Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3101
Peer-review started: May 15, 2021
First decision: June 12, 2021
Revised: July 27, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: April 6, 2022
Processing time: 317 Days and 14.6 Hours
Colon cancer is one the most common forms of cancer in both sexes. Due to important progress in the field of early detection and effective treatment, colon and rectal cancer survivors currently account for 10% of cancer survivors worldwide. However, the effects of anti-cancer treatments, especially oxaliplatin-based chemotherapy, on the quality of life (QoL) have been less evaluated. Although the incidence of severe chemotherapy-induced neuropathy (CIPN) in clinical studies is below 20%, data from real-world studies is scarce, and CIPN is probably under-reported due to patient selection and the patients’ fear that reporting side-effects might lead to treatment cessation.
To determine the impact of CIPN on QoL in colorectal cancer patients with a recent history of oxaliplatin-based chemotherapy.
We performed a prospective cross-sectional study in two major Romanian oncology tertiary hospitals—the Regional Institute of Oncology Iași (Iasi, Romania) and the Fundeni Clinical Oncology Institute (Bucharest, Romania). All consecutive patients with colon or rectal cancer, undergoing Oxaliplatin-based chemotherapy that consented to enroll in the study, were assessed by means of two questionnaires—the EORTC QQ-CR29 (quality of life in colon and rectal cancer patients) and the QLQ-CIPN20 (assessment of neuropathy). Several demographical, social, clinical and treatment data were also collected. Statistical analysis was performed by means of SPSS v20. The student t test was used to assess the relationship between the QLQ-CIPN20 and QLQ-CR29 results. Kaplan Meyer-curves were used to report 3-year progression-free survival (PFS) in patients that discontinued chemotherapy vs those that completed the recommended course.
Of the 267 patients that fulfilled the inclusion criteria in the pre-specified time frame, 101 (37.8%) agreed to participate in the clinical study. At the time of the enrolment in the study, over 50% of the patients had recently interrupted their oxaliplatin-based chemotherapy, most often due to neuropathy. Almost 85% of the responders reported having tingling or numbness in their fingers or hands, symptoms that were associated with pain in over 20% of the cases. When comparing the scores in the two questionnaires, a statistically significant relationship (P < 0.001) was found between the presence of neuropathic symptoms and a decreased quality of life. This correlation was consistent when the patients were stratified by sex, disease stage, comorbidities and the presence of stoma or treatment type, suggesting that neuropathy in itself may be a reason for a decreased quality of life. At the 3 year final assessment, median recurrence-free survival in stage III patients was 26.88 mo. When stratified by completion of chemotherapy, median recurrence free-survival of stage III patients that completed chemotherapy was 28.27 mo vs 24.33 mo in patients that discontinued chemotherapy due to toxicity, a difference that did not reach statistical significance.
CIPN significantly impacts QoL in colorectal cancer patients. CIPN is also the most frequent reason for treatment discontinuation. Physicians should actively assess for CIPN in order to prevent chronic neuropathy.
Core Tip: Oxaliplatin-induced neuropathy (OIN) is a serious acute and chronic complication of colorectal cancer chemotherapy. This study aims to offer a real-world perspective on the incidence of acute OIN during chemotherapy and its impact on the quality of life. We found that more than 50% of colon cancer patients discontinue chemotherapy, and the main reason is neuropathy. Also, individuals with neuropathy have a lower quality of life, independent of other factors. All in all, as the number of cancer survivors increases, guidelines should be reviewed in order to minimize the risk of chronic OIN as much as possible.
- Citation: Prutianu I, Alexa-Stratulat T, Cristea EO, Nicolau A, Moisuc DC, Covrig AA, Ivanov K, Croitoru AE, Miron MI, Dinu MI, Ivanov AV, Marinca MV, Radu I, Gafton B. Oxaliplatin-induced neuropathy and colo-rectal cancer patient’s quality of life: Practical lessons from a prospective cross-sectional, real-world study. World J Clin Cases 2022; 10(10): 3101-3112
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3101.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3101
Colon cancer is one the most common forms of cancer in both sexes and its incidence is constantly increasing, especially in developed countries, with approximately 1.8 million new cases diagnosed annually worldwide[1]. In Romania, although epidemiological data are scarce[2], colorectal cancer is the second neoplasia in terms of prevalence (13.5% of all cancer cases) and the second cause of death after lung cancer[3].
Notable advances in treatment have decreased mortality and subsequently increased the number of individuals cured of cancer, with colon and rectal cancer survivors currently accounting for 10% of cancer survivors worldwide[4]. However, while significant resources have been focused on cancer cures, the effects of anti-cancer treatment on the quality of life and well-being of these individuals have been less evaluated. Both local and systemic treatments, such as surgery, radiotherapy or chemotherapy can have debilitating long-term side-effects, and their therapeutic management is remarkably challenging.
Oxaliplatin is a platinum-based alkylating agent. Its use, in combination with fluoropyrimidines as an adjuvant treatment in high-risk stage II and stage III colon and rectal cancer, has improved survival, with a 23% reduction in relative risk of recurrence when compared to the previous standard of 5-fluorouracil regimens as reported in the MOSAIC trial[5]. However, oxaliplatin can induce both acute and chronic neurologic toxicities, and chemotherapy-induced neuropathy (CIPN) is often cited as the main reason for discontinuation of anti-cancer treatment or dose modification[6]. Even so, available data regarding CIPN are very heterogeneous, and studies have a very high variability when reporting incidence. While reports from clinical studies suggest that up to 18% of colon and rectal cancer patients experience grade III neuropathy throughout treatment and 4% discontinue treatment due to neuropathy[7], available real-world data suggest that the incidence of CIPN is much higher and leads to discontinuation of treatment significantly more often. There is currently no accepted prophylactic treatment and the management of CIPN with anti-depressants and/or analgesics is unsatisfactory[8]. Additionally, the impact of CIPN on an individual’s quality of life may be under-estimated in clinical trials due to patient selection and the patients’ fear that reporting side-effects might lead to cessation of treatment.
The aim of this study is to determine the impact of CIPN on the quality of life in colon and rectal cancer patients with a recent history of oxaliplatin-based chemotherapy. Another aim of the study is to determine treatment discontinuation rates secondary to CIPN and to assess the impact of chemotherapy discontinuation on progression-free survival and relapse-free survival in stage III colon and rectal cancer patients.
We performed a prospective cross-sectional study in two major Romanian oncology tertiary hospitals—the Regional Institute of Oncology Iași (Iasi, Romania) and the Fundeni Clinical Oncology Institute (Bucharest, Romania). Both units are high-volume reference centers for oncology patients—one for North-Eastern Romania and the other for Southern Romania. The study inclusion criteria were as follows: (1) Cancer patients with a pathology-confirmed high-risk stage II, stage III or stage IV colon or rectal cancer for which the tumor board recommended a minimum of 6 mo of oxaliplatin-based chemotherapy, either in an adjuvant or metastatic setting; (2) A minimum of at least two oxaliplatin-based chemotherapy cycles already administered, the last of which was a maximum of 21 d prior to the study inclusion; and (3) Ability and willingness to give informed consent. The oxaliplatin-based regimens included in the study were FOLFOX (oxaliplatin in combination with folinic acid/Leucovorin), CapeOX (oxaliplatin in combination with capecitabine) or other oxaliplatin-containing regimens, such as chronoFLO or FLOX; dose reductions were allowed up to 80%. Exclusion criteria: (1) Personal history of neuropathy, secondary to diabetes mellitus (SM) or heavy alcohol use (patients with DM or alcohol abuse were included as long as they had a neurological assessment confirming no neuropathy at the beginning of chemotherapy); (2) Personal history of acute/chronic neuropathy; (3) Cognitive impairment or other neurological conditions that could impair the ability to give consent and understand the informed consent form (patients with brain metastases were included as long as they were stable and had received surgery/radiotherapy prior to study inclusion); and (4) Personal history of another type of malignancy (basal cell carcinoma excepted).
The patients were assessed by means of two questionnaires: the updated European Organisation for Research and Treatment of Cancer (EORTC) questionnaire module for colorectal cancer, also known as the EORTC QLQ-CR29 and the EORTC questionnaire module for chemotherapy-induced peripheral neuropathy (CIPN), also known as the EORTC QLQ-CIPN20. Both questionnaires are validated patient-reported outcome measures designed to assess quality of life and the presence of neuropathy, respectively.
The EORTC QLQ-CR29 is a shorter version of the QLQ-CR38 that can be easily completed by the patient within 15 min and offers information regarding health-related quality of life issues. It has 18 items that are common for all colon cancer patients and some additional items personalized either for stoma vs non-stoma patients (items 49-55). Questions 56-59 are gender-specific—questions 56-57 are only for men, and questions 58-59 are only for women. Questions 56-59 assess the interest and the pleasure derived from intercourse. In the questionnaire, the patients rated their experience for each item during the previous week (questions 31-54) or month (questions 56-59) using scores from 1 (not at all) to 4 (very much). Their responses can be added up, in which case a higher score is associated with a lower quality of life, or they can be linearly converted into a 0-100 score using standard EORTC guidelines[9].
The EORTC QLQ-CIPN20 questionnaire contains 20 items on which patients rate their experience of several neuropathic symptoms during the previous week using scores from 1 (not at all) to 4 (very much). The questionnaire contains 20 items and assesses sensory (items 31, 32, 33, 34, 35, 36, 39, 40, and 48), motor (items 37, 38, 41, 42, 43, 44, 45, and 49) and autonomic (items 46, 47, and 50) changes suggestive of neuropathy and has been shown to correlate with the physician-reported CTCAE neuropathy assessment[10]. By adding up all the items, one can assess the intensity of neuropathy—the higher the score, the more severe the neuropathy.
The Romanian versions of both questionnaires are available from the EORTC main website, and written permission was obtained for their use prior to the commencement of the study.
The study was approved by the Ethical Committee of the Regional Institute of Oncology Iași. All the patients were required to read and sign an informed consent form prior to study’s inclusion and were specifically informed of the possibility to withdraw consent at any given time.
In-between February-March 2018, all the patients that were admitted to either of the two reference hospitals and respected the inclusion criteria were screened. The patients that gave consent to participate in the study were assessed by means of the EORTC QLQ-CR29 and the EORTC QLQ-CIPN20 in the same day as they gave consent to participate in the study. Additional demographical, social, clinical and treatment data were collected after obtaining consent, either by means of the observation sheet or by directly asking the study subject. The patients were afterwards followed-up periodically (clinical and imaging assessment every 3-6 mo) up to three years or until disease progression, recurrence or death. The study aimed to determine the incidence of oxaliplatin-induced acute neuropathy and its impact on the quality of life in the selected population. A secondary end-point was the assessment of progression-free survival in stage III patients that completed 6 mo of oxaliplatin-based chemotherapy vs patients that discontinued the treatment due to toxicity.
A statistical analysis was performed by means of SPSS v20 (IBM; Chicago, IL, United States). A student t test was used to assess the relationship between the QLQ-CIPN20 and QLQ-CR29 results. Kaplan Meyer-curves were used to report the 3-year PFS (missing data was censored and included in the final report). The statistical significance was set at 0.05 a priori to study analysis. The graphics and tables were created by means of the GraphPad prism, SPSS or Excel, as suitable. The continuous results are reported as mean ± standard deviation and median. The non-continuous data are reported as frequencies.
Of the 267 patients that fulfilled the inclusion criteria in the pre-specified time frame, 101 (37.8%) agreed to participate in the clinical study. Table 1 presents the main characteristics of the study population. The mean age was 60.6 years, with a median of 63 years. The study group was well balanced in terms of gender (43.6% women and 56.4% men) and consisted mostly of individuals with upper secondary education (53%) and with a good performance status (median PS 90%) (Table 1). The patients were rather equally divided between curative and palliative treatments (56.4% palliative). Some stage III patients were ineligible for curative treatment and some stage IV patients were treated with a curative intent (oligometastatic, liver-only disease). The patients were most often treated by means of FOLFOX (85 mg/m2 Oxaliplatin every 2 wk) or CapeOX (130 mg/m2 Oxaliplatin every 3 wk) chemotherapy protocols, with 17.1% receiving other oxaliplatin-based adjuvant or palliative treatment regimens. All adjuvant oxaliplatin-based regimens were prescribed for 6 mo (12 cycles FOLFOX or 8 cycles CapeOX) and the treatment recommendations for metastatic cases consisted of oxaliplatin-based chemotherapy until disease progression or unacceptable toxicity, whichever came first.
| Age of patients | yr |
| Median | 63 |
| Mean | 60.6 |
| Minimum | 32 |
| Maximum | 82 |
| Level of education | |
| Primary education | 21% |
| Upper secondary education | 53% |
| Master/doctoral education | 26% |
| Gender | |
| Male | 56.4% |
| Female | 43.6% |
| Initial stage | |
| Stage II high risk | 3.9% |
| Stage III | 34.7% |
| Stage IV | 61.4% |
| Purpose of treatment | |
| Curative | 41.6% |
| Palliative | 56.4% |
| Undefined | 2.0% |
| Karnofsky performance status | |
| 100% | 5.9% |
| 90% | 45.5% |
| 80% | 36.5% |
| 70% | 6.9% |
| 60% | 5% |
| Chemotherapy | |
| FOLFOX | 37.4% |
| CapeOX | 45.5% |
| Other | 17.1% |
| Relevant comorbidities | |
| None | 55.2% |
| Diabetes mellitus | 10.3% |
| Alcohol abuse | 3.3% |
| Cardio-vascular diseases | 20.1% |
| Other | 11.1% |
| Diabetes-insulin treatment | |
| Yes | 8.4% |
| No | 91.6% |
Of note, most of the patients (55.2%) had no comorbidities. Of those with pre-existing conditions, arterial hypertension and other cardiac diseases were most prevalent (20.1%), while other types of comorbidities (such as renal impairment or chronic obstructive pulmoriary disease) were found in 11.1% of the patients. A little over 10% of the study population had type 2 diabetes (there were no patients with type 1 diabetes) and 3.3% were chronic alcohol users (Table 1).
At the time of the study’s enrolment, over 50% of the patients had recently interrupted oxaliplatin-based chemotherapy, most often due to neuropathy. Pre-existing diabetes and alcohol consumption were not statistically linked to the probability of oxaliplatin interruption. The mean value of the oxaliplatin dose that the patients received prior to oxaliplatin discontinuation was 871.4 mg (median of 815 mg). Another 35.7% were still undergoing treatment, while 16.8% had recently finished the planned therapy course (patients receiving a predetermined number of cycles as part of their adjuvant treatment). 16.7% of the patients had discontinued chemotherapy due to other types of toxicity, most often digestive (mucositis, diarrhea, constipation, etc), hematologic (anemia, neutropenia and/or thrombocytopenia) or dermatologic. Most of these patients also had neuropathic symptoms, only the severity of neuropathy was not the main reason for treatment discontinuation (Table 2).
| Treatment status | |
| Still going on | 35.7% |
| Stopped due to neuropathy | 30.8% |
| Stopped due to toxicity | 16.7% |
| Finished chemotherapy | 16.8% |
| Types of toxicity | |
| Neuropathy | 30.8% |
| Hematologic | 28.2% |
| Digestive | 12.4% |
| Dermatologic | 3.5% |
| Mixed | 25.1% |
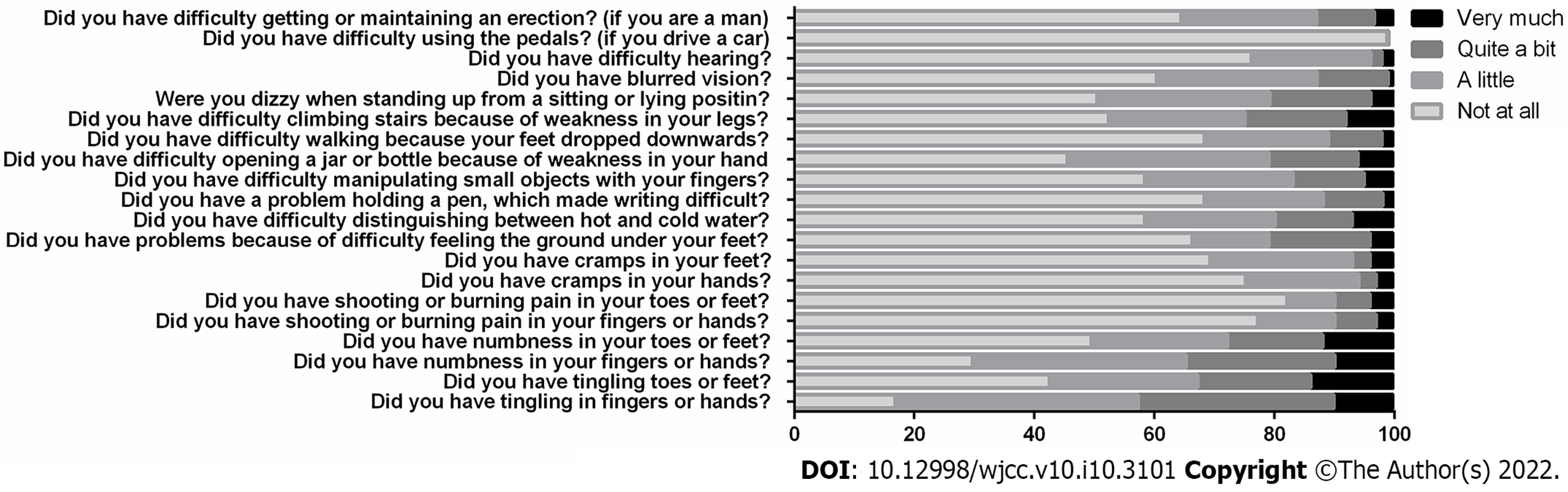
In the QLQ-CIPN20 questionnaire, almost 85% of the responders reported having tingling or numbness in their fingers or hands, and almost 50% of the responders reported having tingling or numbness in their toes or feet. These neuropathic symptoms were associated with pain in over 20% of the cases and cramps in almost 30%. Daily activities were affected by neuropathy—standing, walking or climbing stairs was impaired in over 30% of the patients, over 40% had difficulties manipulating small objects with their fingers or opening jars/bottles, and almost 50% reported feeling dizzy when standing up from a sitting or lying position. Finally, the difficulty of maintaining an erection was admitted and described as being mild by 22.5% of the men, by 9.6% of them as being moderate and by 3.2% of them as being significant (Figure 1).
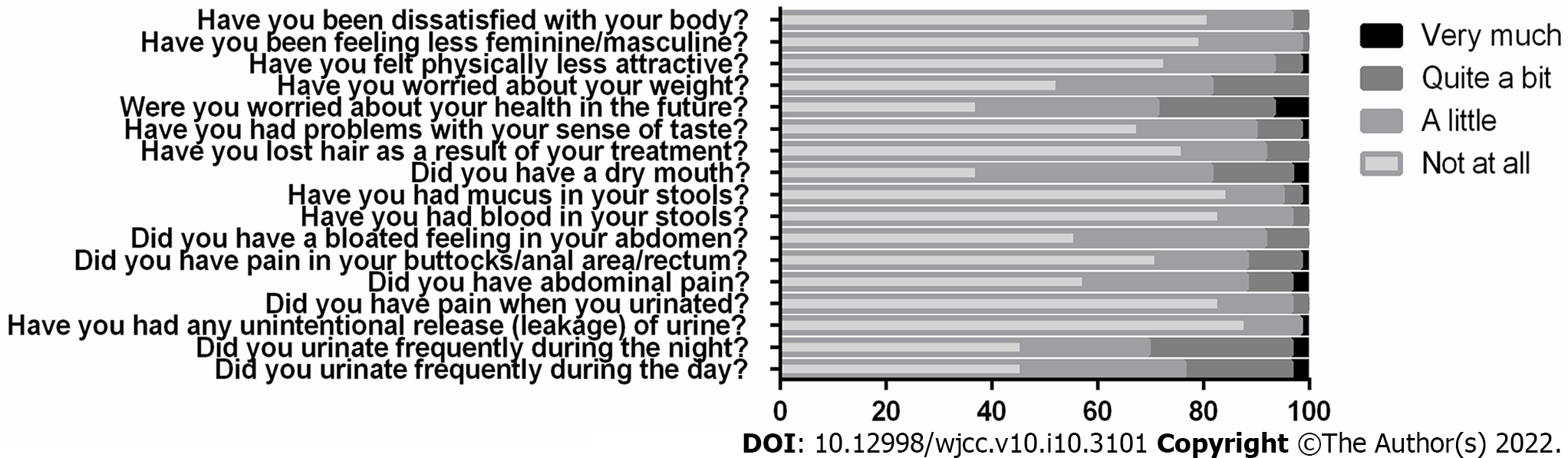
In the QLQ-CR29 questionnaire, over 20% of the patients reported trouble urinating during the day or during the night, and almost 30% reported at least some trouble with their sense of taste. Most of the patients (72.9%) stated that they did not feel physically less attractive as a result of their disease or treatment, and a similarly high percent reported little to no dissatisfaction with their body (Figure 2).
When comparing the scores in the two questionnaires, a statistically significant relationship (P < 0.001) was found between the presence of neuropathic symptoms and a decreased quality of life. This correlation was consistent when the patients were stratified by sex, stage, comorbidities, presence of stoma or treatment type, suggesting that neuropathy in itself may be a reason for a decreased quality of life.
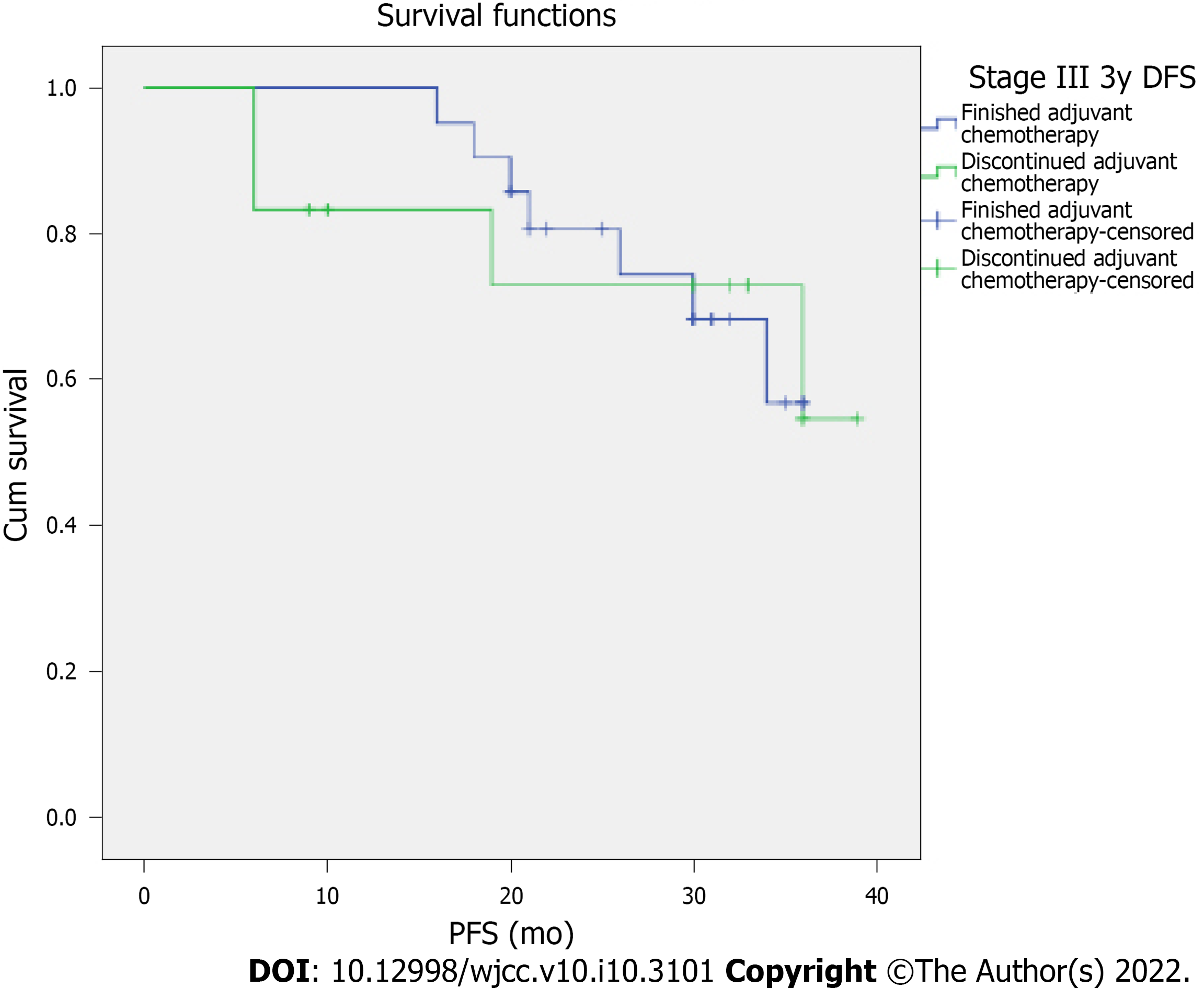
At the three-year final assessment, the median recurrence-free survival in stage III patients was 26.88 mo, with 26.4% of the patients (9 out of 34) showing no signs of recurrence three years after enrolling in the study. When stratified by the completion of chemotherapy, median recurrence-free-survival of stage III patients that completed chemotherapy was 28.27 mo vs 24.33 mo in the patients that discontinued chemotherapy due to toxicity, a difference that did not reach statistical significance (Figure 3). Regarding the stage IV patients, the median progression-free survival time was 16.08 mo.
CIPN is a debilitating and dose-limiting side-effect of Oxaliplatin chemotherapy that can occur in an acute or chronic manner. Clinical signs of acute CIPN include paresthesia felt in the fingers and toes, on the lips or throat that are cold-induced or cold-exaggerated and are most often reported by patients shortly after administration[6,11]; acute CIPN can last from minutes to days and even weeks and can disappear spontaneously. After several cycles of treatment, symptoms can gradually increase in intensity and duration[12]. It is likely that the mechanism of action is represented by an alteration of the functionality of the sodium, calcium and magnesium channels on the cell membrane of the neurons because of oxaliplatin metabolites[13,14]. In our study, almost 85% of the responders reported having tingling or numbness in their fingers or hands in the week prior to the study’s inclusions, which are symptoms consistent with grade II neuropathy as assessed by the CTCAE scale[15]. Although grade II neuropathy is usually not a reason for cessation of treatment, it should be a warning sign for physicians, since there were several articles in the last years that correlated the incidence of acute neuropathy with the likelihood of chronic post-treatment symptoms[11]. Attal et al[16] performed a prospective study on 48 colon cancer patients undergoing cisplatin-based or oxaliplatin-based chemotherapy and concluded that the duration and the intensity of acute neuropathy during the first three cycles can be correlated with the incidence of chronic neuropathy, as assessed at the one year follow-up. A larger prospective study focused specifically on oxaliplatin-induced neuropathy and found that acute symptoms were reported in 85.9% of the patients, most often expressed as cold-induced perioral or pharyngolaryngeal dysesthesias and that they were predictive when it came to the development and the severity of chronic neuropathy[17]. A third body of data comes from the study of Alejandro et al[18] that enrolled 50 colorectal cancer patients treated with mFOLFOX (oxaliplatin dose 85 mg/m2) and concluded that 74% of the patients reported acute and 48% of the patients reported chronic neuropathic symptoms. It is important to also underline the fact that acute neuropathic symptoms can also be associated with prolonged infusion times and treatment delay[19], changes that probably have an impact on treatment efficacy.
In our study, there was a direct correlation between a decreased quality of life and a high score on the OLQ-CIPN20 scale, suggestive for more severe acute neuropathic symptoms. This is in agreement with the available data that highlight the negative impact of neuropathy in cancer patients. A recently published study directly compared colon and rectal cancer patients with neuropathy with those without neuropathy during chemotherapy and reported that patients with neuropathy have significantly lower functioning and more symptoms. Although anxiety and depression are similar in these two groups, the quality of life of patients with neuropathy is significantly lower[20]. Moreover, this relationship seems to persist in the long-term, with several authors reporting that chronic oxaliplatin-induced neuropathy negatively impacts quality of life and functional status in cancer survivors[12,18,19,21].
There are several well-known risk factors that increase the likelihood of CIPN, pre-existing neuropathy, alcoholism and diabetes being most frequent. Although we excluded some patients with these conditions, non-neuropathic individuals with chronic alcohol consumption made up 3.3% of our study group. This percent is somewhat surprising, since in North-East Romania, 60% to 80% of the population are active drinkers[22]. This suggests a lack of confidence when reporting alcohol consumption and fear of being judged. Another possible explanation is that active drinkers are more likely to have a pre-existing neuropathy, and this was an exclusion criteria in our study. The rates of diabetes mellitus patients were 13.3%, and this high incidence can be explained by the etiologic relationship between diabetes and colorectal cancer. However, these pre-existing conditions were not correlated with oxaliplatin interruption, most likely due to the limited number of study participants or the short amount of time between the diagnosis of colon cancer and enrolment in the study.
In this present study, there were no significant differences in terms of recurrence-free survival in stage III patients that completed adjuvant chemotherapy vs those that did not. It is important to underline the fact that all the patients that discontinued chemotherapy had received at least two cycles of oxaliplatin-based chemotherapy prior to discontinuation and that most had received four cycles of treatment before interruption.
These results are in concordance with data published in the IDEA trials. The TOSCA trial was one of the first trials to show that only 35% of the patients were able to complete the planned treatment without any dose modifications in the arm with 3 mo of treatment, while only 12% of the patients completed the 6 mo treatment without any dose modifications or treatment delays[23]. The results were also similar in the SCOT trial. Moreover, it seems that 3 mo of oxaliplatin-based adjuvant chemotherapy were non-inferior to 6 mo of the same therapy for patients with high-risk stage II and stage III colorectal cancer. This suggests that similar results may be achieved with less treatment, reduced toxicity, less costs and improved quality of life[24]. A different profile of data was reported in the IDEA France trial, in which 6 mo of FOLFOX6 were superior to 3 mo of the same treatment, especially in the high-risk population defined as T4 or N2. Two important aspects need to be considered: The dose intensity of oxaliplatin might be the key when it comes to explaining the results, and there is a clear relation between toxicity and the polymorphism of the drug’s metabolism[25]. A similar 3-year DFS rate was seen in these two groups of patients (3 mo 73.2% vs 6 mo 74.9%) in the HORG trial, but when the data was analyzed, based on the protocol, the patients receiving CAPEOX had similar benefits regardless of the treatment duration, while in patients receiving FOLFOX6, there was a difference of 5.9% in the 3-year DFS in favor of the 6 mo treatment[26]. The ACHIEVE trial, which included Asian patients, showed similar benefit for 3 mo of treatment when compared to 6 mo, but most of the patients received CAPEOX (75% of patients), rather than FOLFOX[27]. In conclusion, the IDEA trials have some limitations, but probably 3 mo of CAPEOX is enough at least for low-risk patients, while 6 mo of FOLFOX might be superior to 3 mo of treatment, although more toxic[28].
Even though we focused on peripheral sensory neurotoxicity, other side effects like diarrhea, febrile neutropenia, mucositis, fatigue or hand-foot syndrome may contribute to an early discontinuation of treatment. Still, the neuropathy is the dominant symptom which, in some cases, may be present, years after the end of the treatment (in some trials, up to two-thirds of patients remain with symptoms) and has a huge impact on the quality of life[29]. Patients should be informed about the risks of acute neuropathy and should be actively encouraged to report neuropathic symptoms at the earliest onset.
Given the fact that there is no strategy for prevention or an effective treatment, we believe that one way we can avoid the consequences of oxaliplatin induced neuropathy is treatment discontinuation right before the chronic changes take place. In order not to unnecessarily reduce the effectiveness of oxaliplatin therapy, discontinuation of treatment should be as close as possible to the time of its chronicity.
Acute CIPN significantly impacts quality of life in colon and rectal cancer patients and occurs with extremely high frequency, being the most frequent reason for treatment discontinuation. In order to prevent under-reporting and overlooking the onset of neuropathy, physicians should actively seek and assess for CIPN in order to quickly adjust treatment so that chronic neuropathy can be prevented. More trials that compare shorter-term with longer-term chemotherapy in the adjuvant setting should be encouraged, especially since the drugs most used can cause long-term damage.
As the number of cancer survivors increases worldwide, long-term side effects of anti-cancer treatments have become more and more important, since they can impact quality of life and the success of social reinsertion. Currently, chemotherapy-induced neuropathy (CIPN), following Oxaliplatin treatment, can have debilitating side-effects with long-term consequences, but its assessment is heterogeneous and there are few to no prophylaxis or treatment methods.
Using real-world data, this study was designed to underline the impact of Oxaliplatin-induced neuropathy on the quality of life in colon and rectal cancer patients. Adding this information to the existing data can contribute to increased awareness for the risks of over-using chemotherapy and its long-term consequences.
To assess the impact of CIPN on the QoL in colon and rectal cancer patients. A significant impact on the QoL is especially relevant for cancer survivors with a long life expectancy. Currently, there are very few treatment options for chronic CIPN. To Assessing treatment discontinuation rates secondary to CIPN and their impact on progression-free survival and relapse-free survival. If a significant percentage of patients cannot complete the recommended chemotherapy regimen due to neurological toxicity, perhaps less aggressive or less intensive chemotherapy regiments should be evaluated by means of novel clinical trials.
We performed a prospective cross-sectional study in two oncology tertiary hospitals. Consecutive colorectal cancer patients undergoing oxaliplatin-based chemotherapy were assessed by means of two questionnaires (EORTC for quality of life and QLQ-CIPN20 for neuropathy) and potential correlations were analyzed by means of a student t test. Additional demographical, social, clinical and treatment data were collected from patient charts and Kaplan-Meyer curves were used for three-year PFS. Statistical analysis was performed by means of SPSS v20.
101 patients agreed to participate in the study. Of these, most had recently discontinued oxaliplatin-based chemotherapy. The reason for not finishing the recommended number of cycles was neuropathy in most cases; even in cases where neuropathy was not the main reason for treatment discontinuation, all patients had some neuropathic symptoms. The presence and severity of neuropathy correlated with the quality of life in all patients. Stage III patients that interrupted chemotherapy had a slightly lower recurrence-free survival (24.33 mo vs 28.27 mo) when compared with patients that underwent 6 mo of chemotherapy, although this difference did not reach statistical significance.
CIPN is significantly more frequent outside clinical trials due to heterogeneous types of assessments being performed world-wide and under-reporting by both physicians and patients. Acute CIPN can have long-term consequences due to its impact on the QoL and its association with treatment discontinuation that can, in turn, affect progression-free survival. Physicians should actively search and assess for signs and symptoms of CIPN in order to prevent chronic complications.
Large, real-world prospective studies are needed to identify the best method for diagnosing and monitoring CIPN. Additionally, there is an urgent need for more data regarding the optimal number of adjuvant chemotherapy cycles and the dose at which Oxaliplatin is still effective, but less neurotoxic in order to minimize this important side-effect of anti-cancer treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elghali MA S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 2. | Mare LV, Andrese EP, Monu AM, Adam MG, Solovastru LG, Vata D. Update on Epidemiology of Non-Melanocytic Skin Tumors. Rev Chim. 2019;70:3050-3052. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Ionescu EM, Tieranu CG, Maftei D, Grivei A, Olteanu AO, Arbanas T, Calu V, Musat S, Mihaescu-Pintia C, Cucu IC. Colorectal Cancer Trends of 2018 in Romania-an Important Geographical Variation Between Northern and Southern Lands and High Mortality Versus European Averages. J Gastrointest Cancer. 2021;52:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila). 2011;4:502-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2731] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 6. | Gebremedhn EG, Shortland PJ, Mahns DA. The incidence of acute oxaliplatin-induced neuropathy and its impact on treatment in the first cycle: a systematic review. BMC Cancer. 2018;18:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2950] [Cited by in RCA: 2825] [Article Influence: 113.0] [Reference Citation Analysis (1)] |
| 8. | Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, Peters GJ. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, Johnson CD, Pilkington SA, Arraras J, Ben-Josef E, Pullyblank AM, Fayers P, Blazeby JM; European Organisation for the Research and Treatment of Cancer Quality of Life Group. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009;45:3017-3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 10. | Le-Rademacher J, Kanwar R, Seisler D, Pachman DR, Qin R, Abyzov A, Ruddy KJ, Banck MS, Lavoie Smith EM, Dorsey SG, Aaronson NK, Sloan J, Loprinzi CL, Beutler AS. Patient-reported (EORTC QLQ-CIPN20) vs physician-reported (CTCAE) quantification of oxaliplatin- and paclitaxel/carboplatin-induced peripheral neuropathy in NCCTG/Alliance clinical trials. Support Care Cancer. 2017;25:3537-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Kang L, Tian Y, Xu S, Chen H. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol. 2021;268:3269-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 12. | Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother. 2005;39:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Gamelin L, Capitain O, Morel A, Dumont A, Traore S, Anne le B, Gilles S, Boisdron-Celle M, Gamelin E. Predictive factors of oxaliplatin neurotoxicity: the involvement of the oxalate outcome pathway. Clin Cancer Res. 2007;13:6359-6368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Kaplow R, Iyere K. Grading chemotherapy-induced peripheral neuropathy in adults. Nursing. 2017;47:67-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Attal N, Bouhassira D, Gautron M, Vaillant JN, Mitry E, Lepère C, Rougier P, Guirimand F. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain. 2009;144:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, Alberti P, Bergamo F, Cortinovis D, Cazzaniga M, Santos C, Papadimitriou K, Kalofonos HP. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer. 2013;119:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Alejandro LM, Behrendt CE, Chen K, Openshaw H, Shibata S. Predicting acute and persistent neuropathy associated with oxaliplatin. Am J Clin Oncol. 2013;36:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Storey DJ, Sakala M, McLean CM, Phillips HA, Dawson LK, Wall LR, Fallon MT, Clive S. Capecitabine combined with oxaliplatin (CapOx) in clinical practice: how significant is peripheral neuropathy? Ann Oncol. 2010;21:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Hsu HT, Wu LM, Lin PC, Juan CH, Huang YY, Chou PL, Chen JL. Emotional distress and quality of life during folinic acid, fluorouracil, and oxaliplatin in colorectal cancer patients with and without chemotherapy-induced peripheral neuropathy: A cross-sectional study. Medicine (Baltimore). 2020;99:e19029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Miaskowski C, Mastick J, Paul SM, Topp K, Smoot B, Abrams G, Chen LM, Kober KM, Conley YP, Chesney M, Bolla K, Mausisa G, Mazor M, Wong M, Schumacher M, Levine JD. Chemotherapy-Induced Neuropathy in Cancer Survivors. J Pain Symptom Manage. 2017;54:204-218.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 22. | GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015-1035. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2194] [Cited by in RCA: 2092] [Article Influence: 298.9] [Reference Citation Analysis (0)] |
| 23. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1643] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 24. | Iveson TJ, Kerr RS, Saunders MP, Cassidy J, Hollander NH, Tabernero J, Haydon A, Glimelius B, Harkin A, Allan K, McQueen J, Scudder C, Boyd KA, Briggs A, Waterston A, Medley L, Wilson C, Ellis R, Essapen S, Dhadda AS, Harrison M, Falk S, Raouf S, Rees C, Olesen RK, Propper D, Bridgewater J, Azzabi A, Farrugia D, Webb A, Cunningham D, Hickish T, Weaver A, Gollins S, Wasan HS, Paul J. 3 vs 6 mo of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2018;19:562-578. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 25. | André T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, Fratte S, Hug de Larauze M, Paget-Bailly S, Chibaudel B, Bez J, Dauba J, Louvet C, Lepere C, Dupuis O, Becouarn Y, Mabro M, Egreteau J, Bouche O, Deplanque G, Ychou M, Galais MP, Ghiringhelli F, Dourthe LM, Bachet JB, Khalil A, Bonnetain F, de Gramont A, Taieb J; for PRODIGE investigators, GERCOR, Fédération Française de Cancérologie Digestive, and UNICANCER. Three Versus 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Patients With Stage III Colon Cancer: Disease-Free Survival Results From a Randomized, Open-Label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J Clin Oncol. 2018;36:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Souglakos J, Boukovinas I, Kakolyris S, Xynogalos S, Ziras N, Athanasiadis A, Androulakis N, Christopoulou A, Vaslamatzis M, Ardavanis A, Emmanouilides C, Bompolaki I, Kourousis C, Makrantonakis P, Christofyllakis C, Athanasiadis E, Kentepozidis N, Karampeazis A, Katopodi U, Anagnosopoulos A, Papadopoulos G, Prinarakis E, Kalisperi A, Mavroudis D, Georgoulias V. Three- vs six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann Oncol. 2019;30:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Yoshino T, Yamanaka T, Oki E, Kotaka M, Manaka D, Eto T, Hasegawa J, Takagane A, Nakamura M, Kato T, Munemoto Y, Takeuchi S, Bando H, Taniguchi H, Gamoh M, Shiozawa M, Mizushima T, Saji S, Maehara Y, Ohtsu A, Mori M. Efficacy and Long-term Peripheral Sensory Neuropathy of 3 vs 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Colon Cancer: The ACHIEVE Phase 3 Randomized Clinical Trial. JAMA Oncol. 2019;5:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 28. | Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 685] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 29. | Soveri LM, Lamminmäki A, Hänninen UA, Karhunen M, Bono P, Osterlund P. Long-term neuropathy and quality of life in colorectal cancer patients treated with oxaliplatin containing adjuvant chemotherapy. Acta Oncol. 2019;58:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |