Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3047
Peer-review started: October 16, 2021
First decision: November 17, 2021
Revised: December 13, 2021
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 6, 2022
Processing time: 164 Days and 6.8 Hours
The epidemiological and clinical characteristics of coronavirus disease 2019 (COVID-19) patients have been widely reported, but the assessment of dose-response relationships and risk factors for mortality and severe cases and clinical outcomes remain unclear.
To determine the dose-response relationship between risk factors and incidence of COVID-19.
In this retrospective, multicenter cohort study, we included patients with confirmed COVID-19 infection who had been discharged or had died by February 6, 2020. We used multivariable logistic regression and Cox proportional hazard models to determine the dose-response relationship between risk factors and incidence of COVID-19.
It clarified that increasing risk of in-hospital death were associated with older age (HR: 1.04, 95%CI: 1.01-1.09), higher lactate dehydrogenase [HR: 1.04, 95% confidence interval (CI): 1.01-1.10], C-reactive protein (HR: 1.10, 95%CI: 1.01-1.23), and procalcitonin (natural log-transformed HR: 1.88, 95%CI: 1.22-2.88), and D-dimer greater than 1 μg/mL at admission (natural log transformed HR: 1.63, 95%CI: 1.03-2.58) by multivariable regression. D-dimer and procalcitonin were logarithmically correlated with COVID-19 mortality risk, while there was a linear dose-response correlation between age, lactate dehydrogenase, D-dimer and procalcitonin, independent of established risk factors.
Higher lactate dehydrogenase, D-dimer, and procalcitonin levels were independently associated with a dose-response increased risk of COVID-19 mortality.
Core Tip: This study showed that older age, higher lactate dehydrogenase and creatinine, and elevated procalcitonin and D-dimer at admission were risk factors for the mortality from coronavirus disease 2019 (COVID-19). These findings suggested that higher lactate dehydrogenase, D-dimer and procalcitonin levels were independently associated with a dose-response increased risk of COVID-19 incidence.
- Citation: Zhao SC, Yu XQ, Lai XF, Duan R, Guo DL, Zhu Q. Dose-response relationship between risk factors and incidence of COVID-19 in 325 hospitalized patients: A multicenter retrospective cohort study. World J Clin Cases 2022; 10(10): 3047-3059
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3047.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3047
In December 2019, many cases of unknown viral pneumonia were reported[1]. A novel coronavirus, capable of infecting humans, was detected in January 2020[2,3] and the disease caused was termed coronavirus disease 2019 (COVID-19) by WHO[2]. As of March 19, 2020 > 200000 Laboratory-confirmed cases had been documented globally[1,4-7]. With the increasing awareness of COVID-19 pneumonia, a variety of diagnostic protocols and guidelines have evolved to guide clinical practice[1,5,8-10].
Many patients in some case series which had been published, were hospitalized at the time of reporting. Studies in patients who were not discharged may have misclassified outcomes due to patients developing severe disease or dying during subsequent hospitalization. Consequently, It might be inaccurate and unreliable to estimate of risk factors for severe illness and death in these early case series. Furthermore, although several large studies have reported risk factors for mortality and severe disease in COVID-19 patients, studies that systematically explored the potential associations were limited. Thus, the association of risk factors with COVID-19 outcomes remained unknown.
Therefore, we detail all laboratory-confirmed COVID-19 patients admitted to two designated hospitals as of February 2020, along with clear clinical outcomes (death or discharge). The purpose of this study was to investigate risk factors for death in hospital and to clarify hospitalization characteristics of COVID-19 patients.
This retrospective cohort study included two cohorts of adult inpatients (≥ 20 years old) from two designated hospitals. All patients who were diagnosed with COVID-19 according to the WHO interim guideline were screened[2], and those who died or were discharged by February 6, 2020 were included in the study. We extracted demographic, clinical, laboratory, treatment, and outcome data from the hospital electronic medical records using a standardized data collection form modified from the version of the WHO/International Severe Acute Respiratory and Emerging Infection Consortium. The primary endpoint was in-hospital death occurring beyond 24 h but within 28 d and composite severe cases referred to the admission to intensive care unit, intubation, or death during hospitalization. Patients, who had normalized temperature for over 3 days, relief of clinical symptoms, substantial improvement in the imaging of both lungs and throat-swab samples negative twice for at least 24h apart, were allowed to be discharged. All data were collected by two physicians, double-checked independently, and verified by a third researcher. The computed tomography (CT) demonstrations were described according to the internationally standard nomenclature defined by the Fleischner Society and peer-reviewed literature on viral pneumonia, using the terms including ground glass opacity (GGO), crazy-paving pattern, and consolidation[11,12]. A semi-quantitative scoring system was used to quantitatively estimate the pulmonary involvement of all these abnormalities on the basis of the area involved[13].
The total CT score was the sum of the individual lobar scores and ranged from 0 (no involvement) to 25 (maximum involvement). The distribution of lung abnormalities was recorded as predominantly subpleural (involving mainly the peripheral one-third of the lungs), random (without predilection for subpleural or central regions), or diffuse (continuous involvement without respect to lung segments)[14].
This study was approved by the Ethics of Committees of Zhongnan Hospital of Wuhan University, and in accordance with the Helsinki Declaration. Written informed consent was obtained from all patients before examination. The anonymous data was collected and analyzed to optimize clinical decision and treatment.
Continuous and categorical variables were presented as median and n (%), respectively. We used the Mann-Whitney U test, χ² test, or Fisher’s exact test to compare differences between survivors and non-survivors where appropriate. To explore the risk factors associated with in-hospital death, multivariable logistic regression models and the Cox proportional hazards model was used to determine the independent factors, which were based on the variables selected by a univariate analysis. To generate the Receiver operating characteristic (ROC) curves, patients were classified as survivor or non-survivors and CT total score of different stages excluded patients who were lost to follow-up.
We compared patients’ characteristics between the two hospitals and used a generalized linear model to adjust for possible differences in patients’ characteristics and treatment between the two study centers. Statistical tests and P values were two-sided. Differences were considered significant with a value of P < 0.05. All statistical analyses were carried out using the SAS software (version 9.4), unless otherwise indicated. We assessed potential dose-response associations of incident COVID-19 mortality and severe cases risk by restricted cubic splines logistic and Cox regression using 3 knots at 25th, 50th, and 75th percentiles of the corresponding risk factors with the median value of the above risk factors as the reference group[15].
The basic characteristics of the patients are shown in Table 1. Medical workers accounted for 5.8% (19/325), and those who had a history of contact with wildlife accounted for 1.2%. The median incubation period was 6 d (interquartile range, 2-15 d). The median age was 45 years (interquartile range, 34-61 years). Female accounted for 57.8%. 77.5% of patients had fever on admission and 85.5% had fever during hospitalization. The second most common symptoms were cough (63.7%) and fatigue (48.0%), but nausea or vomiting (7.7%) and difficulty breathing (4.6%) were uncommon. In the total population, 21.2 % have at least one co-existing condition (e.g., hypertension and diabetes).
| Characteristic | All patients (n = 325) | Survivornon-survivor (n = 308); (n = 17) | P value | |
| Age | < 0.0011 | |||
| Median (IQR)-yr | 45.0 (34.0-61.0) | 43.0 (33.0-61.0) | 63.0 (57.0-76.0) | |
| Distribution-no./total no. (%) | ||||
| 20-49 yr | 178 | 178 (57.8) | 0 (0.0) | |
| 50-64 yr | 91 | 80 (25.9) | 11 (64.7) | |
| ≥ 65 yr | 56 | 50 (16.2) | 6 (35.3) | |
| Male sex - no./total no. (%) | 137 (42.2) | 124 (40.3) | 13 (76.5) | 0.0031 |
| Smoking history - no./total no. (%) | 21 (6.5) | 18 (5.8) | 3 (17.7) | 0.054 |
| Exposure to source of transmission within past 14 days - no./total no. | 0.0351 | |||
| Yes | 233 (71.7) | 222 (4.9) | 11 (66.8) | |
| No | 92 (28.3) | 86 (0.3) | 6 (28.0) | |
| Median incubation period (IQR) - days | 5.0 ± 4.0 | 5.0 ± 3.9 | 5.2 ± 3.5 | 0.862 |
| Fever on admission | ||||
| Patients - no./total no. (%) | 252 (77.5) | 240 (77.9) | 12 (70.6) | 0.550 |
| Median temperature (IQR) - °C | ||||
| Distribution of temperature - no./total no. (%) | 0.603 | |||
| < 37.3 °C | 77 (23.7) | 72 (22.2) | 5 (1.5) | |
| 37.3-38.0 °C | 106 (32.6) | 103 (31.7) | 3 (0.9) | |
| 38.1-39.0 °C | 124 (38.2) | 116 (35.7) | 8 (2.5) | |
| > 39.0°C | 18 (5.5) | 17 (5.2) | 1 (0.3) | |
| Symptoms - no. (%) | ||||
| Conjunctival congestion | 1 (0.31) | 1 (0.31) | 0 (0.0) | 1.000 |
| Headache | 52 (16) | 51 (16.6) | 1 (5.9) | 0.243 |
| Cough | 207 (63.7) | 199 (64.6) | 8 (47.1) | 0.143 |
| Sputum production | 81 (24.9) | 76 (24.6) | 5 (29.4) | 0.660 |
| Fatigue | 156 (48) | 145 (47.1) | 11 (64.7) | 0.157 |
| Difficulty breathing | 15 (4.6) | 13 (4.2) | 2 (11.8) | 0.149 |
| Shortness of breath | 73 (22.5) | 68 (22.1) | 5 (29.4) | 0.0121 |
| Nausea or vomiting | 25 (7.7) | 21 (6.8) | 4 (23.5) | 0.0121 |
| Diarrhea | 28 (8.6) | 27 (8.8) | 1 (5.9) | 0.680 |
| Myalgia or arthralgia | 92 (28.3) | 88 (28.5) | 4 (23.5) | 0.630 |
| Chills | 55 (16.9) | 54 (17.5) | 1 (5.88) | 0.212 |
| Coexisting disorder - no. (%) | ||||
| Fatty liver | 15 (4.6) | 15 (4.9) | 0 (0) | 1.000 |
| Chronic obstructive pulmonary disease | 17 (5.2) | 16 (5.2) | 1 (5.9) | 0.608 |
| Diabetes | 34 (10.5) | 27 (8.77) | 7 (41.2) | < 0.0011 |
| Hypertension | 69 (21.2) | 58 (18.8) | 11 (64.7) | < 0.0011 |
| Coronary heart disease | 9 (2.8) | 6 (1.9) | 3 (17.7) | < 0.0011 |
| Cerebrovascular disease | 18 (5.5) | 14 (4.6) | 4 (23.5) | 0.0101 |
| Hyperlipidemia | 17 (5.2) | 16 (5.2) | 1 (5.8) | 0.901 |
| Hepatitis B infection | 6 (1.9) | 5 (1.6) | 1 (5.9) | 0.205 |
At admission, the severity of COVID-19 was classified as not severe 265 cases and severe 60 cases. Patients with severe disease had a median age of 16 years older than those without severe disease, and any comorbidities were more common (66.7% vs26.4%), but exposure histories were similar.
On admission, lymphocytopenia, thrombocytopenia and leukopenia were present in 61.8%, 19.4% and 28.6%, respectively. Most patients (69.5%) had increased C-reactive protein (CRP) levels. Laboratory abnormalities, including lymphocytopenia and leukopenia, were more pronounced in critically ill patients than in non-critically ill patients (Table 2).
| Variable | All patients (N = 325) | Survivor non-survivor (n = 308); (n = 17) | P value | |
| Laboratory findings | ||||
| White-cell count (109/L) | 4.6 (3.3-6.0) | 4.6 (3.29-5.9) | 6.4 (3.6-7.4) | 0.090 |
| Red-cell count (1012/L) | 4.3 (4.1-4.7) | 4.3 (4.1-4.7) | 4.2 (4.0-4.6) | 0.557 |
| Hemoglobin (g/L) | 131.0 (120.0-142.0) | 131.0 (121.0-142.5) | 130.0 (114.0-141.0) | 0.360 |
| Platelet count (109/L) | 171.0 (134.0-202.0) | 173.0 (136.0-204.5) | 143.0 (119.0-155.0) | 0.0081 |
| Hematocrit (%) | 39.4 (36.5-42.6) | 39.4 (36.6-42.6) | 40.0 (34.6-42.6) | 0.530 |
| Neutrophil percentage (%) | 64.6 (56.8-75.5) | 64.5 (56.4-75.2) | 73.4 (67.3-81.8) | 0.0071 |
| Lymphocyte percentage (%) | 26.5 ± 14.5 | 26.6 ± 12.3 | 18.6 (11.2-22.5) | 0.0081 |
| Monocyte percentage (%) | 7.9 ± 3.5 | 8.1 ± 3.5 | 6.2 (3.4-6.9) | 0.0081 |
| Eosinophil percentage (%) | 0.1 (0.0-0.6) | 0.1 (0.0-0.55) | 0.0 (0.0-0.8) | 0.953 |
| Basophil percentage (%) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) | 0.2 (0.1-0.3) | 0.946 |
| Mean red blood cell volume (fL) | 90.6 (87.5-93.6) | 90.6 (87.6-93.6) | 88.9 (85.7-93.1) | 0.432 |
| Mean hemoglobin content (pg) | 30.0 (28.8-31.1) | 30.0 (28.9-31.1) | 29.4 (27.5-30.5) | 0.209 |
| Mean hemoglobin concentration (g/L) | 330.0 (323.0-336.0) | 330.0 (323.0-336.0) | 324.0 (321.0-331.0) | 0.0291 |
| RBC distribution width standard deviation (%) | 39.4 (36.7-41.2) | 39.2 (36.5-41.2) | 40.7 (37.5-42.8) | 0.071 |
| RBC distribution width-coefficient of variation (%) | 12.7 (12.2-14.4) | 12.7 (12.1-14.1) | 13.3 (12.6-15.4) | 0.116 |
| Neutrophil count (109/L) | 2.96 (1.92-4.05) | 2.9 (1.9-4.0) | 4.1 (2.7-4.9) | 0.0351 |
| Lymphocyte count (109/L) | 1.13 ± 0.55 | 1.14 ± 0.55 | 0.89 ± 0.58 | 0.0351 |
| Monocyte count (109/L) | 0.34 (0.24-0.46) | 0.3 (0.3-0.5) | 0.3 (0.2-0.5) | 0.828 |
| Eosinophil count (109/L) | 0.01 (0.0-0.02) | 0.01 (0.0-0.02) | 0.0 (0.0-0.06) | 0.642 |
| Basophil count (109/L) | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) | 0.060 |
| Platelet distribution width (%) | 12.5 (10.6-16.2) | 12.5 (10.6-16.2) | 15.1 (10.9-16.4) | 0.452 |
| Large platelet ratio (%) | 11.1 (9.8-21.2) | 11.1 (9.8-21.4) | 10.0 (10.0-12.9) | 0.405 |
| Mean platelet volume (fL) | 19.0 (10.0-28.7) | 18.5 (9.9-27.9) | 28.9 (18.8-32.4) | 0.0181 |
| Platelet hematocrit (%) | 0.17 (0.14-0.20) | 0.17 (0.14-0.20) | 0.13 (0.13-0.16) | 0.0161 |
| Distribution of other findings-no./total no. (%) | ||||
| Systolic blood pressure (mmHg) | 123.6 ± 13.6 | 123.0 ± 12.7 | 135.4 ± 21.0 | 0.0221 |
| Diastolic blood pressure (mmHg) | 76.4 ± 9.5 | 76.4 ± 9.3 | 76.2 ± 13.1 | 0.464 |
| Blood glucose concentration (mmol/L) | 6.4 ± 2.6 | 6.2 ± 2.3 | 9.1 ± 4.8 | 0.0091 |
| Total cholesterol (mmol/L) | 3.8 (3.2-4.5) | 3.9 (3.3-4.5) | 2.7 (2.6-3.3) | 0.0031 |
| Triglyceride (mmol/L) | 1.1 (0.8-1.4) | 1.1 (0.8-1.4) | 0.9 (0.8-1.0) | 0.455 |
| High density lipoprotein (mmol/L) | 1.1 (0.9-1.2) | 1.1 (0.9-1.3) | 0.97 (0.94-1.12) | 0.354 |
| Low density lipoprotein (mmol/L) | 2.2 ± 0.7 | 2.2 ± 0.7 | 1.5 ± 0.6 | 0.0021 |
| C-reactive protein (mg/dL) | 1.3 (0.3-3.4) | 1.3 (0.3-3.0) | 5.9 (3.3-8.2) | < 0.0011 |
| Lactate dehydrogenase (U/L) | 178.5 (137.5-236.5) | 173.0 (136.0-229.0) | 275.0 (232.0-324.0) | < 0.0011 |
| Aspartate aminotransferase (U/L) | 22.2 (17.1-32.8) | 21.7 (16.8-32.3) | 31.2 (25.5-36.5) | 0.0191 |
| Alanine aminotransferase (U/L) | 19.1 (12.8-32.6) | 18.9 (12.7-33.2) | 19.9 (15.5-29.7) | 0.957 |
| γ–Glutamyltransferase (U/L) | 19.0 (12.6-38.2) | 19.0 (12.4-38.0) | 27.8 (16.9-69.0) | 0.064 |
| Blood urea nitrogen (mmol/L) | 4.1 (3.2-5.3) | 4.0 (3.2-5.0) | 6.4 (5.3-11.1) | < 0.0011 |
| Creatine kinase (ng/mL) | 76.5 (45.0-140.0) | 77.1 (45.0-138.0) | 74.0 (61.0-203.0) | 0.404 |
| Creatinine (μmol/L) | 63.9 (53.6-76.7) | 63.0 (53.1-74.7) | 83.7 (74.9-254.2) | < 0.0011 |
| α-Hydroxybutyrate dehydrogenase (U/L) | 137.5 (109.0-176.5) | 135.0 (108.0-171.0) | 208.0 (158.0-217.0) | 0.0011 |
| D-dimer (μg/mL) | 0.4 (0.2-0.8) | 0.4 (0.2-0.8) | 1.1 (0.6-6.3) | < 0.0011 |
| Procalcitonin (ng/mL) | 0.05 (0.04-0.09) | 0.05 (0.03-0.08) | 0.3 (0.1-2.8) | < 0.0011 |
| Brain Natriuretic peptide (pg/mL) | 34.4 (13.0-128.0) | 31.6 (12.0-108.0) | 295.8 (177.0-406.1) | < 0.0011 |
| Antihypertensive drugs | < 0.0011 | |||
| Yes | 57 (17.5) | 47 (14.5) | 10 (3.0) | |
| No | 268 (82.5) | 261 (80.3) | 7 (2.2) | |
| Hypoglycemic drugs | < 0.0011 | |||
| Yes | 28 (8.6) | 22 (6.8) | 6 (1.8) | |
| No | 297 (91.4) | 286 (88) | 11 (3.4) | |
| Lipid-lowering drugs | 0.0051 | |||
| Yes | 14 (4.3) | 11 (3.4) | 3 (0.9) | |
| No | 311 (95.7) | 297 (91.4) | 14 (4.3) | |
All patients underwent computed tomography scans at the time of admission, and 97.8% revealed abnormal results. The most common patterns on chest CT were GGO (61.1%) and bilateral patchy shadowing (84.7%). No CT abnormality was found in seven of 308 (2.2%) patients who survived and in none of 17 patients who died. GGO, crazy-paving pattern and consolidation were the most frequent CT findings in mild COVID-19 pneumonia (Supplementary Figure 1). Most patients (279/325), the total CT score increasedabout10 d after the onset of symptoms, and then gradually decreased (Table 3, Supple
| Stage-1 (n = 157) | Stage-2 (n = 194) | Stage-3 (n = 165) | Stage-4 (n = 211) | Stage-5 (n = 204) | Stage-6 (n = 137) | P value | |
| Total CT score of the pulmonary involvement | 2 ± 4 (0-18) | 5 ± 5 (0-22) | 7 ± 7 (0-22) | 7 ± 7 (0-25) | 5 ± 7 (0-24) | 4 ± 6 (0-25) | < 0.00011 |
| Number of involved lobes | 22 ± 2 (0-5) | 3 ± 2 (1-5) | 4 ± 2 (1-5) | 3 ± 2 (1-5) | 3 ± 2 (1-5) | 4 ± 2 (1-5) | < 0.00011 |
| CT score in each lobe | < 0.00011 | ||||||
| Left upper lobe | 0 ± 1 (0-3) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-4) | 1 ± 1 (0-5) | |
| Left lower lobe | 1 ± 1 (0-5) | 1 ± 2 (0-5) | 2 ± 2 (0-5) | 2 ± 1 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | |
| Right upper lobe | 0 ± 1 (0-3) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | |
| Right middle lobe | 0 ± 1 (0-3) | 1 ± 1 (0-5) | 1 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 1 (0-5) | 0 ± 1 (0-5) | |
| Right lower lobe | 1 ± 2 (0-12) | 2 ± 1 (0-5) | 2 ± 2 (0-5) | 1 ± 2 (0-5) | 1 ± 1 (0-5) | 1 ± 1 (0-5) |
| Stage-1 (n = 157) | Stage-2 (n = 194) | Stage-3 (n = 165) | Stage-4 (n = 211) | Stage-5 (n = 204) | Stage-6 (n = 137) | |
| Distribution of pulmonary lesions | ||||||
| No lesion | 12/157 | 1/194 | 0/165 | 1/211 | 2/204 | 0/137 |
| Peripheral | 60/157 | 18/194 | 55/165 | 105/211 | 88/204 | 66/137 |
| Random | 85/157 | 162/194 | 88/165 | 75/211 | 78/204 | 44/137 |
| Diffuse | 0/157 | 13/194 | 22/165 | 30/211 | 36/204 | 27/137 |
| Involvement of the lesions | ||||||
| No involvement | 12/157 | 0/194 | 0/165 | 0/211 | 0/204 | 0/137 |
| Single lobe | 48/157 | 18/194 | 11/165 | 30/211 | 22/204 | 11/137 |
| Bilateral multilobe | ||||||
| GGO | 96/157 | 180/194 | 154/165 | 180/211 | 176/204 | 121/137 |
| None | 24/157 | 0/194 | 22/165 | 30/211 | 47/204 | 49/137 |
| Yes | 133/157 | 194/194 | 143/165 | 181/211 | 157/204 | 88/137 |
| Crazy-paving pattern | ||||||
| None | 120/157 | 104/194 | 110/165 | 180/211 | 183/204 | 126/137 |
| Yes | 36/157 | 90/194 | 55/165 | 31/211 | 121/204 | 11/137 |
| Consolidation | ||||||
| None | 157/157 | 140/194 | 88/165 | 105/211 | 102/204 | 89/137 |
| Yes | 0/157 | 54/194 | 77/165 | 105/211 | 102/204 | 48/137 |
| Fibrosis | ||||||
| None | 157/157 | 180/194 | 143/165 | 150/211 | 102/204 | 37/137 |
| Yes | 0/157 | 14/194 | 22/165 | 61/211 | 102/204 | 100/137 |
After univariate analysis, patients with diabetes or hypertension had a higher chance of death in hospital (Tables 1 and 2). Age, sex, leukocytosis, and elevated glucose level, lactate dehydrogenase (LDH), high-sensitivity C-reactive protein (CRP), D-dimer (DD), total cholesterol, triglyceride, creatinine, and procalcitonin (PCT) were associated with death or severe illness.
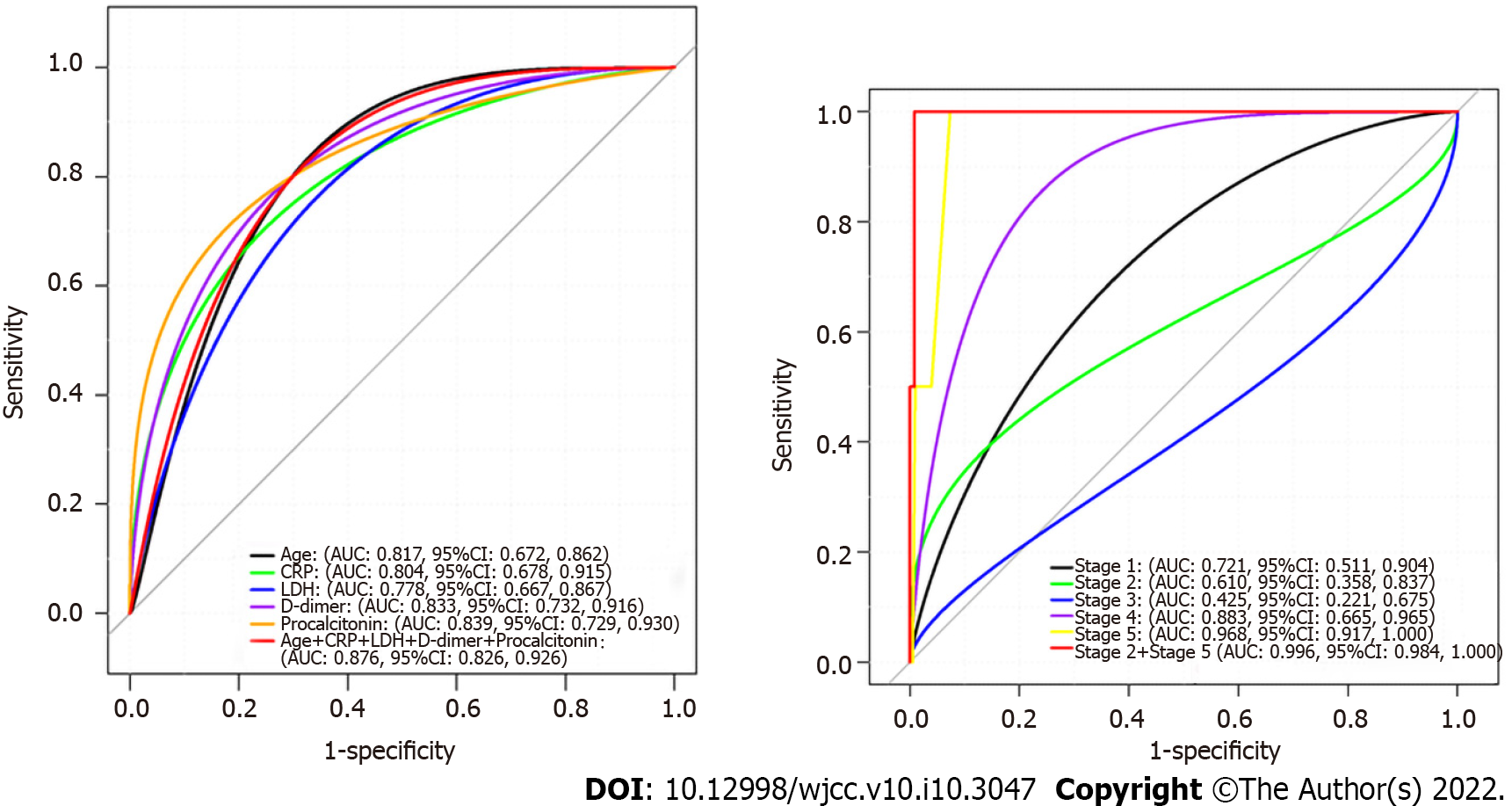
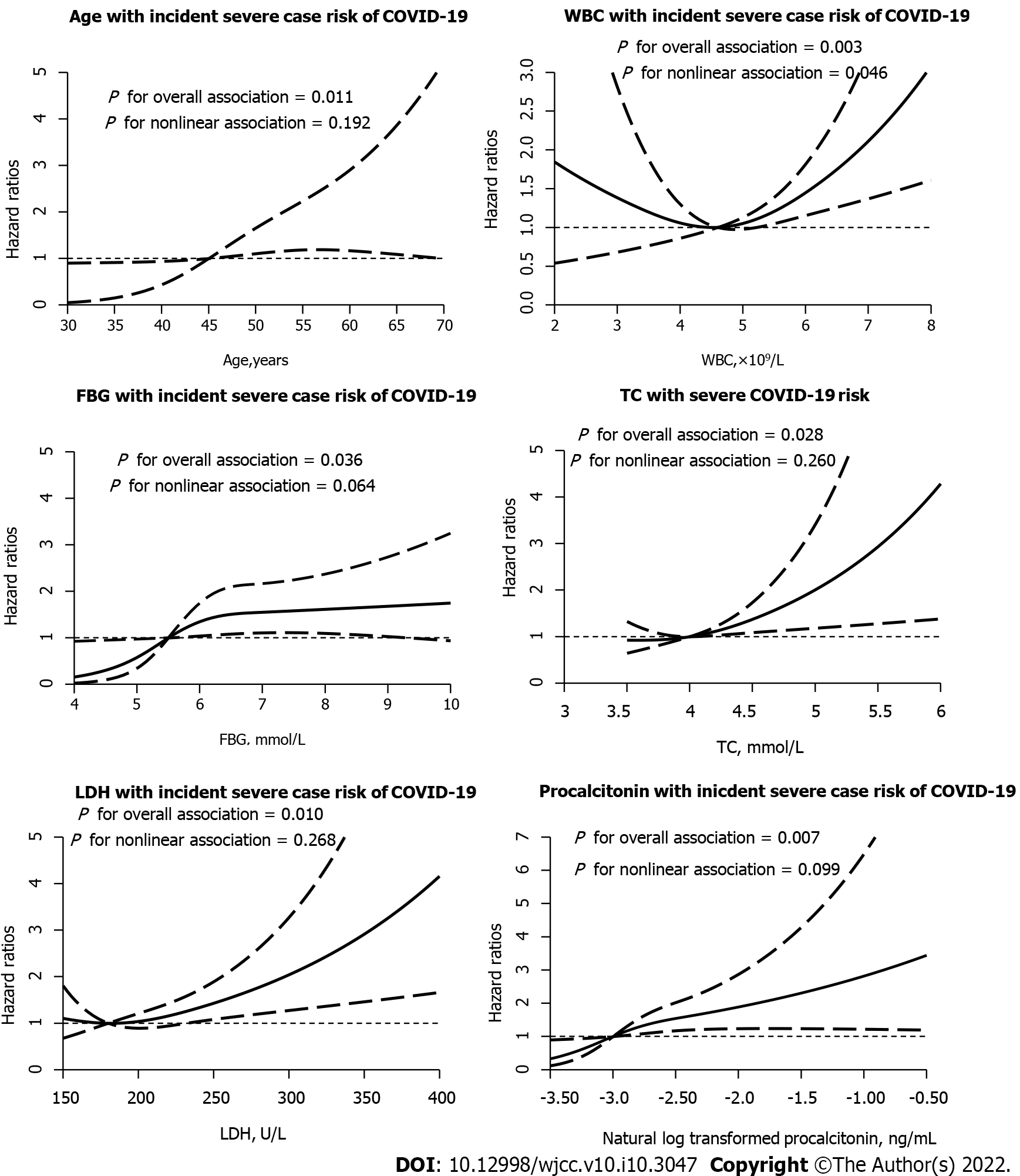
Older age [hazard ratio (HR):1.04, 95% confidence interval (CI): 1.01-1.09], higher LDH (HR: 1.04, 95% 95%CI:1.01-1.10), higher CRP (HR:1.10, 95%CI: 1.01-1.23), and elevated PCT (logn transformed HR: 1.88, 95%CI: 1.22-2.88), and DD > 1 μg/mL at admission (logn transformed HR: 1.63, 95%CI: 1.03-2.58) were associated with increasing odds of in-hospital death (Table 5). Furthermore, DD and PCT were log-linearly correlated with COVID-19 mortality risk, while there were linear dose-response correlations between age, LDH, DD and PCT. In particular, It was evident that the dose-response association of LDH and PCT occurred in severe patients (all P for overall association < 0.05). The dose-response relationship between LDH and PCT was more obvious in severe patients in the meantime (all P for interaction < 0.05) (Figure 2, Tables 5 and 6).
| Variable | HR (95%CI) | P for overall association | P for nonlinear association | |
| Model 1 | Model 2 | |||
| Age (per year increase) | 1.06 (1.03, 1.10) | 1.04 (1.01, 1.09) | 0.080 | 0.805 |
| CRP (per 1 mg/L increase) | 1.15 (1.06, 1.24) | 1.10 (1.01, 1.23) | 0.062 | 0.715 |
| DD (per 1 μg/mL increase of NLT | 1.89 (1.34, 2.69) | 1.63 (1.03, 2.58) | 0.012 | 0.711 |
| LDH (per 10 U/L increase) | 1.06 (1.02, 1.09) | 1.04 (1.01, 1.10) | 0.080 | 0.805 |
| Procalcitonin (per 1 ng/mL increase of NLT) | 2.15 (1.59, 2.90) | 1.88 (1.22, 2.88) | 0.011 | 0.721 |
| Variable | OR (95%CI) | P for overall association | P for nonlinear association | |
| Model 1 | Model 2 | |||
| Age (per year increase) | 1.06 (1.04, 1.08) | 1.04 (1.01, 1.07) | 0.010 | 0.192 |
| WBC (per 1 × 109/L increase) | 1.27 (1.11, 1.46) | 1.20 (1.01, 1.45) | 0.003 | 0.046 |
| FBG (per 1 mmol/L increase) | 1.19 (1.07, 1.33) | 1.15 (1.01, 1.32) | 0.036 | 0.064 |
| Total cholesterol (per 1 mmol/L increase) | 1.43 (1.07, 1.91) | 1.65 (1.09, 2.50) | 0.028 | 0.260 |
| LDH (per 10 U/L increase) | 1.09 (1.05, 1.13) | 1.06 (1.02, 1.10) | 0.009 | 0.268 |
| Procalcitonin (per 1 ng/mL increase of NLT) | 2.26 (1.68, 3.05) | 1.75 (1.16, 2.65) | 0.007 | 0.099 |
ROC curve analysis indicated that the combined AUC for age, sex, high-sensitivity CRP, DD, LDH and PCT (0.947) was higher than that of any one of these variables alone (Figure 1). These results show that combination of age, sex, high-sensitivity CRP, DD, LDH and PCT was more precise in predicting clinical outcome than single factors alone.
Consistent with most studies[1,2,16], we found that the clinical features of COVID-19 were similar to those of SARS. Fever, cough, gastrointestinal symptoms were rare[17]. Lymphocytopenia was common, a finding that was consistent with two recent reports[1,16]. We found that the fatality rate (5.2%) was lower than recently reported[1,16]. This may be due to differences in sample size and case inclusion criteria. Our findings were higher than the national official statistics, which showed a mortality rate of 3.9% among 81003 cases of COVID-19 as of March 13, 2020.
In this study, patients underwent multiple lung CT scans (≥ 3 times), providing reliable dynamic radiographic pattern data. During the first 2 wk, the number and severity of abnormal lesions on chest CT increased. Subsequently, there was a short plateau phase and a gradual decrease in abnormalities. There were six stages of lung involvement in patients who have recovered from COVID-19, which could be more accurately evaluate the time course of lung changes, compared with the previous 4 stages[18]. Combined profiling of stages 2 and 5provides a more precise clinical outcome prediction than conventional stages 1-4 classification[18], suggesting a novel valuable prognostic indicator for COVID-19 patients after antiviral therapy.
Our retrospective cohort study demonstrated several risk factors for death in patients who were hospitalized with COVID-19. Particularly, older age, LDH > 285 U/L, creatinine > 111 ng/mL, PCT > 0.05 ng/mL, and DD > 1 μg/mL on admission were associated with higher odds of in-hospital death. Previously, older age, DD > 1 μg/mL and sequential organ failure assessment (SOFA) score (including creatinine level) have been reported as important independent predictors of mortality in COVID-19[4], which is in accordance with our current study. The most plausible explanation included an age-dependent defect in T-cell and B-cell function and excess type 2 cytokines, which predispose to ischemia and thrombosis, potentially leading to poor outcome[4,19-22]. SOFA score is a good diagnostic marker for renal function, and reflects the state and degree of multiorgan dysfunction[20,23]. In the current study, higher PCT and LDH levels were independently associated with prognosis of COVID-19. Additionally, we found that most patients had lower white blood cell count, and no bacterial pathogens were detected. Viral infections is one of the cause of sepsis syndrome, despite that bacterial infections are used to be the primary cause of sepsis, PCT, as an inflammatory indicator, could better stratify the degree of infection. The level of LDH is important in assessing the risk of cardiac and liver dysfunction, which has great significance for both patient isolation decision-making and guidance around the length of antiviral treatment. Effective antiviral therapy may improve the outcome of COVID-19 in spite of that we did not observe a reduction in viral shedding time after antiviral therapy in the current study. However, further research is needed to investigate the pathogenesis of sepsis in COVID-19.
We showed that CT stage is a powerful indicator in the evaluation of COVID-19 prognosis. We characterized specific factors-prognostic factors model (PFM), age, high-sensitivity CRP, DD, LDH and PCT as a valuable independent prognostic tool of COVID-19 from CT stage. Predictive value of PFM was comparable to that of CT stage. Thus, these results consistently point to the notion that high PFM and CT stage are pivotal factors in evaluating COVID-19, but further research is needed to investigate the prognostic value.
However, no published works were found about the dose-response relationship between mortality and severe illness in adult patients with COVID-19. In recent studies, the relationship of prognostic factors with risk of COVID-19 incidence has not been reported. Of note, we found that higher LDH, DD and PCT levels were independently associated with a dose-response increased mortality risk in patients with COVID-19. Notably, the dose-response relationship between LDH and PCT levels and incidence of COVID-19 was seen in survivors and patients with severe illness. To our knowledge, this is the first study to demonstrate that the higher risk of COVID-19 incidence associated with LDH and PCT levels provides evidence of the dose-response relationship. Several potential mechanisms might explain the association between LDH, DD and PCT levels and COVID-19[4,19,20,22,23]. Although the underlying pathophysiological mechanisms are unclear, it is possible that the presence of COVID-19 risk factors could cover up the effect of LDH, DD and PCT on the risk of COVID-19 among high-risk persons and leave the pernicious effects prominent in relatively healthy adults. Further studies should be performed, which is the key for the development of specific inhibitors targeting COVID-19.
There are some limitations to our study. First, contact histories and laboratory testing records for some cases were incomplete. Second, we could only estimate the incubation period in patients who have recorded information. Uncertainty about the exact date (recall bias) might have inevitably influenced our assessment. Third, since our study did not include patients with mild illness who did not seek medical attention, the case fatality rate would likely have been lower in real-world situations. Meanwhile, during the beginning of the pandemic, we a little about COVID-19, so the treatment regimens have been improving. Also, due to limited medical resources, older patients and patients with serious symptoms may have been preferentially admitted, and this may have resulted in bias. Fourth, data generation was clinically driven and not systematic. Lastly, this was a retrospective study.
To our knowledge, this is the largest retrospective cohort study of COVID-19 patients who have experienced clear results and systematically explored almost all potential risk factors associated with mortality and severe illness. Six stages of lung involvement could be more accurately defined to evaluate the prognosis of COVID-19. The combination of PFM and six stages could provide the rationale for testing novel coronavirus management to improve outcomes. We found that older age, higher LDH and creatinine, and elevated PCT and DD at admission were risk factors for death of patients with COVID-19. These findings suggested that higher LDH, DD and PCT levels were independently associated with increased risk of COVID-19 incidence.
Higher LDH, DD and PCT levels were independently associated with a dose-response increased risk of COVID-19 mortality.
Dose-response assessments and risk factors for mortality, severe cases and clinical outcomes for coronavirus disease 2019 (COVID-19) have not been well described.
To screen for dose-response relationships between risk factors and incidence of COVID-19.
To explore risk factors of in-hospital death and describe the clinical course of symptoms, viral shedding, and temporal changes of laboratory findings during hospitalization.
This retrospective cohort study included two cohorts of adult inpatients from two designated hospitals. Multivariate logistic regression and Cox proportional risk models were used to determine the dose-response relationship between risk factors and the incidence of COVID-19.
D-dimer and procalcitonin were log-linear correlated with the risk of death from COVID-19, while there was a linear dose-response relationship between age, LDH, D-dimer and procalcitonin, independent of identified risk factors.
High lactate dehydrogenase, D-dimer and procalcitonin levels were independently associated with an increased dose-response risk of death from COVID-19.
This study provides ideas and basis for prospective observation of dose-response relationships between risk factors and incidence of COVID-19.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious Diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Balaban DV, Romania; Kim KH, South Korea S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30116] [Article Influence: 6023.2] [Reference Citation Analysis (3)] |
| 2. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9319] [Article Influence: 1863.8] [Reference Citation Analysis (0)] |
| 3. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17646] [Article Influence: 3529.2] [Reference Citation Analysis (0)] |
| 4. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18199] [Article Influence: 3639.8] [Reference Citation Analysis (0)] |
| 5. | Lv Z, Cheng S, Le J, Huang J, Feng L, Zhang B, Li Y. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020;22:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19:100682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 7. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 8. | Lau H, Khosrawipour V, Kocbach P, Mikolajczyk A, Schubert J, Bania J, Khosrawipour T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med. 2020;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 623] [Article Influence: 124.6] [Reference Citation Analysis (0)] |
| 9. | Wang J, Qi H, Bao L, Li F, Shi Y; National Clinical Research Center for Child Health and Disorders and Pediatric Committee of Medical Association of Chinese People's Liberation Army. A contingency plan for the management of the 2019 novel coronavirus outbreak in neonatal intensive care units. Lancet Child Adolesc Health. 2020;4:258-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8:e11-e12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 11. | Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2674] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 13. | Thomas M, Price OJ, Hull JH. Pulmonary function and COVID-19. Curr Opin Physiol. 2021;21:29-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 14. | Wu X, Dong D, Ma D. Thin-Section Computed Tomography Manifestations During Convalescence and Long-Term Follow-Up of Patients with Severe Acute Respiratory Syndrome (SARS). Med Sci Monit. 2016;22:2793-2799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 15. | Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 876] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 16. | Guo CX, He L, Yin JY, Meng XG, Tan W, Yang GP, Bo T, Liu JP, Lin XJ, Chen X. Epidemiological and clinical features of pediatric COVID-19. BMC Med. 2020;18:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Aishwarya M, Singh M, Panda PK. Primary to tertiary COVID-19 transmission in a hospital - A cluster outbreak analysis. J Family Med Prim Care. 2021;10:1489-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Duzgun SA, Durhan G, Demirkazik FB, Akpinar MG, Ariyurek OM. COVID-19 pneumonia: the great radiological mimicker. Insights Imaging. 2020;11:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41 Suppl 7:S504-S512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 302] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 20. | Armstrong BA, Betzold RD, May AK. Sepsis and Septic Shock Strategies. Surg Clin North Am. 2017;97:1339-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Fountoulaki K, Tsiodras S, Polyzogopoulou E, Olympios C, Parissis J. Beneficial Effects of Vaccination on Cardiovascular Events: Myocardial Infarction, Stroke, Heart Failure. Cardiology. 2018;141:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 23. | Wang H, Kang X, Shi Y, Bai ZH, Lv JH, Sun JL, Pei HH. SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy. Ren Fail. 2020;42:638-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |