Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3088
Peer-review started: September 13, 2021
First decision: January 18, 2022
Revised: January 31, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 6, 2022
Processing time: 196 Days and 21.2 Hours
Pleural effusions occur for various reasons, and their diagnosis remains challenging despite the availability of different diagnostic modalities. Medical thoracoscopy (MT) can be used for both diagnostic and therapeutic purposes, especially in patients with undiagnosed pleural effusion.
To assess the diagnostic efficacy and safety of MT in patients with pleural effusion of different causes.
Between January 1, 2012 and April 30, 2021, patients with pleural effusion underwent MT in the Department of Respiratory Medicine, The Second Affiliated Hospital of Xi'an Jiaotong University (Shaanxi, China). According to the discharge diagnosis, patients were divided into malignant pleural effusion (MPE), tuberculous pleural effusion (TBPE), and inflammatory pleural effusion (IPE) groups. General information, and tuberculosis- and effusion-related indices of the three groups were analyzed. The diagnostic yield, diagnostic accuracy, performance under thoracoscopy, and complications of patients were compared among the three groups. Then, the significant predictive factors for diagnosis between the MPE and TBPE groups were analyzed.
Of the 106 patients enrolled in this 10-year study, 67 were male and 39 female, with mean age of 57.1 ± 14.184 years. Among the 74 thoracoscopy-confirmed patients, 41 (38.7%) had MPE, 21 had (19.8%) TBPE, and 32 (30.2%) were undiagnosed. Overall diagnostic yield of MT was 69.8% (MPE: 75.9%, TBPE: 48.8%, and IPE: 75.0%, with diagnostic accuracies of 100%, 87.5%, and 75.0%, respectively). Under thoracoscopy, single or multiple pleural nodules were observed in 81.1% and pleural adhesions in 34.0% with pleural effusions. The most common complication was chest pain (41.5%), followed by chest tightness (11.3%) and fever (10.4%). Multivariate logistic regression analyses showed effusion appearance [odds ratio (OR): 0.001, 95%CI: 0.000-0.204; P = 0.010] and carcinoembryonic antigen (OR: 0.243, 95%CI: 0.081-0.728; P = 0.011) as significant for differentiating MPE and TBPE, with area under the receiver operating characteristic curve of 0.977 (95%CI: 0.953-1.000; P < 0.001).
MT is an effective, safe, and minimally invasive procedure with high diagnostic yield for pleural effusion of different causes.
Core Tip: To evaluate the efficacy and safety of medical thoracoscopy (MT) for pleural effusion of different causes, this study retrospectively analyzed the medical records of 106 patients with pleural effusion who underwent MT at our hospital. The results showed that MT had high diagnostic value and a good safety profile, especially for malignant pleural effusion. Due to its clinical practicability, it is worth continually improving and vigorously promoting this technology.
- Citation: Liu XT, Dong XL, Zhang Y, Fang P, Shi HY, Ming ZJ. Diagnostic value and safety of medical thoracoscopy for pleural effusion of different causes. World J Clin Cases 2022; 10(10): 3088-3100
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3088.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3088
Pleural effusion, an abnormal build-up of fluid in the pleural space[1], is a common clinical symptom caused by cancer, tuberculous pleurisy, inflammation, and dysfunction of organs such as the heart, liver, and kidney[2]. The main manifestation in patients is dyspnea, and other presenting manifestations are largely determined by the underlying diseases. Previously, pleural effusion was mainly diagnosed by clinical history, physical examination, imaging techniques, thoracentesis, and percutaneous pleural biopsy. However, these methods have low diagnostic yield and delayed diagnosis of pleural effusion, which are associated with markedly higher morbidity and mortality. Currently, medical thoracoscopy (MT), a minimally invasive procedure that is efficient, safe, simple, and cost-effective, has distinctive advantages in diagnosing and treating pleural effusion and pleural diseases[3]. Thus, it is currently the gold standard for the diagnosis of pleural effusion[4].
Our study collected relevant clinical data of patients in The Second Affiliated Hospital of Xi'an Jiaotong University (Shaanxi, China), who underwent MT for diagnosis and/or treatment. We evaluated the diagnostic value and safety of MT by analyzing the diagnostic yield and complications in patients with pleural effusion of different causes.
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Xi'an Jiaotong University. This study involved patients admitted to the Department of Respiratory Medicine at our institute between January 1, 2012 and April 30, 2021 to undergo MT for diagnosing and/or treating pleural effusion. Inclusion criteria were: patients with pleural effusion confirmed by chest computed tomography (CT) before admission or before thoracoscopy; patients with undiagnosed pleural effusion (UPE) that could not be determined by various methods such as thoracentesis, closed pleural biopsy (CPB), or bronchoscopy or those who had been diagnosed but needed thoracoscopy for treatment; and patients who underwent MT twice with data collected only after the first MT, and those who underwent pathological tissue biopsy under MT. Exclusion criteria were: incomplete clinical data; no pleural space, extensive pleural adhesions, or late empyema; poor physical condition accompanied by severe cardiopulmonary insufficiency, and inability to tolerate thoracoscopy; and severe hyperemia, bleeding tendency, or refractory cough.
The general patient information included age, sex, length of hospitalization, length of time from onset to hospitalization, history of smoking, history of cancer (personal and family), tuberculosis; history of chronic diseases including hypertension, diabetes mellitus, chronic coronary and lung diseases, others; tumor-related biomarkers such as carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), carbohydrate antigen 125, squamous cell carcinoma-associated antigen, pro gastrin releasing peptide (PROGPR), cytokeratin fragment (CYFRA); tuberculosis-related indices such as erythrocyte sedimentation rate (ESR), tuberculosis DNA test, tuberculosis triple antibody test, tuberculosis immunoglobin G (IgG) antibody test, rapid microbial resistance test [X-pert mycobacterium tubercu
All analyses were performed with SPSS Statistics software (version 18.0; IBM Co., Armonk, NY, United States). Data not normally distributed are presented as M (Q1, Q4), and the Kruskal-Wallis H test was used for comparisons among groups. The enumeration data are presented as n (%) and were subjected to the c2 test for comparisons among groups for bidirectional unordinal variables, noting that more than one expected grid frequency was less than 5. The Fisher’s exact test or Kruskal-Wallis H test was used for single directional ordinal variables. Logistic regression analysis was used to analyze the significant predictive factors for diagnosis between the MPE and TBPE groups. Variables in logistic regression analysis were those in which P was less than 0.05 between the MPE and TBPE groups. Prior to analyses, the logit-converted values needed to meet a linear relationship between continuous independent and dependent variables, and the multiple commonalities needed to be excluded between independent and dependent variables. P < 0.05 was considered statistically significant. The receiver operating characteristic (ROC) curve was drawn according to the logistic regression analyses.
Between January 1, 2012 and April 30, 2021, 106 patients with pleural effusion successfully underwent MT, and pleural biopsy samples were obtained for diagnostic evaluation. There were 67 men and 39 women (age range, 21-82 years; mean age, 57.1 ± 14.184 years; mean length of hospitalization, 15.57 ± 5.386 d; mean time from onset to hospitalization, 57.04 ± 97.35 d). In the 106 patients, there were 41 (38.7%) smokers, 12 (11.3%) with a history of cancer, 2 (1.9%) with a family history of cancer, 2 (1.9%) with a history of tuberculosis, 6 (5.7%) with a history of coronary disease, 19 (17.9%) with hypertension, and 11 (10.4%) with diabetes mellitus. The effusion size was small in 2 (1.9%), moderate in 32 (30.2%), and large in 72 (67.9%). Pleural effusion occurred only on the left side in 31 (29.2%), only on the right in 52 (49.1%), and on both sides in 23 (21.7%) patients. The pleural effusion appearance was bloody in 39 (36.8%) patients and non-bloody in 67 (63.2%).
In the aforementioned indices, age was statistically different among groups (P = 0.025), mainly between the MPE and TBPE groups. Time in the MPE group was longer than that in the TBPE and IPE groups (P = 0.021). The incidence of cancer history in the MPE group was higher than that in the TBPE and IPE groups (P = 0.029). Regarding the tuberculous indices, the positive rates of tuberculosis DNA, triple antibody, IgG antibody, and MTB/RIF were not significantly different among the three groups, with the exception of T-SPOT (P < 0.001) and ESR (P = 0.004). Regarding the tumor biomarkers, CEA, PROGPR, and CYFRA were statistically different between the MPE and TBPE groups (P < 0.05). Regarding the effusion-related examinations, LDH, protein, and glucose were not statistically significant among the three groups, with the exception of the ADA index (P < 0.001). Additionally, there were higher numbers of nucleated and mononucleated cells in the TBPE group than in the MPE and IPE groups (P = 0.001 and P < 0.001, respectively). The patients’ data are shown in Table 1.
| Variables | Total | Malignant (n = 54) | Tuberculous (n = 43) | Inflammatory (n = 9) | χ2/Z
| P value |
| Age in yr | 59 (49-68) | 62 (54-70) | 54 (43-66)a | 58 (51-72) | 7.359 | 0.025 |
| Sex | 1.723 | 0.427 | ||||
| Male | 67 (63.1) | 34 | 29 | 4 | ||
| Female | 39 (36.8) | 20 | 14 | 5 | ||
| Hospital stay in d | 15.00 (12.00-17.25) | 16.00 (14.00-19.25) | 13.00 (11.00-17.00)a | 15.00 (12.50-23.00) | 7.773 | 0.021 |
| Disease duration in d | 30.00 (15.00-60.00) | 30.00 (20.00-60.00) | 30.00 (10.00-60.00) | 30.00 (8.00-30.00) | 3.375 | 0.185 |
| Smoking history | 41(38.7) | 19 | 18 | 4 | 0.688 | 0.756 |
| Personal tumor | 12 (11.3) | 9 | 1a | 2 | 6.815 | 0.029 |
| Family tumor | 2 (1.9) | 2 | 0 | 0 | 1.735 | 0.583 |
| Tuberculous history | 2 (1.9) | 0 | 1 | 1 | 4.351 | 0.076 |
| Hypertension | 19 (17.9) | 9 | 8 | 2 | 0.431 | 0.930 |
| Diabetes | 11 (10.4) | 5 | 4 | 2 | 1.760 | 0.428 |
| Coronary disease | 6 (5.7) | 3 | 3 | 0 | 0.336 | 1.000 |
| Chronic lung disease | 3 (2.8) | 0 | 2 | 1 | 4.561 | 0.061 |
| Other chronic diseases | 25 (23.6) | 12 | 10 | 3 | 0.745 | 0.744 |
| Tuberculosis DNA | 1 (0.9) | 0 | 1 | 0 | 2.831 | 0.643 |
| Tuberculosis triple antibody | 13 (12.3) | 3 | 10 | 0 | 7.807 | 0.080 |
| Tuberculosis IgG antibody | 5 (4.7) | 2 | 3 | 0 | 4.518 | 0.310 |
| MTB/RIF | 4 (3.8) | 1 | 1 | 2 | 8.230 | 0.061 |
| T-SPOT | 45 (42.5) | 13 | 29 a | 3 | 19.217 | < 0.001 |
| ESR in mm/h | 28.00 (12.00-44.00) | 25.50 (12.00-39.25) | 27.00 (12.00-34.00) | 62.00 (50.00-71.50)a, b | 11.009 | 0.004 |
| Side of effusion | 6.521 | 0.151 | ||||
| Left | 31 (29.2) | 19 | 10 | 2 | ||
| Right | 52 (49.1) | 28 | 21 | 3 | ||
| Bilateral | 23 (21.7) | 7 | 12 | 4 | ||
| Size of effusion | 5.333 | 0.069 | ||||
| Small | 2 (1.9) | 0 | 1 | 1 | ||
| Moderate | 32 (30.2) | 12 | 17 | 3 | ||
| Large | 72 (67.9) | 42 | 25 | 5 | ||
| Effusion appearance | 14.815 | < 0.001 | ||||
| Bloody | 39 (36.8) | 29 | 7 | 3 | ||
| Non-bloody | 67 (63.2) | 25 | 36a | 6 | ||
| CEA in ng/mL | 2.22 (1.25-4.66) | 3.28 (1.73-9.10) | 1.40 (0.89-2.3)a | 1.45 (0.94-4.85) | 21.293 | < 0.001 |
| NSE in ng/mL | 16.29 (10.96-20.94) | 16.44 (10.91-20.80) | 16.65 (13.00-23.19) | 11.40 (9.94-14.30) | 3.934 | 0.140 |
| CA125 in U/mL | 75.63 (33.02-229.63) | 65.15 (31.64-231.88) | 84.89 (40.61-285.80) | 84.73 (36.91-112.84) | 0.830 | 0.660 |
| SCCA in ng/mL | 0.70 (0.50-0.90) | 0.70 (0.50-0.80) | 0.70 (0.50-1.00) | 0.80 (0.55-1.15) | 1.785 | 0.410 |
| PROGPR in pg/mL | 24.40 (17.28-35.38) | 29.80 (19.58-37.38) | 20.10 (15.80-27.50)a | 25.90 (17.45-55.75) | 7.874 | 0.020 |
| CYFRA in ng/mL | 3.35 (2.02-8.70) | 5.51 (3.35-14.46) | 2.06 (1.48-2.90)a | 2.54 (1.38-9.90) | 33.461 | < 0.001 |
| Characteristics of pleural effusion | ||||||
| LDH in U/L | 279.00 (182.50-485.25) | 333.50 (187.00-495.75) | 245.00 (183.00-383.00) | 699.00 (106.00-947.50) | 2.950 | 0.229 |
| Protein in g/L | 43.50 (36.90-48.48) | 42.15 (36.98-46.58) | 46.00 (37.20-49.90) | 43.00 (29.40-47.40) | 2.008 | 0.366 |
| Glucose in mmol/L | 6.16 (4.97-7.31) | 6.37 (5.15-7.33) | 5.87 (5.07-7.08) | 5.57 (3.97-10.67) | 0.218 | 0.897 |
| ADA in U/L | 14.00 (8.00-31.00) | 9.00 (7.00-13.00) | 27.00 (16.00-37.00)a | 17.00 (6.50-34.50) | 26.167 | < 0.001 |
| Nucleated cell | 1345.50 (770.75-2847.25) | 950.00 (569.50-2089.25) | 2283.00 (1312.00-3631.00)a | 1000.00 (245.00-4430.00) | 15.049 | 0.001 |
| Mononucleated cell | 1079.00 (584.25-2317.50) | 753.50 (420.25-1629.50) | 1889.00 (1057.00-3018.00)a | 880.00 (206.50-3658.00) | 17.186 | < 0.001 |
| Multinucleated cells | 99.00 (48.00-304.75) | 108.50 (54.75-257.25) | 88.00 (37.00-335.00) | 120.00 (47.50-1140.50) | 1.331 | 0.514 |
Of the 106 patients, 54 were diagnosed with MPE, 43 with TBPE, and 9 with IPE, according to clinical history, imaging and pleural effusion examination, MT and other inspections. Under MT, 41 patients were confirmed to have MPE, with pleural origin in 5, lung origin in 32 (28 adenocarcinomas, 1 squamous carcinoma, 3 small cell carcinomas), and non-lung origin in 4 (2 kidney cancer, 1 breast cancer, 1 malignant lymphoma). Therefore, the diagnostic yield of MPE was 75.9% without the misdiagnosed ones, and the diagnostic accuracy was 100%. In the 43 TBPE patients, 21 were confirmed. The diagnostic yield was 48.8%; however, three exceptions demonstrated inflammation under MT. The pleural effusion in these 3 patients indicated tuberculosis infection and the diagnostic anti-tuberculous chemotherapy was effective; therefore, their diagnoses were subsequently modified to TBPE. Eventually, the diagnostic accuracy was 87.5%. Twelve patients were confirmed to have IPE under MT, one had purulent pleural effusion, and three were ultimately diagnosed with TBPE. The diagnostic yield and diagnostic accuracy were 75.0%. The patients’ data are presented in Tables 2 and 3.
| Etiology | Value | % |
| Malignancy | 41 | 38.7 (41/106) |
| Pleural origin | 5 | 12.2 (5/41) |
| Lung origin | ||
| Adenocarcinoma | 28 | 68.3 (28/41) |
| Squamous carcinoma | 1 | 2.4 (1/41) |
| Small cell carcinoma | 3 | 7.4 (3/41) |
| Non-lung origin | 4 | 9.8 (4/41) |
| Tuberculosis | 21 | 19.8 (21/106) |
| Purulence | 1 | 0.9 (1/106) |
| Nonspecific inflammation | 11 | 10.4 (11/106) |
| Undiagnosed | 32 | 30.2 (32/106) |
| Total | 106 | 100.0 (106/106) |
| Etiology | Malignant | Tuberculous | Inflammatory | |||||||||
| T | D | UD | MD | T | D | UD | MD | T | D | UD | MD | |
| n | 54 | 41 | 13 | 0 | 43 | 21 | 19 | 3 | 9 | 12 | 0 | 3 |
| Diagnostic yield, % | 75.9 | 48.8 | 75.0 | |||||||||
| Accuracy, % | 100 | 87.5 | 75.0 | |||||||||
Under MT, we observed single or multiple nodules in 86 (81.1%) patients, including 49 with malignant etiology, 29 with tuberculous etiology, and 8 with inflammatory etiology. In the 36 (34.0%) patients with pleural adhesions, malignant, tuberculous and inflammatory etiologies were in 15, 15, and 6, respectively. Fibrous connective tissue and fibrous bands were seen in 13 (12.3%) patients, including 8 tuberculous and 5 malignant patients. Plaque-like lesions and carbon foam deposition were seen in 11 (10.4%), including 4 tuberculous and 7 malignant patients. Nine (8.5%) patients had miliary nodules under MT, mainly observed in the TBPE group. Six (5.6%) patients (3 in the MPE and 3 in the TBPE groups) had focal necrosis. Three (2.8%) patients with MPE demonstrated neoformation and 2 (1.9%) with TBPE demonstrated pleural thickening. Only one confirmed MPE patient had pleural hyperemia and edema. In the nine types of thoracoscopic findings above, single or multiple nodules and miliary nodules were statistically significant in the comparison of the three groups (P < 0.05). Particularly, single or multiple nodules were more frequently observed in the MPE group than in the TBPE group (P = 0.004), whereas there were more miliary nodules in the TBPE group than in the MPE group (P = 0.010). The data are presented in Table 4.
| Characteristic | n (%) | Malignant | Tuberculous | Inflammatory | χ2 | P value |
| Single or multiple nodules | 86 (81.1) | 49 | 29a | 8 | 8.335 | 0.011 |
| Miliary nodules | 9 (8.5) | 1 | 8 a | 0 | 8.228 | 0.012 |
| Pleural hyperemia and edema | 1 (0.9) | 1 | 0 | 0 | 1.735 | 1.000 |
| Pleural adhesions | 36 (34.0) | 15 | 15 | 6 | 4.937 | 0.084 |
| Pleural thickening | 2 (1.9) | 0 | 2 | 0 | 2.656 | 0.326 |
| Fibrous connective tissue and fibrous bands | 13 (12.3) | 5 | 8 | 0 | 2.953 | 0.244 |
| Focal necrosis | 6 (5.6) | 3 | 3 | 0 | 0.336 | 1.000 |
| Neoformation | 3 (2.8) | 3 | 0 | 0 | 2.330 | 0.428 |
| Plaque-like lesions and carbon foam deposition | 11 (10.4) | 7 | 4 | 0 | 0.877 | 0.709 |
During this 10-year study, no serious adverse events were recorded in any patient. Local pain was the most common complication in 44 (41.5%) patients, including 21 malignant, 19 tuberculous, and 4 inflammatory patients. Twelve (11.3%) patients had chest tightness, including nine malignant, one tuberculous, and two inflammatory patients. Eleven (10.4%) patients had fever, including seven tuberculous and four malignant patients. Seven (6.6%) patients had subcutaneous emphysema, including three tuberculous, two malignant, and two inflammatory patients. Bleeding, cutaneous infection at the entry site, and prolonged air leak were observed in 2 (1.9%) patients, 1 malignant and 1 tuberculous. Other complications were mainly nausea in 2 patients, vomiting in 1 patient, and arrhythmia (rapid heart rate, rapid atrial fibrillation, frequent atrial fibrillation) in 1 patient. In the eight complications above, only the incidence of chest tightness was statistically different among the three groups (P < 0.05), mainly between the MPE and TBPE groups (P = 0.039). The data are presented in Table 5.
| Complication | n (%) | Malignant | Tuberculous | Inflammatory | χ2 | P value |
| Fever | 11 (10.4) | 4 | 7 | 0 | 4.403 | 0.250 |
| Bleeding | 2 (1.9) | 1 | 1 | 9 | 0.767 | 1.000 |
| Chest tightness | 12 (11.3) | 9a | 1a | 2 | 6.815 | 0.029 |
| Subcutaneous emphysema | 7 (6.6) | 2 | 3 | 2 | 3.905 | 0.128 |
| Local pain | 44 (41.5) | 21 | 19 | 4 | 0.396 | 0.874 |
| Cutaneous infection at the entry site | 2 (1.9) | 1 | 1 | 0 | 0.767 | 1.000 |
| Prolonged air leak | 2 (1.9) | 1 | 1 | 0 | 0.767 | 1.000 |
| Others | 4 (3.8) | 2 | 2 | 0 | 0.365 | 1.000 |
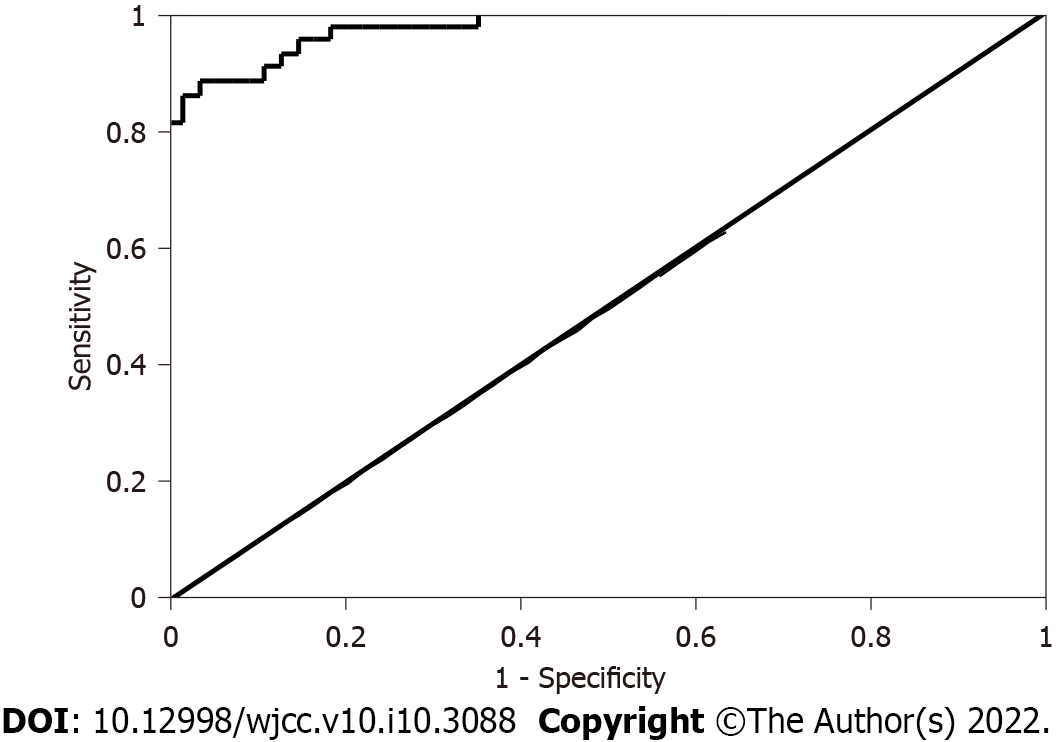
As there were only 9 patients with IPE, the statistically significant differences among the three groups mainly focused on the MPE and TBPE groups, and multivariate analyses were only performed in those two groups. Variables with P < 0.05 in univariate analysis were included in the logistic regression model. Prior to analysis, the logit-converted values had a linear relationship (P > 0.05) between continuous independent and dependent variables. Additionally, multiple commonalities (tolerance > 0.1 and variance expansion factor < 10) were excluded between independent and dependent variables; therefore, 15 variables met the inclusion criteria. Logistic regression analyses showed that the effusion appearance [odds ratio (OR): 0.001, 95%CI: 0.000-0.204; P = 0.010) and CEA level (OR: 0.243, 95%CI: 0.081-0.728; P = 0.011) were statistically significant; that is, bloody pleural effusion and CEA played predictive roles in the differential diagnosis of MPE and TBPE. ROC results showed that the area under the ROC was 0.977 (95%CI: 0.953-1.000; P < 0.001). When the Youden index was 0.847, the sensitivity was 88.4% and the specificity was 96.3%. The data are presented in Table 6 and Figure 1.
| Variable | β | SE | P value | OR | 95%CI | |
| Lower | Upper | |||||
| Age in yr | -0.042 | 0.037 | 0.259 | 0.959 | 0.892 | 1.031 |
| Hospital stay in d | -0.032 | 0.125 | 0.800 | 0.969 | 0.758 | 1.238 |
| Personal tumor | -1.223 | 1.865 | 0.512 | 0.294 | 0.008 | 11.385 |
| T-SPOT | -2.259 | 1.467 | 0.124 | 0.105 | 0.006 | 1.854 |
| ESR | 0.007 | 0.032 | 0.814 | 1.008 | 0.946 | 1.072 |
| Effusion ADA | 0.212 | 0.109 | 0.052 | 1.236 | 0.998 | 1.530 |
| Effusion appearance | -6.710 | 2.613 | 0.010 | 0.001 | 0.000 | 0.204 |
| CEA | -1.413 | 0.559 | 0.011 | 0.243 | 0.081 | 0.728 |
| PROGPR | -0.097 | 0.051 | 0.060 | 0.908 | 0.821 | 1.004 |
| CYFRA | -0.064 | 0.038 | 0.091 | 0.938 | 0.870 | 1.010 |
| Nucleated cell | 0.001 | 0.000 | 0.263 | 1.001 | 1.000 | 1.002 |
| Mononucleated cell | -0.001 | 0.001 | 0.354 | 0.999 | 0.998 | 1.001 |
| Single or multiple nodules | 2.143 | 2.008 | 0.286 | 8.526 | 0.167 | 436.395 |
| Miliary nodules | -1.982 | 2.936 | 0.500 | 0.138 | 0.000 | 43.469 |
| Chest tightness | 5.356 | 2.817 | 0.057 | 211.770 | 0.848 | 52896.349 |
Pleural effusions are caused by various reasons, but the common causes are congestive heart failure, malignancy, pneumonia, and pulmonary embolism[2]. In addition to mesothelioma, pleural metastatic carcinomas from the lung, breast, and lymph nodes are common causes of MPE[5], while in benign pleural effusion, tuberculosis is the most common cause. As reported, 16.7% of patients with MPE likely develop an effusion during their disease[6], with 15% at presentation, 50% during lung cancer[5], and 90% in malignant pleural mesothelioma[7]. Regarding TBPE, in tuberculosis endemic areas, the incidence of pleural involvement approaches 30%, and is 3%-5% in non-endemic areas[8]. In our study, for etiological analysis of pleural effusion, malignant origin ranked first (38.7%), mainly subclassified into pleural metastatic carcinomas originating from the lung, tuberculous origin (19.8%), and inflammatory origin (11.3%), generally in accordance with the results of similar studies from our adjacent hospitals (cancer metastasis 43.0%, tuberculous pleuritis 23.3%, and non-specific pleuritis 15.1%)[9]. In a meta-analysis involving 2380 patients in the etiological analysis of patients with pleural effusion who underwent MT, malignant, tuberculous, and inflammatory causes accounted for 56.2%, 21.6%, and 17.5% cases, respectively. In patients with malignant causes, 38.7% of patients were due to metastatic carcinomas, mainly from the lung (78.1%)[10], consistent with another large sample study (lung cancer accounts for 85.2% of metastatic cancer in MPE)[11]. Although the samples in our study were small, the results were similar to those of large samples. Of note, in our study, MPE accounted for a considerable proportion of cases, as the majority of patients with MPE had obtained a definitive diagnosis and underwent MT in order to seek present or subsequent treatment as well as symptomatic relief. TBPE accounted for less than 20% of cases, as these patients mainly had UPE before MT in our study. As reported in a portion of the literature, UPE still leaves a low diagnostic level even under MT[8,12]. There was a low number of IPE patients confirmed under MT in this study, partly due to enrolling some patients who had no evidence of malignancy or tuberculosis except inflammation.
Differentiating benign from malignant pleural effusions is critical for diagnosis establishment, management guidance, and prognosis judgement[13]. Over the past several years, the primary diagnostic methods were effusion examination and CPB, coupled with clinical history, blood biochemistry, and imaging examination to distinguish between benign and MPE, despite the low diagnostic yield. Studies have shown that thoracocentesis yields a diagnosis of pleural effusion in 60% of cases, and CPB in 45%. By contrast, the combined diagnostic yield can be improved to 75%. Recently, the use of thoracic ultrasound and/or CT to provide real-time image guidance has been increasingly adopted, which should be the best practice to optimize diagnostic yield and patients’ safety, avoiding subsequent invasive procedures such as MT, which are unequivocally supported by national guidelines[14]. A randomized controlled trial revealed that CT-guided biopsy improves the diagnostic yield of 40% compared with unassisted CPB in patients with MPE (87% vs 47%)[15]. In terms of safety, another observational cohort study demonstrated that performing ultrasound-guided thoracentesis could reduce the risk of pneumothorax by 19% and bleeding complications by 68%[16]. Nonetheless, 8%-25% have UPE[17], probably as image-guided biopsy allows limited access to adequate quantities of tissue compared with thoracoscopy, particularly for those in whom additional molecular analysis is required or histological diagnosis is challenging. Fortunately, MT has brought about revolutionary improvements in the diagnosis and management of pleural effusion, and the diagnostic yield for UPE has reportedly increased 80%-99%[18,19]. Among the 106 patients in our study, MT yielded a diagnosis in 74 (69.8%), with an undiagnostic yield in 30.2%. A systematic review including four articles, each with 21-68 patients, yielded a diagnosis by MT in patients with UPE, ranging from 66.7% to 97%. Simultaneously, the study performed an analogic single-center study in 48 congener patients revealing a diagnostic yield of 66.7%, a proportion similar to that in our study[20]. For example, a systematic review and meta-analysis of published studies that included data on the yield and diagnostic safety of pleural cryobiopsy compared procedures performed using conventional flexible forceps, and found a diagnostic yield of 95% and 91%, respectively[18]. However, some reports revealed a higher diagnostic yield of MT up to 95%[21]. We took into account the development level of MT and operators’ technical proficiency in different regions and hospitals. Regardless of the diagnostic yield, the diagnostic sensitivity and specificity of MT remain high[22]. It is noticeable that the diagnostic yield of MT for MPE is relatively high, in contrast to TBPE. Regarding our study on pleural effusion with different etiologies, the diagnostic yield for MPE, TBPE, and IPE were 75.9%, 48.8%, 75.0%, respectively, with diagnostic accuracy of 100%, 87.5%, and 75.0%, respectively. Relevant studies have reported a diagnostic yield of 65.8% and 34.2% for MPE and benign pleural effusion, respectively, with a diagnostic accuracy of 97.4%[23]. Another study showed similar results (diagnostic yield for malignant 68.3%, benign 31.7%, and diagnostic accuracy 97.6%)[24]. The diagnosis of TBPE is difficult, largely due to the paucibacillary nature of these effusions and low yield on mycobacterium tuberculosis culture, because of the compartmentalization of pleural effusion and effective containment of this bacilli by cytokine milieu[8]. Thoracoscopic pleural pathology is the gold standard method for TBPE diagnosis[25], but tissue material selection is not always available, which limits its diagnosis. Furthermore, the effects of variables on predicting benign and MPE are differential. In our study, we used indices such as age, blood and effusion-related indices, and effusion appearance in univariate analyses between the benign and malignant group. Only bloody effusion and CEA had predictive value after multivariate analysis, consistent with clinical practice. It remains challenging to distinguish TBPE from MPE due to the lack of specificity of clinical features, despite some indices such as CEA, LDH, ADA, T-SPOT, mononuclear cell count, interferon gamma, interleukin 12, and X-pert MTB/RIF, which are potential predictors[8,26,27].
Unlike thoracocentesis and CPB, MT permits biopsy for suspicious lesions with direct visualization to improve the diagnostic yield of pleural effusion, which can be targeted accurately[28]. In our study, single or multiple nodules (81.1%) were the most common findings under MT, following by pleural adhesions (34.0%). For MPE, single or multiple nodules (46.2%), pleural adhesions (14.2%), plaque-like lesions and carbon foam deposition (6.6%) were reported successively. For TBPE, in addition to single or multiple nodules (27.4%) and pleural adhesions (14.2%), frequent reports were miliary nodules (7.5%), and fibrous connective tissue and fibrous bands (7.5%) were also reported. Regarding IPE, only nodules and pleural adhesions were observed. Sakr et al[29] reported the thoracoscopic findings of 107 patients with MPE, revealing pleural nodules (81.3%) and pleural adhesions (40.2%). In a study by Wang et al[30], 333 patients were diagnosed with tuberculous pleurisy by MT, which revealed pleural nodules (69.4%), pleural adhesions (66.7%), hyperemia (60.7%), and plaque-like lesions (6.0%). The common findings in our study were similar to those in previous reports. Pleural nodules and pleural adhesions were the most frequent pleural abnormalities under MT in both reported studies and the current study. Pleural nodules, one of the MT indications, classified as benign or malignant, are generally caused by tumors, tuberculosis, or inflammation. Unlike with lung nodules, radiologic methods such as CT often fail to pick up early pleural abnormalities, given the similar density between the apposed pleura and adjacent pleural effusion[13]. Therefore, MT has been the optimal choice due to its direct visible access to the lesions. Pleural adhesions refer to the two layers of pleura sticking together, along with pleural thickening if the fibrin in the effusion is deposited on the pleura. The presence of pleural adhesions may prevent full examination of pleural effusions and/or pleural diseases. A retrospective analysis of 540 patients with MPE who underwent MT, found a high frequency of significant adhesions (40%) and an inverse correlation between the extent of pleural adhesions and the sensitivity of MPE cytology; when the grade of adhesions ranged from 0 to 4, the cytologic sensitivity of MPE decreased from 71% to 20%[31]. Moreover, in clinical practice, pleural adhesions can also increase the frequency of thoracentesis. Therefore, pleural adhesions have been challenging the diagnosis and management of pleural effusions and/or pleural diseases. Serious pleural adhesion that leaves no pleural space is an absolute contraindication for MT[32]. In our study, the extent of pleural adhesions permitted conducting MT, which is why the proportion of pleural adhesions was lower than that in the literature. Other findings under MT were less than 15% in our study. Representative characteristics of different pleural diseases under MT are generally not distinct; however, visual judgement for differential performance of lesions coupled with professional cognition for diseases can partly help pulmonologists obtain preliminary inference.
MT is now increasingly common in pleural interventional practice, where recent years have seen rapid and unprecedented variations in access to diagnosis and treatment with yields and safety levels akin to or even surpassing those provided by other methods[14]. To a great extent, its safety should be attributed to the procedure’s standard operating specification, such as special semi-rigid instruments that allows a single small skin incision for insertion of a disposable flexible trocar, adequate patient preparation prior to the procedure, local anesthesia, moderate sedation and analgesic, and spontaneous ventilation, accompanied by electrocardiographic and oxygen saturation monitoring throughout the procedure[32]. However, it is generally acknowledged that complications are inevitable, and the reasons should be considered in two situations: when the operators do not extensive knowledge on the anatomical structure of the thoracic cavity and proficient operational techniques that require a learning process to master, and when the patients have special physical constitutions. Therefore, careful assessment of the patient’s condition, adequate training of pulmonologists’ operating skills, careful consideration of contraindications and prevention of complications cannot be overemphasized prior to the procedure[21]. The Medicine Thoracoscopy Diagnostic Specifications by Chinese Medical Doctor Association lists 23 possible complications including 3 prior to MT, 7 during MT, and 13 after MT[32]. By contrast, our study obtained more complications after MT due to limited data collection methods that relied on electronic medical records. In our retrospective study, the results revealed pain at the entry site (41.5%), slight chest tightness (11.3%), fever (10.4%), and subcutaneous emphysema (6.6%), and other reported complications less than 5% recovered soon after symptomatic treatments, and there was no thoracoscopy-related death. A large sample study with 1926 patients with pleural effusion undergoing MT reported that the most common complications were pain (38.9%), fever (20.8%), cutaneous infection at the entry site (7.1%), and subcutaneous emphysema (3.2%); however, the rare complications were prolonged air leakage, bleeding, lung laceration, pulmonary re-expansion edema, mediastinal emphysema and mortality, whose incidences were less than 0.5%[33]. By contrast, other recorded complications such as prolonged air leak, cutaneous infection at the entry site, and bleeding in our study were also less than 2%. Collectively, it is generally agreed that MT appears to be relatively safe and deserves to be vigorously promoted clinically.
This study had several limitations. First, it was a single-center retrospective study, whose results were from a population in local regions and hospitals and thus may not be applicable to other populations from different regions and hospitals. Second, the timespan for the sample selection was large but the sample size was small. Our study included eligible patients from 2012 to 2021, when the MT technology was being developed and used in our hospital. At the beginning of development, the limitations such as operators’ technical proficiency, equipment configuration, and team’s co-ordination discounted the results of diagnosis and management. This may be why the diagnostic yield of pleural effusion of different causes in our study was lower than that confirmed by MT in the latest literature. Small samples of patients with inflammatory pleural effusion render it unable to conduct multivariate analysis, which will be improved in future studies.
In conclusion, MT appears to be efficient and relatively safe in the management of pleural diseases. Compared with effusion examination and pleural biopsy, its advantages lie in the factors including higher diagnostic yield and safety, easier use, lower cost and better tolerability to patients, which confer a significant clinical value. Presently, domestic MT has been proficient but is still in limited use and slow uptake by respiratory physicians in non-first-tier cities and non-large hospitals, which has delayed the diagnosis and management of thoracoscopy-adapted diseases. With the rapidly evolving development, it is vital that knowledge of MT is disseminated as widely and as efficiently as possible, and this novel pleural technique will also usher in more potential benefits.
In clinical practice, most patients with pleural effusion can be diagnosed definitively according to clinical history, symptoms, signs, and relevant examinations, but some undiagnosed and misdiagnosed patients still remain and miss the best time for treatment. However, minimally invasive techniques such as medical thoracoscopy (MT) have significantly improved the diagnostic yield and cure rate, especially in patients with undiagnosed pleural effusion. Therefore, evaluating the effectiveness and safety of MT has been key in the extensive development of this technology.
This study retrospectively analyzed the diagnostic efficacy and safety of MT in patients with pleural effusion, to comprehensively evaluate the practicability of MT and provide evidence support for large-scale clinical application.
This study investigated the diagnostic value of MT in patients with pleural effusion and evaluated its safety.
We obtained the clinical data of patients from the electronic medical system of our hospital, and summarized the baseline characteristics, MT results, and adverse reactions of 106 patients with pleural effusions. In addition, SPSS 18.0 software was used to analyze the single and multiple factors of patients with pleural effusions and establish the receiver operating characteristic curve (ROC) model to predict the value of these factors in differential diagnosis.
MT improved the diagnostic yield of pleural effusion (69.8%), especially malignant pleural effusion (75.9%) but not tuberculous pleural effusion (38.7%). We found that the incidence of adverse reactions was low, and chest pain at the entry site was largely seen. Logistic regression analysis identified bloody pleural effusion, and carcinoembryonic antigen had good predictive value in differentiating between malignant and tuberculous pleural effusion with an area under the ROC of 0.977 (P < 0.001).
MT is an effective, safe, minimally invasive procedure with high diagnostic yield for pleural effusion of different causes.
In recent years, increasingly improved diagnostic yield and cure rate of pleural effusions have been due to MT. However, some restrictions from promotion and technology itself contribute to undiagnosis and misdiagnosis. In the future, we should be committed to continuously innovating this technology to improve its clinical benefits.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors are grateful to the Department of Respiratory Medicine at The Second Affiliated Hospital of Xi'an Jiaotong University, Shaanxi, China and in particular the invaluable tutor, Professor Xilin Dong, for his assistance and guidance in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fekri MS, Soriano-Ursúa MA S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Semaan R, Feller-Kopman D, Slatore C, Sockrider M. Malignant Pleural Effusions. Am J Respir Crit Care Med. 2016;194:P11-P12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Jany B, Welte T. Pleural Effusion in Adults-Etiology, Diagnosis, and Treatment. Dtsch Arztebl Int. 2019;116:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Lee P, Folch E. Thoracoscopy: Advances and Increasing Role for Interventional Pulmonologists. Semin Respir Crit Care Med. 2018;39:693-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Anevlavis S, Froudarakis ME. Advances in pleuroscopy. Clin Respir J. 2018;12:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 6. | Walker S, Mercer R, Maskell N, Rahman NM. Malignant pleural effusion management: keeping the flood gates shut. Lancet Respir Med. 2020;8:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Koegelenberg CFN, Shaw JA, Irusen EM, Lee YCG. Contemporary best practice in the management of malignant pleural effusion. Ther Adv Respir Dis. 2018;12:1753466618785098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Shaw JA, Diacon AH, Koegelenberg CFN. Tuberculous pleural effusion. Respirology. 2019;24:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Chen RL, Zhang YQ, Wang J, Wu H, Yang SM. Diagnostic value of medical thoracoscopy for undiagnosed pleural effusions. Exp Ther Med. 2018;16:4590-4594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Zhang S, Tian HD, Liang B, Jiang SJ. Diagnostic value of medical thoracoscopy in 2380 patients with pleural effusion. Shiyong Yixue Zazhi. 2013;29:1316-1810. |
| 11. | Wu YB, Xu LL, Wang XJ, Wang Z, Zhang J, Tong ZH, Shi HZ. Diagnostic value of medical thoracoscopy in malignant pleural effusion. BMC Pulm Med. 2017;17:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Shrestha BK, Adhikari S, Thakur BK, Kadaria D, Tamrakar KK, Devkota M. Medical Thoracoscopy for Undiagnosed Exudative Pleural Effusion: Experience from Two Tertiary Care Hospitals of Nepal. JNMA J Nepal Med Assoc. 2020;58:158-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Fysh ETH, Thomas R, Tobin C, Kuok YJ, Lee YCG. Air in the Pleural Cavity Enhances Detection of Pleural Abnormalities by CT Scan. Chest. 2018;153:e123-e128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Bhatnagar R, Corcoran JP, Maldonado F, Feller-Kopman D, Janssen J, Astoul P, Rahman NM. Advanced medical interventions in pleural disease. Eur Respir Rev. 2016;25:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 240] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Agarwal A, Prasad R, Garg R, Verma SK, Singh A, Husain N. Medical thoracoscopy: a useful diagnostic tool for undiagnosed pleural effusion. Indian J Chest Dis Allied Sci. 2014;56:217-220. [PubMed] |
| 18. | Botana Rial M, Lojo Rodríguez I, Mouronte Roibás C, Leiro Fernández V, Núñez Delgado M, Salgado Barreira Á, Pereira Torrado A, Fernández Villar A. Diagnostic Yield and Safety of Pleural Cryobiopsy during Medical Thoracoscopy to Diagnose Pleural Effusion. A Systematic Review and Meta-Analysis. Arch Bronconeumol. 2020;56:784-791. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Dixon G, de Fonseka D, Maskell N. Pleural controversies: image guided biopsy vs. thoracoscopy for undiagnosed pleural effusions? J Thorac Dis. 2015;7:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 20. | Nattusamy L, Madan K, Mohan A, Hadda V, Jain D, Madan NK, Arava S, Khilnani GC, Guleria R. Utility of semi-rigid thoracoscopy in undiagnosed exudative pleural effusion. Lung India. 2015;32:119-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Burrows NJ, Ali NJ, Cox GM. The use and development of medical thoracoscopy in the United Kingdom over the past 5 years. Respir Med. 2006;100:1234-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Mohan A, Chandra S, Agarwal D, Naik S, Munavvar M. Utility of semirigid thoracoscopy in the diagnosis of pleural effusions: a systematic review. J Bronchology Interv Pulmonol. 2010;17:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Rozman A, Camlek L, Kern I, Malovrh MM. Semirigid thoracoscopy: an effective method for diagnosing pleural malignancies. Radiol Oncol. 2014;48:67-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Rozman A, Camlek L, Marc-Malovrh M, Triller N, Kern I. Rigid versus semi-rigid thoracoscopy for the diagnosis of pleural disease: a randomized pilot study. Respirology. 2013;18:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Lei X, Wang J, Yang Z, Zhou S, Xu Z. Diagnostic Value of Pleural Effusion Mononuclear Cells Count and Adenosine Deaminase for Tuberculous Pleurisy Patients in China: A Case-Control Study. Front Med (Lausanne). 2019;6:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Yang X, Feng M, Shen Y, Deng B, He Y, Cao G. Clinical characteristics and potential indicators for definite diagnosis of tuberculous pleural effusion. Artif Cells Nanomed Biotechnol. 2019;47:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12:1753466618808660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Shaikh F, Lentz RJ, Feller-Kopman D, Maldonado F. Medical thoracoscopy in the diagnosis of pleural disease: a guide for the clinician. Expert Rev Respir Med. 2020;14:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Sakr L, Maldonado F, Greillier L, Dutau H, Loundou A, Astoul P. Thoracoscopic assessment of pleural tumor burden in patients with malignant pleural effusion: prognostic and therapeutic implications. J Thorac Oncol. 2011;6:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Wang Z, Xu LL, Wu YB, Wang XJ, Yang Y, Zhang J, Tong ZH, Shi HZ. Diagnostic value and safety of medical thoracoscopy in tuberculous pleural effusion. Respir Med. 2015;109:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol. 2008;3:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Jin F, Wang H, Li Q, Li S, Lai G, Huang J, Huang Y, Jiang T, Bai C, Li W, Lu Y, Song Y, Sun R, Chen C, Zhang J, Zhang X, Zhou R, Zhou X, Chen Y, Du Y, Hu C, Zhou H. Expert consensus for diagnosis and treatment using medical thoracoscopy in China. J Thorac Dis. 2020;12:1799-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Wan YY, Zhai CC, Lin XS, Yao ZH, Liu QH, Zhu L, Li DZ, Li XL, Wang N, Lin DJ. Safety and complications of medical thoracoscopy in the management of pleural diseases. BMC Pulm Med. 2019;19:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |