Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3131
Peer-review started: July 12, 2021
First decision: October 22, 2021
Revised: October 26, 2021
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 6, 2022
Processing time: 260 Days and 6.7 Hours
Oral potential malignant disorders (OPMDs) are a precancerous condition of oral disease. Several studies have found that betel quid chewing, smoking and alcohol drinking might be the risk factors of OPMDs. But the relationships of them, especially their interaction are still inconclusive.
To evaluate the relationship between betel quid chewing and OPMDs and to explore the interaction of smoking and alcohol drinking on the relationship.
We searched PubMed, Web of Science, Embase and the Cochrane Library databases with items complete until January 2021 for relevant studies. The research data were extracted according to the inclusion criteria. The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the effect size. Subgroup analysis was performed to assess interactions between exposures and OPMDs. Relative excess risk of interaction (RERI) was used to estimate the size of interaction.
Nine articles were selected in the final meta-analysis. The results showed that betel quid chewing (pooled OR: 8.70, 95%CI: 5.18-14.61), alcohol consumption (pooled OR: 1.95, 95%CI: 1.5-2.55), and smoking (pooled OR:4.35, 95%CI: 3.06-6.2) could significantly increase the risk of OPMDs compared to individuals without these behaviors. Smoking and alcohol drinking synergistically increased the association between betel quid chewing and OPMDs (pooled OR(BQ+SM):14.38, 95%CI: 7.14-28.95; pooled OR(BQ+DK): 11.12, 95%CI: 8.00-15.45, respectively). The RERI(BQ+SM) and RERI(BQ+DK) were 2.33 and 1.47, respectively.
The synergistic effects between smoking/drinking and betel quid highlights the importance of focusing on individuals with multiple exposures. Further study should be conducted to confirm these interactions.
Core Tip: Betel quid chewing, smoking and drinking could significantly increase the risk of oral potential malignant disorders (OPMDs). And smoking and drinking synergistically increased the association between betel quid chewing and OPMDs.
- Citation: Lin HJ, Wang XL, Tian MY, Li XL, Tan HZ. Betel quid chewing and oral potential malignant disorders and the impact of smoking and drinking: A meta-analysis. World J Clin Cases 2022; 10(10): 3131-3142
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3131.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3131
Oral cancer is the sixth most common cancer worldwide[1], occurring predominantly in developing countries. It has a high incidence in young adults creating a major economic burden to families and society. Diagnosis time is the most important factor affecting the prognosis; therefore, early diagnosis is crucial. Oral squamous cell carcinoma (OSCC) is the most common type of oral cancer and the majority of OSCCs are transformed from oral potential malignant disorders (OPMDs). OPMDs are a precancerous condition of oral disease which can be transformed and cured. Hence, controlling the disease in a precancerous state is the most effective prevention strategy. A meta-analysis found that the global prevalence of OPMDs was 4.47% higher in Asia and South America[2]. Leukoplakia, erythroplakia, oral submucous fibrosis (OSF) and oral lichen planus are four common categories of OPMDs focused upon in the study. Among them, OSF and leukoplakia, especially erythroleukoplakia, have a higher risk of malignant transformation[3-5].
A number of studies have shown that betel quid and areca nut chewing is associated with OSF, leukoplakia and other premalignant disorders[6-8]. In contrast, the effects of smoking and alcohol consumption on OPMDs were mixed in studies, with some suggesting that smoking or alcohol increases the risk of diseases[9-11], while others reporting the opposite conclusion[10,12-13]. At present, about 600 million people in the world have the habit of betel quid chewing[14], accounting for about 10% of the global population with a younger age trend[15,16].
On the other hand, they generally have multiple exposures, such as most areca nut chewers are smokers[17-19]. If there is a joint effect among multiple exposure factors, the incidence of disease will greatly increase when multiple exposure factors exist. Lee et al[12] found that when smoking and betel quid were combined, the risks of OPMDs were greater than the sum of each of them alone, indicating the potential interaction between smoking and betel quid. However, Ray et al[20] did not observe such an interaction. Similarly, the same situation was observed in studies of betel nut and alcohol consumption exposure. Hence, whether smoking and alcohol consumption play a role in the relationship between betel quid and OPMDs is uncertain. The purpose of this meta-analysis was to evaluate the relationship between betel quid chewing and OPMDs and to explore the effects of smoking and alcohol drinking on this process.
We systematically searched four databases: PubMed, Web of Science, Embase, and the Cochrane Library. The search terms were: (1) Oral precancerous lesions OR oral premalignant lesions OR oral potentially malignant disorders OR oral leukoplakia OR oral submucous fibrosis OR oral lichen planus OR oral erythema OR erythroplakia; (2) Betel nut OR betel quid OR areca nut OR pan chewing; (3) Alcohol OR drinking OR alcoholic beverage OR ethanol; and (4) Smoking OR cigarettes OR tobacco. The specific search strategy is shown in Table 1. The study language was limited to English. The data retrieval process was completed in January 2021.
| Number | Contents |
| 1 | Oral potentially malignant disorders OR oral precancerous lesions OR oral premalignant lesions OR oral leukoplakia OR oral submucous fibrosis OR oral lichen planus OR oral erythema OR erythroplakia |
| 2 | Betel quid OR betel nut OR areca nut OR pan chewing |
| 3 | Alcohol OR drinking OR alcoholic beverage OR ethanol |
| 4 | Tobacco OR smoking OR cigarettes |
| 5 | (#1) AND (#2) AND (#3) |
| 6 | (#1) AND (#2) AND (#4) |
| 7 | (#5) OR (#6) |
The study inclusion criteria were: (1) The diagnosis of oral potentially malignant disorders is confirmed by a clinician and histological biopsy; (2) The exposure factors included both betel quid chewing and smoking, or both betel quid chewing and alcohol drinking; and (3) The study provided ORs and 95% confidence intervals (CIs) or sufficient information to calculate them.
After using Endnote software and manually to remove duplicates, literature screening was carried out. Firstly, two authors (Hui-Jun Lin and Xiao-Lei Wang) conducted preliminary screening by reading the titles and abstracts respectively, and then carefully read the full text to select the final literatures. Finally, the third author (Meng-Yuan Tian) was consulted to make the decision when the conclusions between the two authors were inconsistent.
The outcome indicators were oral potential malignant disorders, including oral leukoplakia, oral erythema, OSF and OLP. Though the specific mechanism of betel quid to every precancerous lesion were different, we believed that betel quid could cause all these diseases just with an inconsistent degree of damage after reading literatures. Therefore, it was reasonable to include the four precancerous lesions in the meta-analysis. Several precancerous lesions might be present in a study, or only one of them. The exposure categories were divided into five types: betel quid chewing (BQ), smoking (SM), alcohol drinking (DK), betel quid chewing and smoking (BQ + SM), betel quid chewing and alcohol drinking (BQ + DK). The presence of all three exposures is rarely mentioned in studies, this case was not considered in this meta-analysis. BQ included exposure to betel quid, betel nut, areca nut and other products containing betel quid; SM referred to exposure to cigarettes, bidi and other tobacco products; and DK stood for exposure to all alcoholic beverages, including beer, white wine, etc. BQ + SM means exposure to both SM and BQ. BQ + DK means exposure to both BQ and DK. All exposures were defined as having lasted at least 1 year. The odds ratios (ORs) and 95% confidence intervals (CIs) were extracted because the number of cases/controls in each exposure category were not specified. In the data extraction of BQ, SM, and DK, we chose the OR and 95%CI of the single exposure category, such as smoking only. If the single exposure category data were not provided in the article, the adjusted total population exposure data were selected. Other information, such as study design and types of oral precancerous lesions, were also collected in a standardized manner.
Newcastle-Ottawa Scale (NOS) criteria was used for the quality assessment of case-control studies respectively. NOS scale is a tool for evaluating the quality of case-control study with ten questions, consisting of three main components: Selection, Comparability and Exposure. The quality of cross-sectional study was assessed using the Agency for Healthcare Research and Quality (AHRQ) guidelines (11 questions) recommended by AHRQ. Each study was evaluated independently by two authors (Hui-Jun Lin and Xiao-Lei Wang), and they would make a discussion when the results were inconsistent. If no agreement could be reached, a third author (Meng-Yuan Tian) would join to discuss and reach a consensus. The higher the score of both scales, the better the quality. And the score of < 6, 6-7, and > 7 indicated that the studies had low, medium and high quality, respectively. Only literatures of medium quality or above could be included in this meta-analysis.
The data were analyzed using Stata 12.0 (Statacorp, Cannon Creek, TX, United States). We measured the effect size with ORs and 95%CIs, and performed heterogeneity tests using I2 statistics. If I2 was more than 50%, indicating greater heterogeneity, a random-effects model was used to calculate pooled effects; otherwise, a fixed-effects model was used. Sensitivity analysis was performed using the trim-and-fill method, which investigated whether the pooled OR changed significantly when the studies were eliminated one by one. Publication bias was assessed using Egger’s regression and funnel plots, lnOR in the X-axis, standard error of lnOR in the Y-axis. Asymmetric funnel plots suggested the existence of bias.
Subgroup analysis was conducted to analyze the association between SM or DK and OPMDs, and preliminarily explore the interaction effect of SM/DK and BQ using stratified analysis. Relative excess risk of interaction (RERI) was used to estimate the size of interaction. A and B are denoted two exposure factors. A and B stand for their absence, with formula (If RERI = 0, there was no interaction[21]).
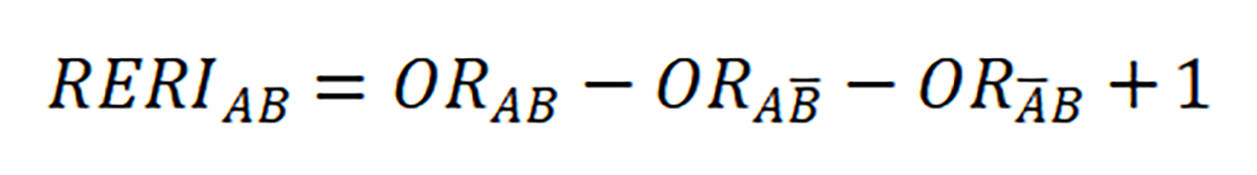
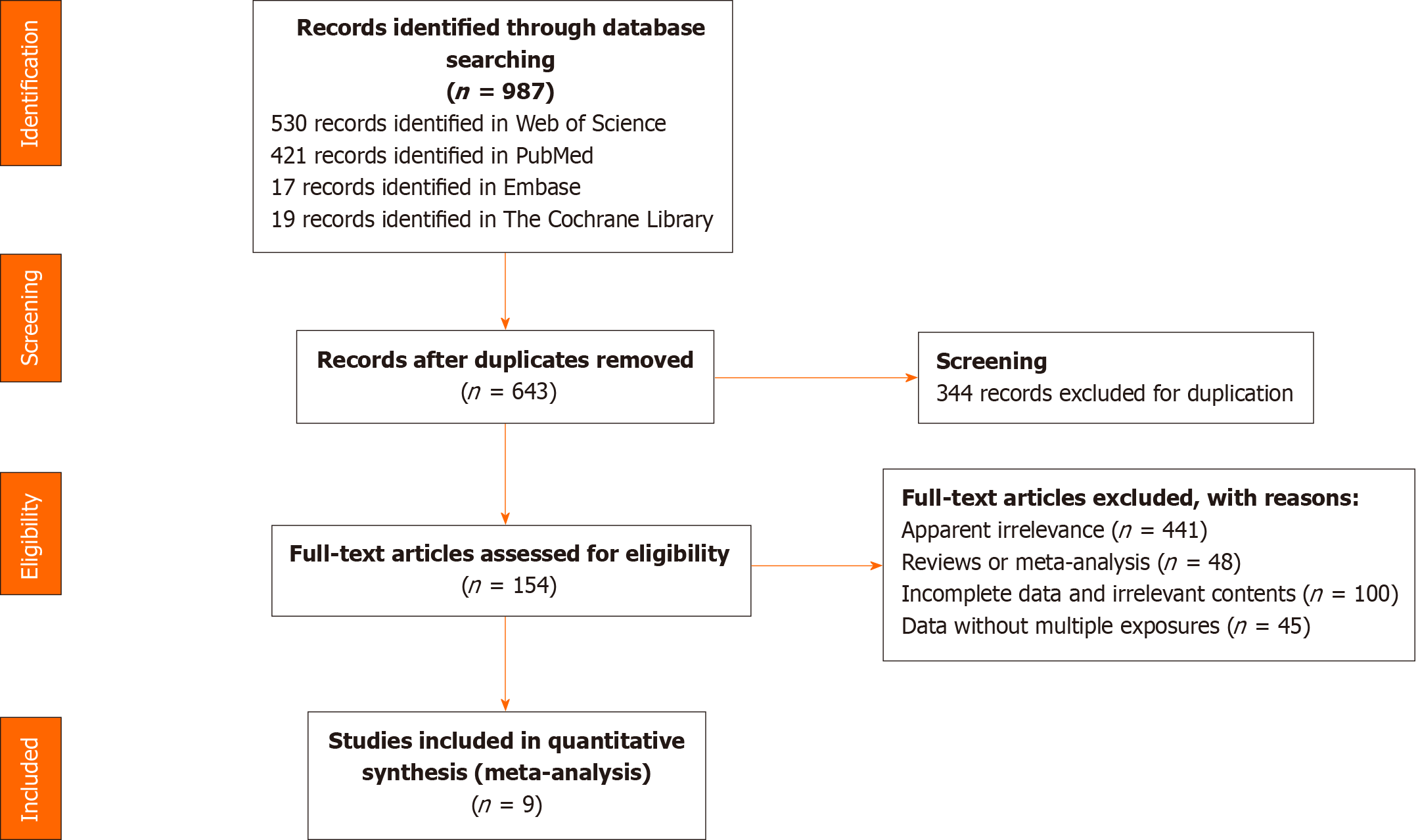
A total of 987 studies were retrieved, of which 344 were duplicates. We excluded 499 articles when reading titles and abstracts including 441 completely unrelated articles and 48 reviews or meta-analysis. And an additional 145 articles were excluded after reading full text. Finally, nine articles were included in the meta-analysis. The article selection process is shown in Figure 1.
Table 2 presents the characteristics of the final nine studies. Only one study contained four precancerous lesions[22], the other studies only included fewer condition[7,12,13,20,23-26]. One of the studies reported leukoplakia and OSF as two separate diseases, and discussed the harm of betel quid to these two respectively[13], so the effect sizes were extracted separately. Among the studies, 9 studies reported the effect size of BQ, 9 of SM, 6 of DK, 8 of BQ + SM, 9 of BQ + DK. All studies included both males and females.
| Ref. | Publication year | Study diseases | Study area | Study design | Sample size | Age | ORs and 95% confidence intervals | NOS/AHRQ scale | ||||
| BQ | SM | DK | BQ + SM | BQ + DK | ||||||||
| Yang et al[12] | 2001 | Leukoplakia, OSF | Taiwan | Cross sectional | 312 | 20 and above | 4.51 (1.30-15.62) | 1.05 (0.5-2.23) | 1.8 (0.88-3.73) | 3.75 (0.99-14.19) | 7.49 (1.95-28.69) | 8 |
| Lee et al[13] | 2003 | Leukoplakia | Taiwan | 1:4 case control | 125:876 | 15 and above | 22.3 (11.3-43.8) | 6.1 (3.4-10.6) | 1.8 (1.1-2.8) | 40.2 (16.3-99.2) | 16.8 (7.2-39.5) | 8 |
| OSF | 1:4 case control | 94:876 | 15 and above | 40.7 (16-103.7) | 7 (3.5-14.3) | 1.8 (1.1-3.1) | 57.9 (16-209.6) | 31.7 (10.1-99.3) | ||||
| Yang et al[26] | 2005 | OSF | Taiwan | 1:1 case control | 62:62 | 20 and above | 4.51 (1.20-16.94) | —— | —— | 8.68 (1.87-40.23) | —— | 7 |
| Chung et al[25] | 2005 | Leukoplakia | Taiwan | Cross sectional | 525 | 15 and above | —— | 3.69 (1.61-8.43) | 2.36 (0.91-6.13) | 8.54 (2.58-28.23) | 5.82 (1.10-30.84) | 7 |
| Thomas et al[7] | 2008 | Leukoplakia | PNG | Case control | 197:1282 | 18 and above | 3.8 (1.7-8.4) | 6.4 (4.1-9.9) | —— | 24.3 (8.7-67.4) | —— | 6 |
| Mehrotra et al[27] | 2013 | OSF | India | Case control | 448:2688 | 15 and above | 6.38 (4.29-9.48) | 4.31 (2.84-6.55) | 3.19 (1.36-7.45) | —— | 9.79 (6.51-14.75) | 8 |
| Chher et al[23] | 2018 | OPMDs | Cambodia | Cross sectional | 1614 | 18 and above | 6.75 (3.32-13.72) | 3.74 (1.89-7.41) | 1.88 (0.81-4.36) | —— | 5.40 (0.64-45.36) | 7 |
| Ikeda et al[24] | 1995 | OSF, lichen planus | Cambodia | Cross sectional | 1319 | 15 and above | 21.66 (5.06-92.75) | 8.89 (1.78-44.42) | —— | 17.16 (3.09-95.48) | —— | 7 |
| Ray et al[21] | 2013 | Leukoplakia, OSF | India | Case control | 698:766 | 10 and above | 5.15 (2.63-10.09) | 4.38 (2.14-8.96) | —— | 5.75 (2.32-14.27) | —— | 7 |
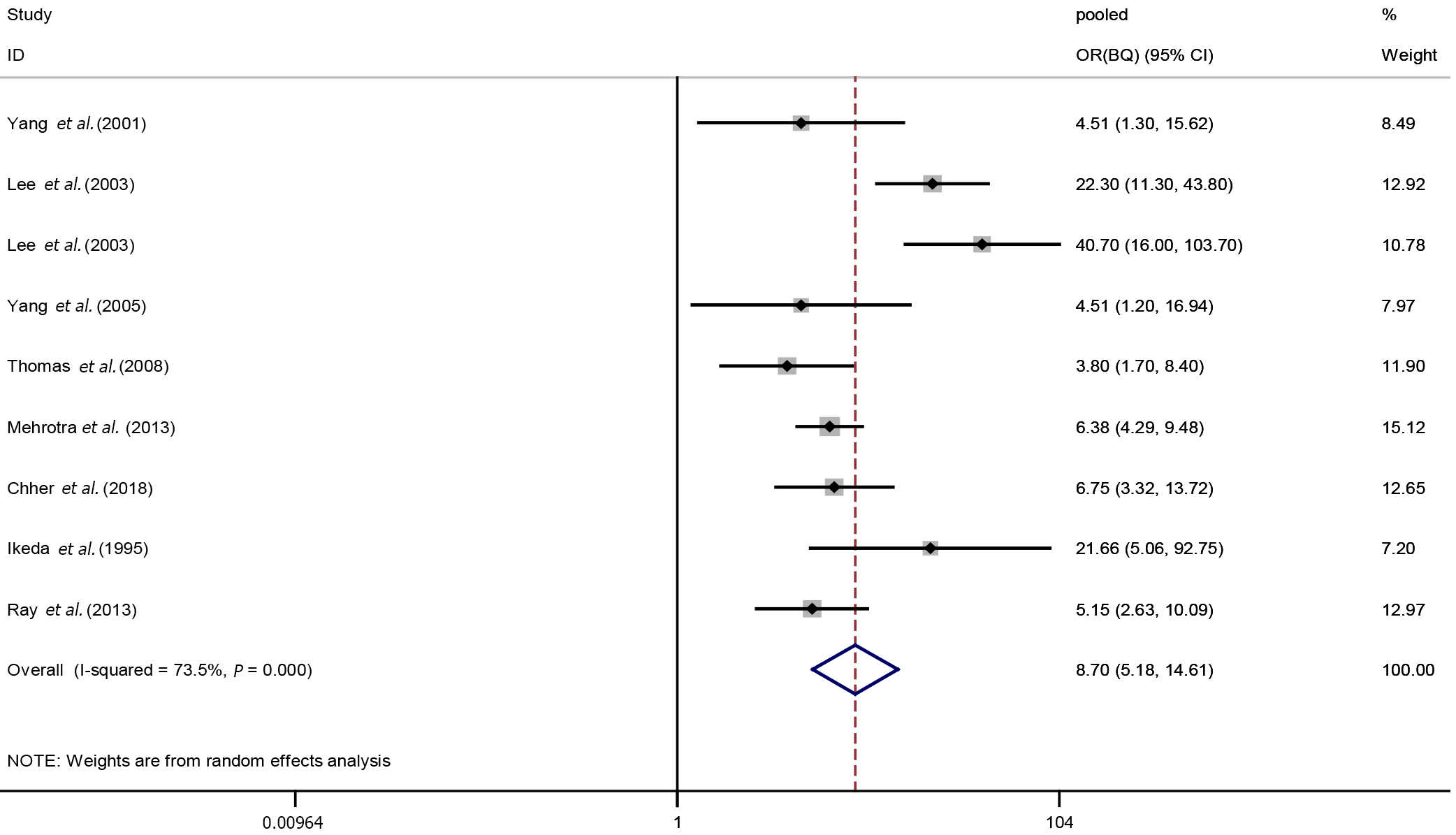
Nine studies were included in our meta-analysis of the relationships between BQ exposure and OPMDs. The analysis revealed great heterogeneity (I2 = 73.5%, P < 0.001) between studies, so a random-effects model was used to estimate the pooled OR and 95%CI. The results suggested BQ was associated with an increased risk of OPMDs (pooled OR(BQ): 8.70, 95%CI: 5.18-14.61). A forest plot of the results of the individual studies and the pooled result was shown in Figure 2.
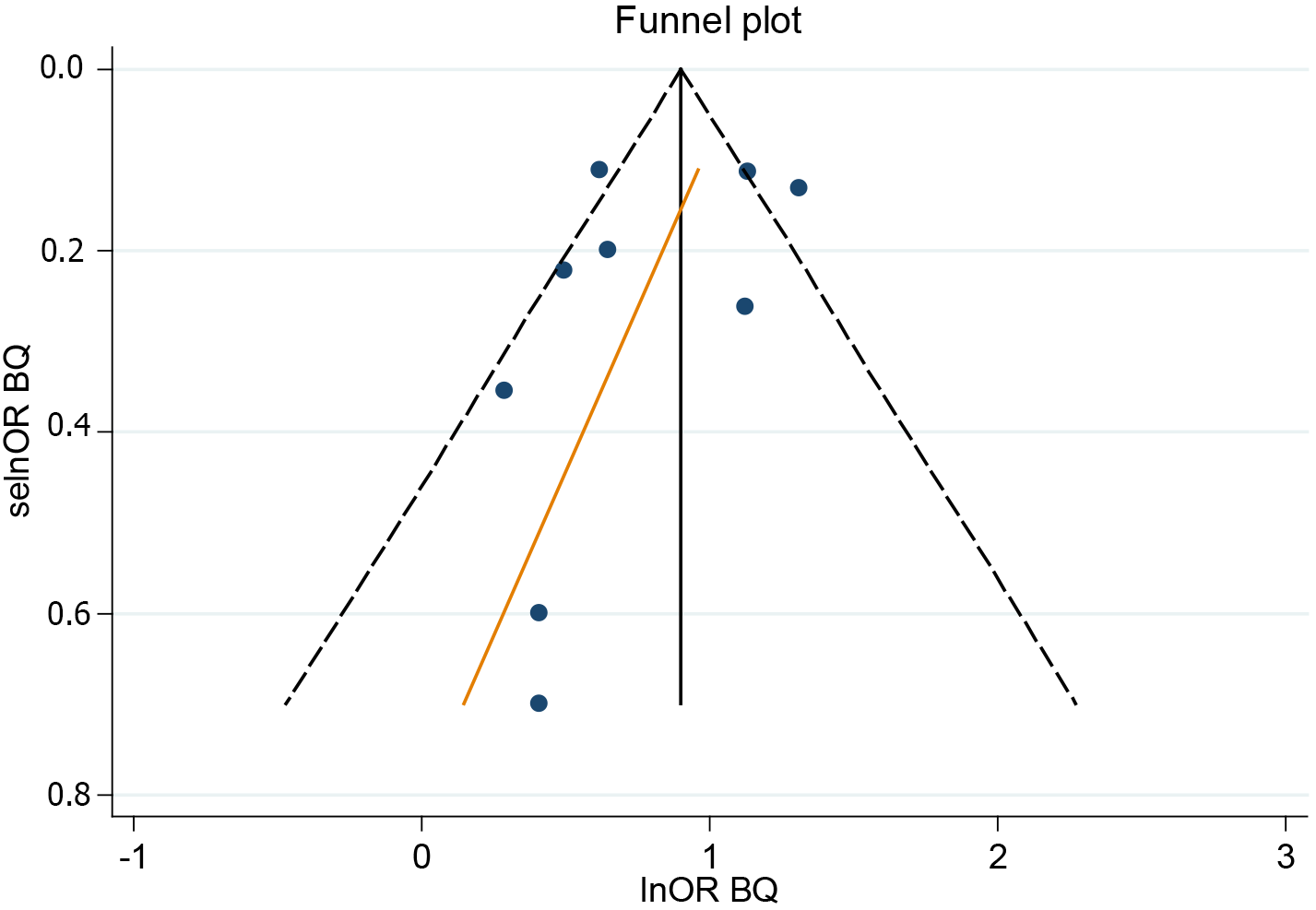
Sensitivity analysis suggested that the combined OR(BQ) was stable and that none of the individual studies disproportionately influenced the results. The funnel plot and Egger’s regression test showed no evidence of publication bias (Figure 3).
Separate effects of smoking or alcohol drinking: In order to better explore the role of SM or DK on the association between BQ and OPMDs, we first analyzed the separate effects of SM or DK with the disease. Nine studies were included in meta-analysis of the relationships between SM exposure and OPMDs, six of DK. Heterogeneity tests showed a lack of heterogeneity in DK (I2 = 0.0%, P = 0.889), but significant heterogeneity in SM (I2 = 61.6%, P = 0.008). We found both SM and DK increased the risk of OPMDs (pooled OR(SM): 4.35, 95%CI: 3.06-6.2; pooled OR(DK): 1.95, 95%CI: 1.5-2.55). There was no evidence of publication bias and the sensitivity analysis showed that the results were stable.
Effects of combined exposure with oral potential malignant disorders: In the assessment of combined BQ and SM, or BQ and DK, there was no evidence of publication bias and the sensitivity analysis showed the results were stable.
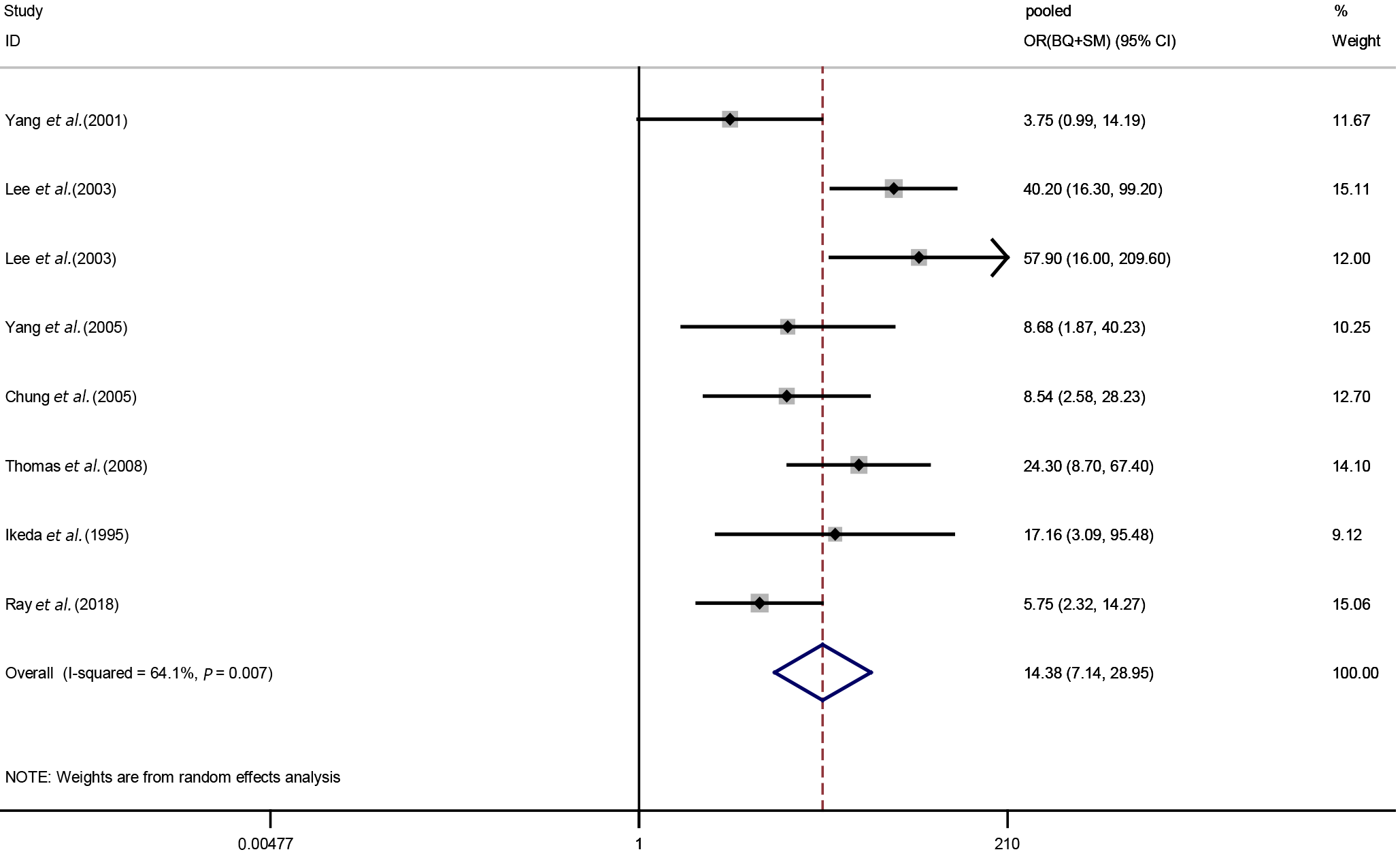
Effects of smoking and betel quid chewing on oral potential malignant disorders: Eight studies were included in our meta-analysis of the relationships between BQ + SM exposure and OPMDs. After the heterogeneity test, a random-effects model was used to calculate the pooled OR. The results showed that smoking and betel quid exposure increased the incidence of OPMDs (pooled OR (BQ+SM): 14.38, 95%CI: 7.14-28.95), and a higher risk degree than BQ alone (Figure 4).
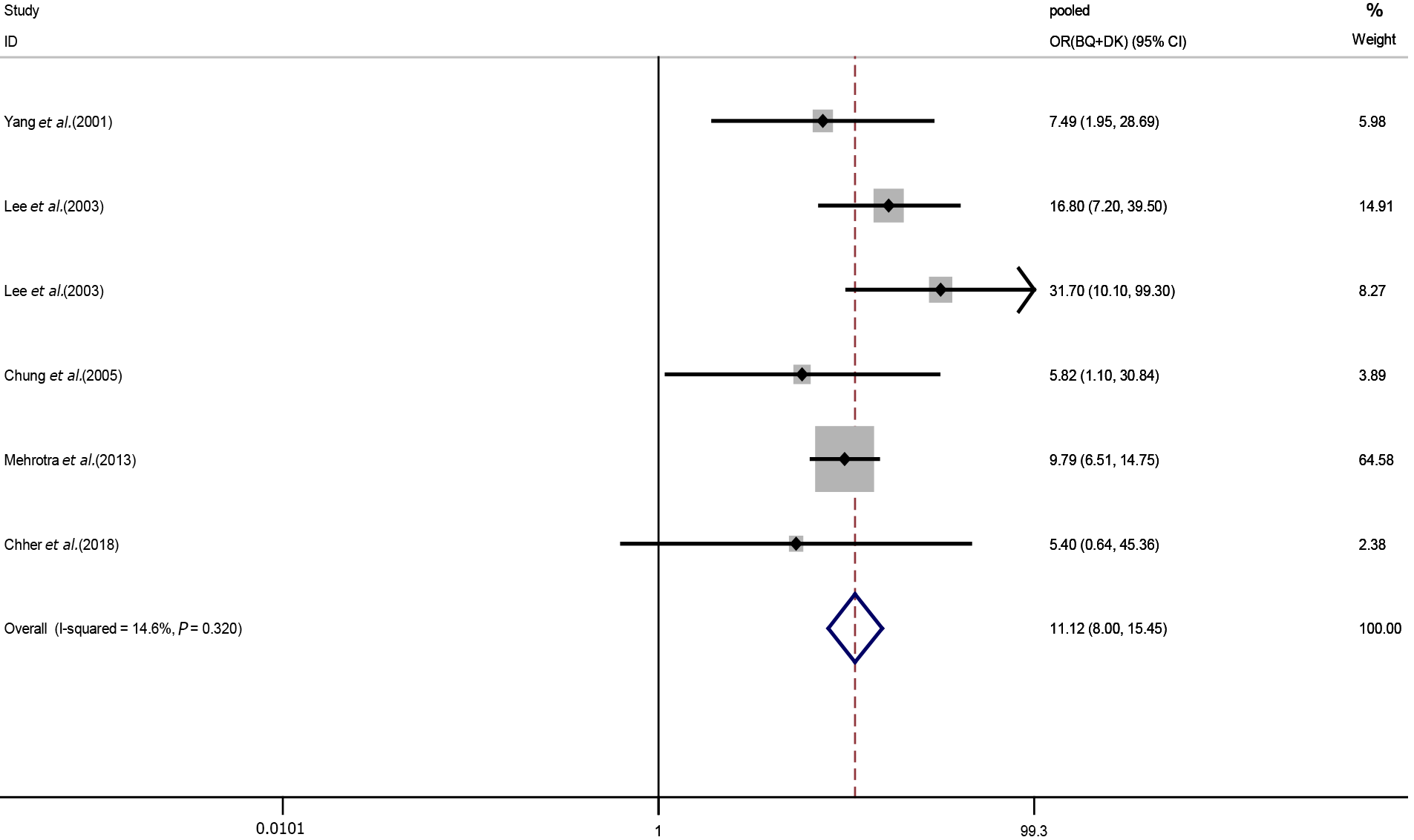
Effect of alcohol drinking and betel quid chewing on oral potential malignant disorders: Nine studies were included in our meta-analysis of the relationships between BQ + DK exposure and OPMDs. A fixed-effects model was used to calculate the pooled OR for the risk of both BQ and DK exposure. We discovered alcohol consumption reinforced the effect of betel nut on OPMDs (pooled OR (BQ+DK): 11.12, 95%CI: 8.00-15.45) (Figure 5).
As shown in Table 3, pooled OR(BQ) plus pooled OR(SM) was smaller than pooled OR(BQ+SM), and pooled OR(BQ) plus pooled OR(DK) was smaller than pooled OR(BQ+DK). We speculated that both SM and DK had synergistic effects with BQ. After calculation, RERI(BQ+SM) was 2.33, and RERI(BQ+DK) was 1.47, providing further evidence of an interaction.
| Exposure category | Pooled OR | 95%CI |
| BQ | 8.70 | 5.18-14.61 |
| SM | 4.35 | 3.06-6.20 |
| DK | 1.95 | 1.50-2.55 |
| BQ and SM | 14.38 | 7.14-28.95 |
| BQ and DK | 11.12 | 8.00-15.45 |
Our study found that betel quid chewing was associated with OPMDs. There was an interaction between smoking and betel quid chewing, and alcohol drinking and betel quid chewing with respect to their association with OPMDs. The data extracted from the original study was adjusted for confounding factors such as gender, age, and education status, though varies from article to article. And the sensitivity analysis also showed that our study results were stable with no significant bias. This meta-analysis provides a new approach for the prevention of OPMDs. It revealed that individuals with multiple exposures had very high risk because of synergistic interaction, which remind us that we should pay more attention to these people.
Oral potential malignant disorders are a kind of disease with the risk of malignant transformation. It is of great significance for the prevention of oral cancer to realize the related risk factors and avoid them in daily life so as to reduce the occurrence of the disease. Areca nut is the fourth largest psychoactive substance in the world. The International Agency for Research on Cancer recognized that betel quid and areca nut were carcinogens in 2004[27], and studies have found that betel quid chewing is a risk factor for many diseases, including oral cancer, oropharyngeal cancer[28] and esophageal cancer[29]. And a variety of oral diseases have also been linked to betel quid chewing[30,31]. Studies found that betel quid harms the cavity mainly in two aspects: first, arecoline, the active ingredient in betel quid, would produce a variety of nitrosamines in the acidic environment of the oral cavity and stomach. And nitrosamines could interact with DNA, protein or other biological macromolecules, and then induce oxidative stress and participate in the canceration of oral mucosa. Causes of damage to the oral mucosa include oxidative stress caused by nitrification of arecoline and mechanical stimulation of the mucosa[32]. In addition, hydrated lime in betel quid could alter intracellular calcium homeostasis, causing abnormal activation of calcium-mediated transduction cascades and leading to oral cancer. Second, the mechanical stimulation of betel quid to oral mucosa caused by repeated chewing. Moreover, the current studies found that chewing betel quid could cause the changes in oral microbiota which might be related to oral diseases[33,34].
We found betel quid chewing has significantly increased the risk of OPMDs which is consistent with the majority of studies[35-37]. But the effects of betel quid varied greatly across studies and there was a large variation of the ORs[36,38,39]. It might be related to the study area and population. The production methods and ingredients of betel quid vary in different countries and the degree of oral damage also differed. But all the countries in the literature we included chew the raw betel quid, supplemented with such auxiliary materials as betel nut leaves and flowers, and all of them do not contain tobacco. Therefore, the results of our meta-analysis were robust and reliable.
In the subgroup analysis, we found that smoking alone was also associated with an increased risk of OPMDs, which is consistent with other studies[9,40]. There was an interaction between smoking and betel quid chewing, and tobacco enhanced the toxicity of betel quid to the oral mucosa. And studies showed that the risk of OPMDs in betel quid with tobacco was greater than that in betel quid alone[37]. Several potential mechanisms can explain this synergistic effect. The site in the oral cavity where tobacco is placed becomes keratinized due to friction. With the prolongation of tobacco use, the degree of mucosal keratosis deepens and plaques are formed[41]. The mechanical damage caused by betel quid chewing could accelerate this process. Alternatively, both smoking and betel quid chewing could lead to the accumulation of nitrosamines in the cavity and increase oxidative stress[32], which might exert a synergistic effect. Additionally, nicotine has been reported to have a synergistic effect on betel quid cytotoxicity[42]. And the synergistic effect has been reported in other diseases. Liu et al[43] found the interactions between betel quid and cigarette or alcohol in malignant transformation of OSF. Similarly, the same synergistic effects in oral cancer were suggested in the study of Petti et al[44].
In the relationship between alcohol consumption and OPMDs, different studies had different results[10,40,45]. Our subgroup analysis found that alcohol consumption alone had an association with OPMDs. It was mainly due to the direct stimulation to oral mucosa by alcohol, which could be enhanced if there is damage in the mouth. The results of our meta-analysis showed that betel quid chewing interacted with alcohol consumption. Animal experiments have shown that chronic alcohol exposure can cause atrophy of the oral mucosa, making it more sensitive to stimulation[46], such as the mechanical stimulation of betel nut chewing. The increased permeability caused by alcohol could accelerate the absorption of arecoline by the mucosa. Additionally, both betel quid chewing and alcohol drinking could change the composition of the oral microbiome[47]. Betel quid chewing combined with heavy alcohol drinking has been reported to change the diversity of the oral microbiome[33]. Oral microorganisms normally maintain oral health through immune regulation[48] and microbial disorders are associated with various diseases including OPMDs[49,50].
There are still some limitations to our study. The selected studies had cross-sectional and case-control study design, which provide weak evidence of causality. The number of people in each subgroup may have been inadequate because of having five exposure categories, though the direct number were not reported. Further investigation should be conducted with a large cohort study to accurately assess the impact of smoking and alcohol drinking on the association between betel quid chewing and OPMDs.
The meta-analysis suggested that betel quid chewing is associated with an increased risk of oral potential malignant disorders. There was a synergistic effect between smoking or alcohol drinking and betel quid chewing. Therefore, we should focus on high-risk groups with multiple exposure, especially individuals with smoking and betel quid exposed, and provide health education about the harmful effects of unhealthy behavior. Governments should develop policies to quit betel quid, smoking and alcohol, especially in individuals with multiple exposure, in order to control and reduce the incidence of oral potential malignant disorders.
Studies have found that betel quid chewing, smoking and alcohol drinking might be the risk factors of oral potential malignant disorders (OPMDs). But the relationships of them, especially the interaction are still inconclusive.
This meta-analysis was to determine the damage of betel quid on OPMDs and to explore the potential interaction between smoking, alcohol drinking and betel quid. It could provide new ideas for the prevention of OPMDs.
To evaluate the relationship between betel quid chewing and OPMDs and to explore the interaction of smoking and drinking on the relationship.
We searched four database and finally selected 9 from 987 studies. The research data were extracted according to the inclusion criteria. The pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the effect size and relative excess risk of interaction (RERI) was used to estimate the size of interaction.
The results showed that betel quid chewing (pooled OR: 8.70, 95%CI: 5.18-14.61) could significantly increase the risk of OPMDs. And smoking and drinking synergistically increased the association between betel quid chewing and OPMDs (pooled OR(BQ+SM):14.38, 95%CI: 7.14-28.95, RERI(BQ+SM): 2.33; pooled OR(BQ+DK): 11.12, 95%CI: 8.00-15.45, RERI(BQ+DK): 1.47, respectively).
Betel quid chewing is associated with an increased risk of oral potential malignant disorders. There was a synergistic effect between smoking or drinking and betel quid chewing.
Governments should focus on individuals with multiple exposure.
We are thankful to Tan HZ for his support guidance and Wang XL, Tian MY and Li XL to contributions in data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Public, environmental and occupational health
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeng J S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Guo X
| 1. | Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010;46:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Mello FW, Miguel AFP, Dutra KL, Porporatti AL, Warnakulasuriya S, Guerra ENS, Rivero ERC. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J Oral Pathol Med. 2018;47:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Jayasooriya PR, Dayaratne K, Dissanayake UB, Warnakulasuriya S. Malignant transformation of oral leukoplakia: a follow-up study. Clin Oral Investig. 2020;24:4563-4569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Iocca O, Sollecito TP, Alawi F, Weinstein GS, Newman JG, De Virgilio A, Di Maio P, Spriano G, Pardiñas López S, Shanti RM. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020;42:539-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 6. | Ray JG, Chatterjee R, Chaudhuri K. Oral submucous fibrosis: A global challenge. Rising incidence, risk factors, management, and research priorities. Periodontol 2000. 2019;80:200-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Thomas SJ, Harris R, Ness AR, Taulo J, Maclennan R, Howes N, Bain CJ. Betel quid not containing tobacco and oral leukoplakia: a report on a cross-sectional study in Papua New Guinea and a meta-analysis of current evidence. Int J Cancer. 2008;123:1871-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Paulino YC, Hurwitz EL, Warnakulasuriya S, Gatewood RR, Pierson KD, Tenorio LF, Novotny R, Palafox NA, Wilkens LR, Badowski G. Screening for oral potentially malignant disorders among areca (betel) nut chewers in Guam and Saipan. BMC Oral Health. 2014;14:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Kusiak A, Maj A, Cichońska D, Kochańska B, Cydejko A, Świetlik D. The Analysis of the Frequency of Leukoplakia in Reference of Tobacco Smoking among Northern Polish Population. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Shiu MN, Chen TH, Chang SH, Hahn LJ. Risk factors for leukoplakia and malignant transformation to oral carcinoma: a leukoplakia cohort in Taiwan. Br J Cancer. 2000;82:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Maserejian NN, Joshipura KJ, Rosner BA, Giovannucci E, Zavras AI. Prospective study of alcohol consumption and risk of oral premalignant lesions in men. Cancer Epidemiol Biomarkers Prev. 2006;15:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Yang YH, Lee HY, Tung S, Shieh TY. Epidemiological survey of oral submucous fibrosis and leukoplakia in aborigines of Taiwan. J Oral Pathol Med. 2001;30:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Lee CH, Ko YC, Huang HL, Chao YY, Tsai CC, Shieh TY, Lin LM. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br J Cancer. 2003;88:366-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Herzog TA, Pokhrel P. Introduction to the Special Issue: International Research on Areca Nut and Betel Quid Use. Subst Use Misuse. 2020;55:1383-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Oakley E, Demaine L, Warnakulasuriya S. Areca (betel) nut chewing habit among high-school children in the Commonwealth of the Northern Mariana Islands (Micronesia). Bull World Health Organ. 2005;83:656-660. [PubMed] |
| 16. | Wang SC, Tsai CC, Huang ST, Hong YJ. Betel nut chewing and related factors in adolescent students in Taiwan. Public Health. 2003;339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Lee CH, Ko AM, Warnakulasuriya S, Yin BL, Sunarjo, Zain RB, Ibrahim SO, Liu ZW, Li WH, Zhang SS, Kuntoro, Utomo B, Rajapakse PS, Warusavithana SA, Razak IA, Abdullah N, Shrestha P, Kwan AL, Shieh TY, Chen MK, Ko YC. Intercountry prevalences and practices of betel-quid use in south, southeast and eastern Asia regions and associated oral preneoplastic disorders: an international collaborative study by Asian betel-quid consortium of south and east Asia. Int J Cancer. 2011;129:1741-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Hsu KY, Tsai YF, Huang CC, Yeh WL, Chang KP, Ling CC, Chen CY, Lee HL. Tobacco-Smoking, Alcohol-Drinking, and Betel-Quid-Chewing Behaviors: Development and Use of a Web-Based Survey System. Jmir Mhealth Uhealth. 2018;. [DOI] [Full Text] |
| 19. | Wen CP, Tsai SP, Cheng TY, Chen CJ, Levy DT, Yang HJ, Eriksen MP. Uncovering the relation between betel quid chewing and cigarette smoking in Taiwan. Tob Control. 2005;14 Suppl 1:i16-i22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Ray JG, Ganguly M, Rao BS, Mukherjee S, Mahato B, Chaudhuri K. Clinico-epidemiological profile of oral potentially malignant and malignant conditions among areca nut, tobacco and alcohol users in Eastern India: A hospital based study. J Oral Maxillofac Pathol. 2013;17:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Chu H, Nie L, Cole SR. Estimating the relative excess risk due to interaction: a bayesian approach. Epidemiology. 2011;22:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Chher T, Hak S, Kallarakkal TG, Durward C, Ramanathan A, Ghani WMN, Razak IA, Harun MH, Ashar NAM, Rajandram RK, Prak P, Hussaini HM, Zain RB. Prevalence of oral cancer, oral potentially malignant disorders and other oral mucosal lesions in Cambodia. Ethn Health. 2018;23:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Ikeda N, Handa Y, Khim SP, Durward C, Axéll T, Mizuno T, Fukano H, Kawai T. Prevalence study of oral mucosal lesions in a selected Cambodian population. Community Dent Oral Epidemiol. 1995;23:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Chung CH, Yang YH, Wang TY, Shieh TY, Warnakulasuriya S. Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern Taiwan. J Oral Pathol Med. 2005;34:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Yang YH, Lien YC, Ho PS, Chen CH, Chang JS, Cheng TC, Shieh TY. The effects of chewing areca/betel quid with and without cigarette smoking on oral submucous fibrosis and oral mucosal lesions. Oral Dis. 2005;11:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Mehrotra D, Kumar S, Agarwal GG, Asthana A. Odds ratio of risk factors for oral submucous fibrosis in a case control model. Br J Oral Maxillofac Surg. 2013;51:e169-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ko AM, Lee CH, Ko YC. Betel quid-associated cancer: Prevention strategies and targeted treatment. Cancer Lett. 2020;477:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Akhtar S, Sheikh AA, Qureshi HU. Chewing areca nut, betel quid, oral snuff, cigarette smoking and the risk of oesophageal squamous-cell carcinoma in South Asians: a multicentre case-control study. Eur J Cancer. 2012;48:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Giovannoni ML, Valdivia-Gandur I, Lozano de Luaces V, Varela Véliz H, Balasubbaiah Y, Chimenos-Küstner E. Betel and tobacco chewing habit and its relation to risk factors for periodontal disease. Oral Dis. 2018;24:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Parmar G, Sangwan P, Vashi P, Kulkarni P, Kumar S. Effect of chewing a mixture of areca nut and tobacco on periodontal tissues and oral hygiene status. J Oral Sci. 2008;50:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Islam S, Muthumala M, Matsuoka H, Uehara O, Kuramitsu Y, Chiba I, Abiko Y. How Each Component of Betel Quid Is Involved in Oral Carcinogenesis: Mutual Interactions and Synergistic Effects with Other Carcinogens-a Review Article. Curr Oncol Rep. 2019;21:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Hernandez BY, Zhu X, Goodman MT, Gatewood R, Mendiola P, Quinata K, Paulino YC. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS One. 2017;12:e0172196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 34. | Chen MY, Chen JW, Wu LW, Huang KC, Chen JY, Wu WS, Chiang WF, Shih CJ, Tsai KN, Hsieh WT, Ho YH, Wong TY, Wu JH, Chen YL. Carcinogenesis of Male Oral Submucous Fibrosis Alters Salivary Microbiomes. J Dent Res. 2021;100:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Amarasinghe HK, Usgodaarachchi US, Johnson NW, Lalloo R, Warnakulasuriya S. Betel-quid chewing with or without tobacco is a major risk factor for oral potentially malignant disorders in Sri Lanka: a case-control study. Oral Oncol. 2010;46:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Chen PC, Pan CC, Kuo C, Lin CP. Risk of oral nonmalignant lesions associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan: an integrated molecular and epidemiologic study. Arch Pathol Lab Med. 2006;130:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Zaw KK, Ohnmar M, Hlaing MM, Oo YT, Win SS, Htike MM, Aye PP, Shwe S, Htwe MT, Thein ZM. Betel Quid and Oral Potentially Malignant Disorders in a Periurban Township in Myanmar. PLoS One. 2016;11:e0162081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Song H, Wan Y, Xu YY. Betel Quid Chewing Without Tobacco: A Meta-analysis of Carcinogenic and Precarcinogenic Effects. Asia-Pac J Public He. 2015;Np47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Chen PH, Mahmood Q, Mariottini GL, Chiang TA, Lee KW. Adverse Health Effects of Betel Quid and the Risk of Oral and Pharyngeal Cancers. Biomed Res Int. 2017;2017:3904098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Thomas G, Hashibe M, Jacob BJ, Ramadas K, Mathew B, Sankaranarayanan R, Zhang ZF. Risk factors for multiple oral premalignant lesions. Int J Cancer. 2003;107:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Muthukrishnan A, Warnakulasuriya S. Oral health consequences of smokeless tobacco use. Indian J Med Res. 2018;148:35-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Chang YC, Lii CK, Tai KW, Chou MY. Adverse effects of arecoline and nicotine on human periodontal ligament fibroblasts in vitro. J Clin Periodontol. 2001;28:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Liu B, Shen M, Xiong J, Yuan Y, Wu X, Gao X, Xu J, Guo F, Jian X. Synergistic effects of betel quid chewing, tobacco use (in the form of cigarette smoking), and alcohol consumption on the risk of malignant transformation of oral submucous fibrosis (OSF): a case-control study in Hunan Province, China. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Petti S, Masood M, Scully C. The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia. A meta-analysis of observational studies. PLoS One. 2013;8:e78999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Varshney S, Sandhir S, Mishra S. A study of oral pre-malignant lesions and related risk factors. Indan J Community He. 2015;130. |
| 46. | Wight AJ, Ogden GR. Possible mechanisms by which alcohol may influence the development of oral cancer--a review. Oral Oncol. 1998;34:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Uehara O, Hiraki D, Kuramitsu Y, Matsuoka H, Takai R, Fujita M, Harada F, Paudel D, Takahashi S, Yoshida K, Muthumala M, Nagayasu H, Chiba I, Abiko Y. Alteration of oral flora in betel quid chewers in Sri Lanka. J Microbiol Immunol Infect. 2021;54:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Zhong XH, Lu Q, Zhang Q, He Y, Wei WJ, Wang YM. Oral microbiota alteration associated with oral cancer and areca chewing. Oral Dis. 2021;226. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 50. | Deng S, Xu Y, Wang X, Liu M, Li L, Yu X, Wang Y, Wu Y, Wang W, Gao M, Cong B. Study on the Role of Salivary Flora and NF-κB Inflammatory Signal Pathway in Oral Lichen Planus. Inflammation. 2020;43:994-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |