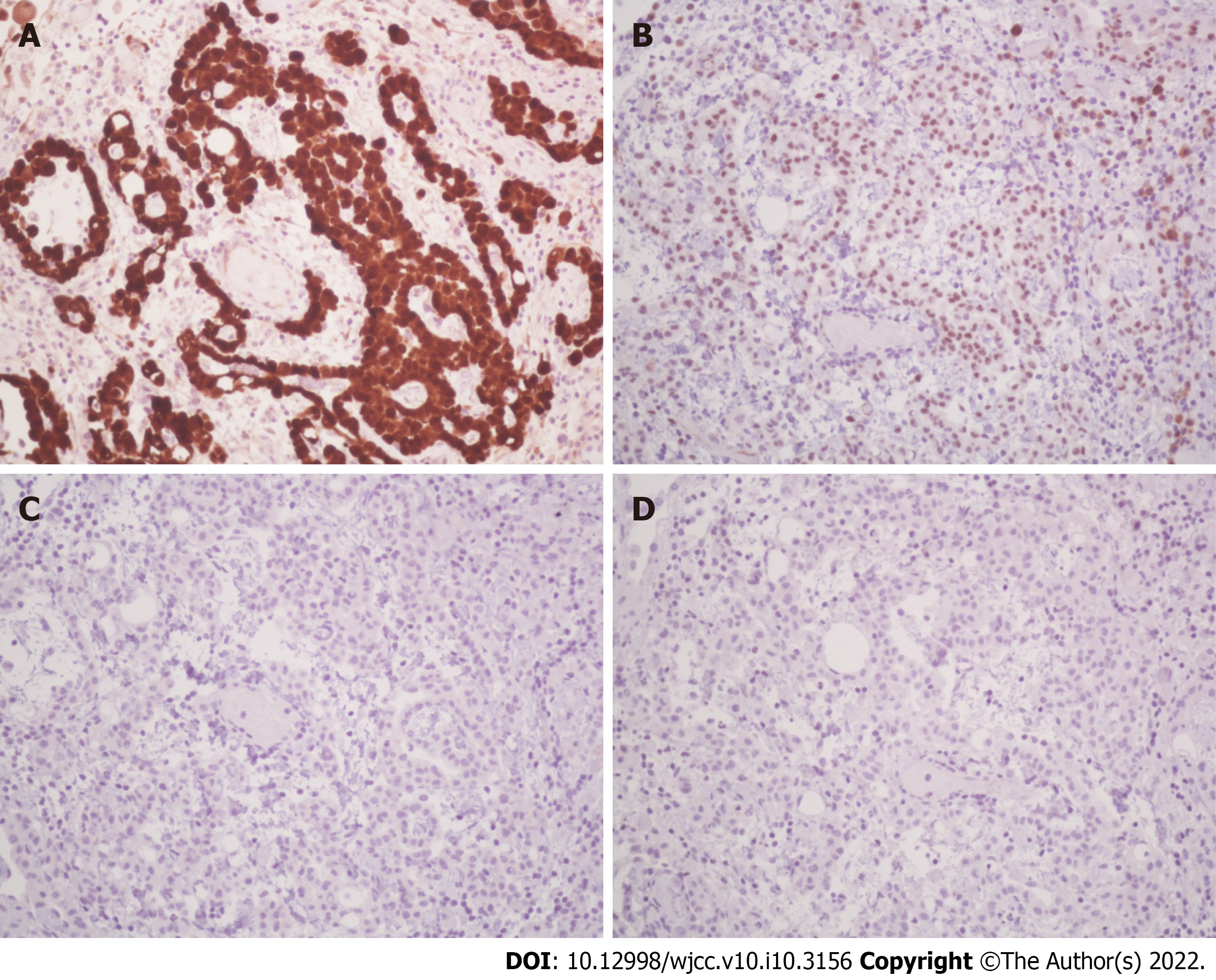
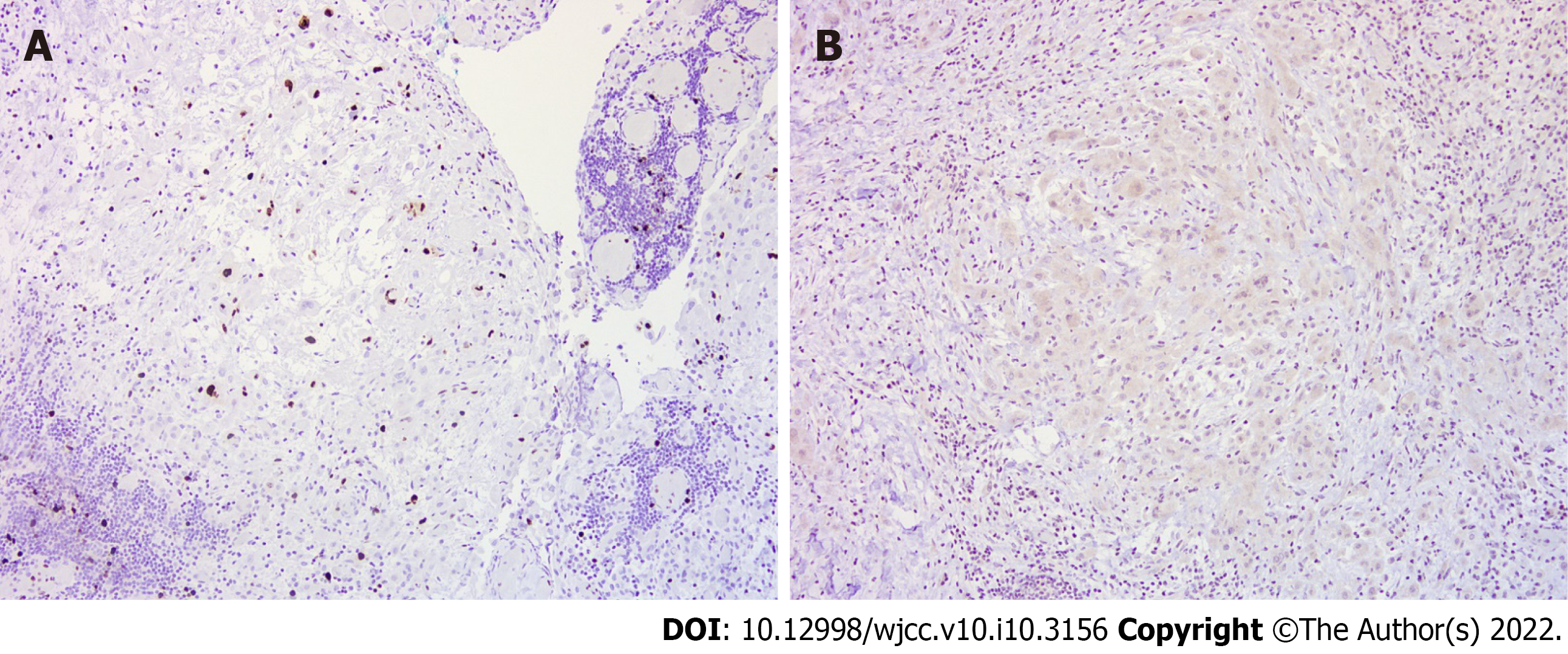
Histologically, according to hematoxylin and eosin (HE) staining, biopsy of the peritoneum revealed soft tissue mesothelioma invasion composed of a papillary, tubular, single-cell arrangement of epithelioid cells with oval-round nuclei, conspicuous nucleoli, and moderate eosinophilic cytoplasm (Figure 3). The immunohistochemical (IHC) staining was positive with calretinin and WT-1 (focal+) (Figure 4A and B), which were specific for MPM and negative for markers of adenocarcinomas, such as PAX-8, TTF-1, and CDX-2 (Figure 4C and D). In addition, Ki-67 proliferative index was < 10% and IHC staining of napsin A was negative (Figure 5). Cytology with cell blocking showed atypical mesothelial cells that were positive for calretinin under IHC staining. No definite microorganism was observed under periodic acid-Schiff staining (PAS), Gomori’s methenamine silver (GMS), and acid-fast staining. The culture of ascites showed no isolated pathogen.
The final diagnosis was MPM.
Intravenous flomoxef was initially started due to the presence of suspicious bacterial peritonitis in the first four days. After encountering negative EGD and colonoscopy findings, a laparoscopic intervention was performed. The patient was discharged on the ninth day after admission and waiting for histopathological results at home.
The patient was then referred to oncology OPD, where he expressed a desire to return to Canada for further treatment.
Mesothelioma is a neoplasm of mesothelial cells in the serous membranes, such as the pleura, peritoneum, pericardium, and tunica vaginalis of the testes, lining the wall of serous cavities[3]. The second most common site of mesothelioma is the peritoneum, following the visceral pleura. MPM occurs in 15%–20% of cases of mesothelioma[3,4].
Metastases to the peritoneum are much more common than primary neoplasms. MPM is an extremely rare and highly lethal disease[3,4]. The first reported case was described by Miller in 1908, where the patient was treated symptomatically and passed away within one year[5]. Boffetta et al[3] reported that the incidence rates of MPM range between 0.5 and 3 cases per million in men and between 0.2 and 2 cases per million in women in industrialized countries.
Toxic exposure to industrial pollutants, especially asbestos, is the main cause of mesothelioma. Other risk factors are exposure to talc, radiation, erionite, mica, and volcanic ash and suffering from chronic peritonitis[3-7]. The presenting symptoms of MPM are nonspecific[4,6]. Abdominal distension is the most common initial complaint, presenting in 30%–80% of patients, while abdominal pain is the second most common symptom, occurring in 27%–58% of patients[5]. In addition, early satiety, nausea, anorexia, weight loss, hernia, fever of unknown origin, night sweats, and the occasional diagnosis found at laparoscopy have been reported in some cases[4-6]. These vague clinical presentations make early diagnosis difficult.
There has been non-specific laboratory data for MPM. Although serum CA-125 Levels were elevated in some cases, the specificity was low[4]. The findings on CT imaging are usually diffuse and have widespread involvement of the peritoneal cavity associated with irregular thickening of the peritoneum, and ascites are present in 60%–100% of newly diagnosed patients[7]. Therefore, clinicians should be suspicious when a radiographic evaluation shows diffuse distribution throughout the abdominal cavity because MPM tends to be more expansive than infiltrative[3].
The CT imaging in our case revealed peritoneal thickening and ascites. Based on these findings, the differential diagnoses of peritoneal carcinomatosis, TBP, and primary peritoneal neoplasms had to be considered[8-10]. Because there were no contributory findings on the upper and lower gastrointestinal endoscopy, peritoneal metastatic carcinomatosis was less likely. TB has always been one of the most severe communicable diseases in Taiwan. Even though TBP is an extremely rare disease and its reported incidence among all forms of TB varies from 0.1%–0.7% worldwide[11], it is one of the differential diagnoses in this situation[12].
The presenting symptoms of TBP are different from the symptoms of MPM in terms of night sweats. Additionally, CA-125 elevation is frequently noticed in TBP[11]. In our case, the gastrointestinal symptoms, such as abdominal pain and ascites, elevated CA-125, and peritoneal thickening that emerged can all present in both MPM and TBP[13]. MPM’s mimicry of these features of TBP makes diagnosis especially difficult in high TB prevalence areas.
CT-guided core needle biopsy or laparoscopic biopsy both provide sufficient material to establish the diagnosis[5,6,14]. In TB, an ascitic fluid culture of the mycobacterium remains the gold standard for diagnosis. Adenosine deaminase of ascitic fluid is a useful tool for identifying patients with a diagnosis of TBP[15]. Histologically, typical lesions of TB are represented by the tuber, which corresponds to caseating epithelioid granuloma containing multinucleated giant cells. By contrast, MPM can be divided into three broad histologic subtypes: Epithelioid, sarcomatoid, and biphasic[15]. The epithelioid type, which is the most common type associated with the best prognosis, forms a tubulopapillary or trabecular pattern of flattened or cuboidal cells with monotonous nuclei that line the papilla or tubules. The sarcomatoid type is typically composed of tightly packed spindle cells. The biphasic type is a mixed form that consists of both epithelial and sarcomatous components[3,5,6,16].
In our case, an ascitic fluid analysis showed no definite microorganism observed under PAS, GMS, or acid-fast staining. The histopathological results revealed mesothelioma invasion into soft tissue composed of a papillary, tubular, single-cell arrangement of epithelioid cells. In addition, immunohistochemical staining was positive for mesothelioma markers and negative for adenocarcinoma markers. Based on the above findings, TBP was excluded. Ultimately, this 42-year-old man was diagnosed with MPM, epithelioid subtype.
The median overall survival of MPM without treatment is generally around 6–12 mo after diagnosis. The mainstream treatment for resectable MPM remains cytoreductive surgery with heated intraperitoneal chemotherapy; this approach has a potential survival outcome greater than five years in carefully selected patients. Even though the disease has shown resistance to standard chemotherapeutic agents, patients with inoperable MPM will be offered systemic treatment[4,5]. Due to its high mortality and poor prognosis, it is essential for physicians to diagnose MPM as early as possible and conduct prompt treatment. Our case highlights the need for high clinical suspicion of MPM in patients who present with clinical features mimicking TBP.
Our patient was a Canadian man who had been living in Taiwan for five years, making it difficult to quantify the detailed influential factors of the disease. For example, while our patient said that many houses had asbestos roofs when he was a child in Canada, we could not weigh this risk factor in our case.
We lost the follow-up of both the treatment and the outcome of this case because the patient decided to return to Canada to receive further treatment.
We reported on a rare case of MPM mimicking TBP and showed that diagnostic laparoscopy provided a precise method for confirming the diagnosis in this patient with unexplained ascites.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu C, Song YH S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Tischoff I, Tannapfel A. [Mesothelioma]. Pathologe. 2017;38:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 2. | Salo SAS, Ilonen I, Laaksonen S, Myllärniemi M, Salo JA, Rantanen T. Malignant Peritoneal Mesothelioma: Treatment Options and Survival. Anticancer Res. 2019;39:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol. 2007;18:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Greenbaum A, Alexander HR. Peritoneal mesothelioma. Transl Lung Cancer Res. 2020;9:S120-S132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Kim J, Bhagwandin S, Labow DM. Malignant peritoneal mesothelioma: a review. Ann Transl Med. 2017;5:236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Anwar A, Kasi A. Peritoneal Cancer. StatPearls. Treasure Island (FL), 2021. |
| 7. | Ros PR, Yuschok TJ, Buck JL, Shekitka KM, Kaude JV. Peritoneal mesothelioma. Radiologic appearances correlated with histology. Acta Radiol. 1991;32:355-358. [PubMed] |
| 8. | Pickhardt PJ, Bhalla S. Primary neoplasms of peritoneal and sub-peritoneal origin: CT findings. Radiographics. 2005;25:983-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Walkey MM, Friedman AC, Sohotra P, Radecki PD. CT manifestations of peritoneal carcinomatosis. AJR Am J Roentgenol. 1988;150:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 113] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Yin WJ, Zheng GQ, Chen YF, Chen DQ, Sun NN, Yang YX, Sun XY, Kang LQ. CT differentiation of malignant peritoneal mesothelioma and tuberculous peritonitis. Radiol Med. 2016;121:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Lo HY, Yang SL, Chou P, Chuang JH, Chiang CY. Completeness and timeliness of tuberculosis notification in Taiwan. BMC Public Health. 2011;11:915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Guirat A, Koubaa M, Mzali R, Abid B, Ellouz S, Affes N, Ben Jemaa M, Frikha F, Ben Amar M, Beyrouti MI. Peritoneal tuberculosis. Clin Res Hepatol Gastroenterol. 2011;35:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Algın MC, Yaylak F, Bayhan Z, Aslan F, Bayhan NA. Malignant peritoneal mesothelioma: clinicopathological characteristics of two cases. Case Rep Surg. 2014;2014:748469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Burgess LJ, Swanepoel CG, Taljaard JJ. The use of adenosine deaminase as a diagnostic tool for peritoneal tuberculosis. Tuberculosis (Edinb). 2001;81:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Levy AD, Arnáiz J, Shaw JC, Sobin LH. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics. 2008;28:583-607; quiz 621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |