Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3014
Peer-review started: September 13, 2021
First decision: November 22, 2021
Revised: December 24, 2021
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: April 6, 2022
Processing time: 197 Days and 0.5 Hours
Dry eye syndrome (DES) is a common disease with various clinical manifestations. DES had a significant association with diabetes. Blink reflex (BR) is also known as trigeminal nerve facial reflex. The stimulation of corneal nerves is one of the origins of BR stimulation. The parasympathetic fibers sent out through the facial nerve are the outlet of tear reflexes. BR can be used to assess the function of the corneal nerve closed-loop; however, whether the BR changes in these patients is unclear.
To understand the morphology and function of the corneal nerve in patients with dry eyes having diabetes or not.
This study enrolled 131 patients who visited the inpatient and outpatient services of ophthalmology and endocrinology departments between January 2019 to August 2020 with subjective symptoms of dry eyes and non-dry eye reasons, as well as volunteers such as colleagues. The patients were divided into four groups: DEwDM, with dry eyes having type 2 diabetes mellitus (T2DM); DMnDE, with T2DM not having dry eyes; DEnDM, with dry eyes not having diabetes; and nDMnDE, with neither dry eyes nor diabetes. The tear film break-up time, Schirmer I test, in vivo confocal microscopy, and BR were performed.
The DEwDM, DMnDE, DEnDM, and nDMnDE groups included 56, 22, 33, and 20 patients, respectively. Sex and age were not statistically different among the four groups. The nerve fiber length (NFL) of patients in the DEwDM, DEnDM, and DMnDE groups reduced (P < 0.001, P = 0.014, and P = 0.001, respectively). No significant difference in corneal nerve fiber density (NFD) (P = 0.083) and corneal nerve branch density (NBD) (P = 0.195) was found among the four groups. The R1 Latency of blink reflexes increased only in the DEwDM group (P = 0.008, P = 0.001, P < 0.001, compared with the DMnDE, DEnDM, and nDMnDE groups, respectively). The NBD and R1 Latency were different between DEwDM and DEnDM groups in patients with moderate and severe dry eyes.
The corneal nerve morphology changed in patients with dry eyes or diabetes, or with both, while the function of corneal nerve closed-loop reduced only in those with dry eyes and diabetes.
Core Tip: This study aimed to understand more about patients with dry eyes by exploring the corneal nerve morphology and function. A total of 131 patients were enrolled and divided into four groups depending on their types accordingly. Then, several tests were employed, including tear film break-up time, Schirmer I, in vivo confocal microscopy, and blink reflex tests. The tests results revealed that the nerve fiber length in three of four groups was reduced, R1 Latency of blink reflexes increased only in one group. Besides, the nerve branch density and R1 Latency differed between the two groups.
- Citation: Fang W, Lin ZX, Yang HQ, Zhao L, Liu DC, Pan ZQ. Changes in corneal nerve morphology and function in patients with dry eyes having type 2 diabetes. World J Clin Cases 2022; 10(10): 3014-3026
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3014.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3014
Dry eye syndrome (DES) is a common disease with various clinical manifestations[1] that decreases the quality of life[2,3]. The prevalence of DES in China and other parts of the world increases with multiple complications[4-6].
The corneal nerves regulate corneal sensitivity, blink reflex, tear production, and epithelial regeneration[7-9]. The relationship between corneal sensation and subbasal nerve morphology depends on the pathophysiological mechanism of ocular surface disease[10].
DES had a significant association with higher blood glucose and higher glycosylated hemoglobin A1c (HbA1c)[11,12]. Although the exact mechanisms underlying dry eyes are unclear in diabetes, corneal nerve dysfunction may be a possible mechanism[13]. Some studies showed different reports about the morphology of corneal nerves in patients with diabetes or neuropathy[14-18]. In this study, the relationship between morphological abnormalities of corneal nerves and dry eye disease in patients with type 2 diabetes mellitus (T2DM) was observed.
Blink reflex (BR) is also known as trigeminal nerve facial reflex. Blinks are vital in the diffusion, mixing, and renewal of tears and stimulate the release of lipids from the meibomian glands. This blink nerve function can indirectly determine whether corneal sensory afferent nerve and facial parasympathetic outflow function abnormally[19,20]. The stimulation of corneal nerves is one of the origins of BR stimulation. It passes through the trigeminal nerve to the trigeminal nucleus of the brainstem. It then transmits nerve impulses to the facial nucleus. The motor fibers of the facial nerve dominate the orbicularis. The parasympathetic fibers sent out through the facial nerve are the outlet of tear reflexes[21,22]. Thus, BR can be used to assess the function of the corneal nerve closed loop, which may occur in patients with dry eyes and diabetes; however, whether the BR changes in these patients is unclear.
In this study, the corneal subbasal nerve plexus morphology was observed by in vivo confocal microscopy (IVCM)[15]. The BR was also measured, which is an examination of the nerve conduction to observe the sensory function of the trigeminal nerve and the motor function of the facial nerve. This cross-sectional study was performed to investigate whether corneal nerve fibers and corneal nerve closed loop function are impaired in patients with dry eyes having or not having T2DM.
This cross-sectional study was conducted on patients visiting the outpatient and inpatient services of the departments of ophthalmology and endocrinology in Xuanwu Hospital of Capital Medical University between January 2019 and August 2020.
Patients aged 18-70 years with subjective symptoms of dry eyes, such as dryness, burning, stinging, and discomfort, and willingness to cooperate with the examination, were included in the study. A signed informed consent form was collected from all included patients, and this study was approved by the ethics committee of the hospital.
Patients selected based on the inclusion criteria were divided into four groups: (1) DEwDM group: Patients with dry eyes having T2DM; (2) DEnDM group: Patients with dry eyes not having diabetes; (3) DMnDE group: Patients with T2DM not having dry eyes; and (4) nDMnDE group: Control patients who visited the ophthalmology department for non-dry eye reasons such as cataracts and myopia, and some patients complained of dry eyes but did not diagnose with dry eyes after examination, as well as volunteers such as colleagues.
Dry eye disease was diagnosed according to the diagnostic criteria of dry eyes in the “2013 Chinese Clinical Diagnosis and Treatment Experts Consensus of Dry Eye”[23]. These were: (1) Patients with one of the subjective symptoms such as dryness, burning, stinging, ocular fatigue, discomfort, vision fluctuation, and tear film break-up time (BUT) ≤ 5 s or Schirmer I test ≤ 5 mm/5 min. (2) patients with one of the subjective dry eye symptoms and 5 s < BUT ≤ 10 s or 5 mm/5 min < Schirmer I test ≤ 10 mm/5 min and the conjunctiva was fluorescein-stained positive. Grouping for diabetes depended on whether the patients were ever diagnosed with T2DM in the endocrinology department of the hospital[24].
The exclusion criteria were as follows: A history of wearing contact lens, laser corneal surgery or other eye surgery, eye trauma, with Sjogren's syndrome, rheumatoid arthritis (RA), Steven–Johnson syndrome, conjunctiva pemphigoid, conjunctival scarring, or other diseases that affect tear production, corneal disease, high intraocular pressure, pigment membrane inflammation, trigeminal neuropathy, facial paralysis, or other confirmed nerve-related ophthalmopathy, or type 1 diabetes mellitus (T1DM). In the non-diabetic group, diabetic retinopathy and diabetic optic neuropathy were excluded. A few patients in the diabetic group had diabetic fundus disease. However, the degree of fundus disease is not included in the data analysis.
Clinical and ophthalmic assessment: All patients answered the Ocular Surface Disease Index (OSDI) questionnaire, and ophthalmological examinations were performed in the right eye of each participant. The slit lamp was used to examine the anterior segment to exclude ocular surface inflammation, corneal disease, pigment membranitis, and other abnormalities. The BUT was assessed using a 77000 keratograph D type comprehensive corneal topography examination instrument (OCULUS, Germany). After three blinks, the system automatically detected the time of the first noninvasive tear film BUT. The corneal fluorescein staining was performed using a wet fluorescein stain paper (Tianjin Jingming New Technology Development Co., Ltd., China) and observed through a slit lamp with a cobalt blue filter. The Schirmer I test was performed in a quiet and dark environment. The Schirmer paper strip was folded and placed at the temporal one-third of the lower-lid margin. The patients were asked to look down or close their eyes gently. After 5 min, the score was measured as the length of the wetted area on the Schirmer paper strip. Fasting or postprandial venous blood glucose test was performed on patients with diabetes using a Hitachi 7600 automatic biochemical analyzer (Hitachi, Japan). HbA1c was tested using a Bio-Rad Variant II HbA1c analyzer (Bio-Rad, Montreal, Quebec, Canada). The blood glucose of fingertip was measured by dry chemical method (ACCU-CHEK Performa, Roche Diabetes Care GmbH, Germany) in patients without diabetes history. If the tests came back normal, we did not think he was diabetic.
Heidelberg Retina Tomograph III with the Rostock Cornea Module (Heidelberg Engineering GmbH, Germany) was used to perform the IVCM imaging on the central subbasal corneal nerve fibers. After local anesthesia, an image of 400 μm2 × 400 μm2 of the central subbasal nerve fibers was acquired according to operation routine by a single examiner, with scanning depth of 50 ± 5 μm. Images with constant scanning, good contrast, most nerve fibers, and without folds or motion were selected for storage. The curve measurement function in ImageJ 32 software was used to trace and measure the central subbasal corneal nerve fibers in the images[25,26]. Then, a pixel to millimeter conversion was performed using the software. The NFL (total length of corneal nerves, mm/mm2), NFD (the number of nerve fibers/mm2), and NBD (the number of branch points on the nerves/mm2) were measured by two different researchers (WF and FL)[27]. As corneal tissue of 400 μm2 × 400 μm2 could be seen in each IVCM image, the corresponding pixel in ImageJ 32 software was 384 × 384 pixels. The conversion formula was the total length of nerve fibers in each picture (μm) = the tracing result/0.96.
Nicolet EDX electromyograph (Thermo Nicolet Corporation, United States) was used to measure the BR[28]. The patient lay flat on the bed with eyes slightly opened or closed. The surface recording electrode was placed in the middle of the bilateral lower eyelid to record bilateral orbicularis signals. The reference electrode was placed outside the lateral canthus. The surface-stimulating electrodes were placed on the supraorbital incisure to stimulate the supraorbital nerve.
A 0.2-ms square-wave pulse was used to stimulate the BR. As the stimulus intensity sufficient to induce response differed among patients, the stimulus was set at 0 mA at the beginning for each patient. It was increased until the response was induced, which only increased the reaction extraction rate without affecting the amplitude and latency. Moreover, the intensity inducing the first response was used for further stimulation and analysis of each patient. The interval between each stimulation was 5 s. The mean intensity that induced the response was 13.6 mA (range: 10-16 mA).
For BR analysis, bilateral signals were recorded at a one-side stimulus, repeated four times, and the average R1 and R2 Latency and amplitude were calculated. The normal values of the latency were as follows: R1 ≤ 11.8 ms, bilateral difference ≤ 2 ms; R2 ≤ 34.4 ms, bilateral difference ≤ 3.6 ms; and R2' ≤ 34.6 ms, bilateral difference ≤ 4.0 ms (data from Union Medical College Hospital)[29]. The R2 wave involved multiple bilateral intracranial structures with a long conduction path and was affected by intracranial lesions. The absolute value of the amplitude was not significant enough for diagnosis, and primarily the R1 Latency was presented in this study[30].
Comparing the Schirmer I score between patients with diabetes for short and long duration in the DEwDM group (< 10 years 5.89 ± 4.68 mm; > 10 years 3.23 ± 3.39 mm) and patients with different durations of diabetes in the DMnDE group (< 10 years 18.38 ± 5.93 mm; > 10 years 10.93 ± 6.17 mm). The significant differences were observed (DEwDM: P = 0.018; DMnDE: P = 0.012); and such differences were not found in BUT between the DEwDM or DMnDE groups with different durations of diabetes (DEwDM: P = 0.586; DMnDE: P = 0.601) (Table 1).
| Parameters | DEwDM | P value | DMnDE | P value | DEwDM versus DMnDE P value | |||
| Duration of diabetes ≤ 10 yr | Duration of diabetes > 10 yr | Duration of diabetes ≤ 10 yr | Duration of diabetes > 10 yr | Duration of diabetes ≤ 10 yr | Duration of diabetes > 10 yr | |||
| Number [N] | 26 | 30 | 8 | 14 | ||||
| Age [yr ± SEM] | 56.8 ± 9.3 | 57.8 ± 6.9 | 0.660 | 51.3 ± 8.9 | 57.1 ± 5.3 | 0.065 | 0.146 | 0.740 |
| Gender | 0.126 | 0.074a | 0.257a | 1b | ||||
| Male [N (%)] | 13 (50.0) | 21 (70.0) | 2 (25.0) | 10 (71.4) | ||||
| Female [N (%)] | 13 (50.0) | 9 (30.0) | 6 (75.0) | 4 (28.6) | ||||
| Blood glucose [mmol/L ± SEM] | 7.9 ± 3.7 | 9.6 ± 4.2 | 0.103 | 7.8 ± 1.1 | 9.7 ± 2.8 | 0.083 | 0.926 | 0.989 |
| Duration of diabetes [years ± SEM] | 5.9 ± 2.6 | 17.8 ± 4.8 | 0.000 | 8.3 ± 1.7 | 17.6 ± 5.4 | 0.000 | 0.021 | 0.940 |
| HbA1c [% ± SEM] | 7.8 ± 2.3 | 8.4 ± 1.8 | 0.257 | 7.7 ± 1.9 | 8.1 ± 1.4 | 0.652 | 0.921 | 0.484 |
| BUT [s ± SEM] | 4.1 ± 2.5 | 3.8 ± 2.5 | 0.586 | 11.2 ± 7.5 | 10.0 ± 2.9 | 0.601 | 0.000 | 0.000 |
| Schirmer test [mm ± SEM] | 5.9 ± 4.7 | 3.2 ± 3.4 | 0.018 | 18.4 ± 5.9 | 10.9 ± 6.2 | 0.012 | 0.000 | 0.000 |
| NFL [mm/mm2 ± SEM] | 17.653 ± 4.569 | 15.851 ± 4.881 | 0.162 | 20.445 ± 4.036 | 15.904 ± 5.238 | 0.047 | 0.131 | 0.974 |
| NFD [num/mm2 ± SEM] | 33.2 ± 12.1 | 30.4 ± 8.486 | 0.323 | 39.1 ± 12.4 | 33.9 ± 10.9 | 0.323 | 0.240 | 0.250 |
| NBD [num/mm2 ± SEM] | 56.7 ± 25.5 | 50.0 ± 25.2 | 0.326 | 61.7 ± 21.0 | 43.3 ± 22.4 | 0.072 | 0.619 | 0.401 |
| R1 [ms] | 11.8 ± 0.9 | 12.2 ± 0.8 | 0.083 | 10.3 ± 0.5 | 11.8 ± 1.0 | 0.001 | 0.000 | 0.183 |
Statistical analysis was performed using SPSS Statistics 16.0 (SPSS Inc., IL, United States). All data from measurements were expressed as mean ± SD unless stated. Kolmogorov-Smirnov D-test was conducted to test the normality of data distribution. The chi-square test was performed on the sex of each group. For other data, the Kruskal–Wallis test and analysis of variance (ANOVA) were performed to compare the four groups. This was followed by the post-hoc multiple-comparison Tukey (with homogeneity of variance) or Tamhane test (with the heterogeneity of variance) when P was less than 0.05 in Kruskal–Wallis test and ANOVA. Two independent-samples t-tests were conducted for statistical comparison between two subgroups. Correlations between the corneal nerve morphological parameters (NFL, NFD, and NBD) or R1 Latency and ocular surface parameters (BUT, Schirmer I test) or duration of diabetes were analyzed by Spearman correlation. A P value < 0.05 indicated a statistically significant difference. Additionally, Bland–Altman analysis in SPSS16.0 statistical software was used to analyze the consistency of data from the two researchers. If the data were consistent, the mean value of the two was taken as the inspection result.
In this study, 131 participants were included. Of these, 56 participants were in the DEwDM group (56.9 ± 7.8 years; 39.3% women), 33 participants were in the DEnDM group (52.3 ± 11.1 years; 57.6% women), 22 participants were in the DMnDE group (55.0 ± 7.2 years; 45.5% women), and 20 participants were in the nDMnDE (52.2 ± 9.4 years; 60% women) group. Sex and age were not statistically different among the four groups (sex, P = 0.248; age, P = 0.071). Table 2 presents the characteristics of the study population. The DMnDE group had significantly lower OSDI scores than the other three groups. No significant difference was found between DEwDM and DMnDE groups. A significant difference (P < 0.001) in the Schirmer I test score and BUT were found among the four groups. In contrast, no significant difference was observed between the DEwDM and DEnDM groups.
| Parameters | DEwDM | DEnDM | DMnDE | nDMnDE | Statistics |
| Number [N] | 56 | 33 | 22 | 20 | |
| Age [yr ± SEM] | 56.9 ± 7.8 | 52.3 ± 11.1 | 55.0 ± 7.2 | 52.2 ± 9.4 | |
| Gender | chi-square test | ||||
| Male [N (%)] | 34 (60.7) | 14 (42.4) | 12 (54.5) | 8 (40.0) | |
| Female [N (%)] | 22 (39.3) | 19 (57.6) | 10 (45.5) | 12 (60.0) | |
| Blood glucose [mmol/L ± SEM] | 7.4 ± 4.2 | NA | 9.0 ± 2.5 | NA | |
| Duration of diabetes [years ± SEM] | 12.3 ± 7.1 | NA | 14.2 ± 6.4 | NA | |
| HbA1c [% ± SEM] | 8.1 ± 2.0 | NA | 7.9 ± 1.6 | NA | |
| OSDI scores ± SEM | 5.8 ± 3.9b,c | 9.7 ± 6.3a,c | 3.6 ± 2.7a,b,d | 10.5 ± 7.1c | Tamhane test |
| BUT [s ± SEM] | 3.9 ± 2.5c,d | 4.9 ± 3.9c,d | 10.4 ± 4.9a,b | 12.0 ± 6.5a,b | Tamhane test |
| Schirmer test [mm ± SEM] | 4.5 ± 4.2c,d | 3.7 ± 3.6c,d | 13.6 ± 7.0a,b | 11.2 ± 4.4a,b | Tamhane test |
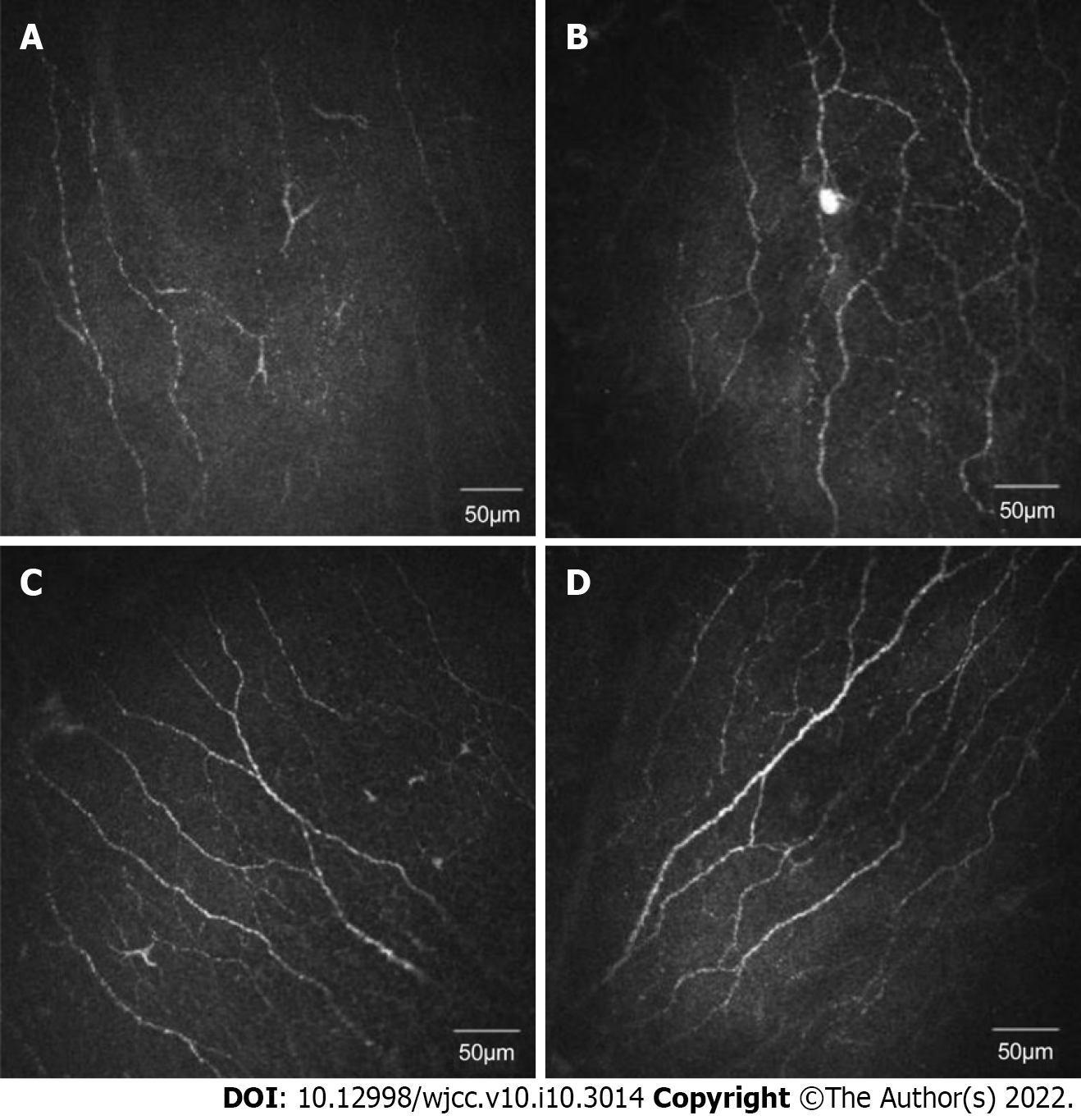
Representative IVCM images of central corneal subbasal nerves from different groups are shown in Figure 1. The corneal NFL of each group had a statistically significant difference (P < 0.001). NFL was significantly shorter in the DEwDM (16.688 ± 4.782 mm/mm2), DMnDE (17.555 ± 5.236 mm/mm2), and DEnDM (16.894 ± 4.855 mm/mm2) groups (P < 0.001, P = 0.014, and P = 0.001, respectively; Table 3) compared with the nDMnDE group (22.017 ± 3.369 mm/mm2). However, no significant variation in the NFL value was found between the groups with dry eyes having or not having diabetes (Table 3). No significant difference in corneal NFD (P = 0.083) and corneal NBD (P = 0.195) was found among the four groups (Table 3).
| Parameters | DEwDM | DEnDM | DMnDE | nDMnDE | Statistics |
| NFL [mm/mm2] | 16.688 ± 4.782d | 16.894 ± 4.855d | 17.555 ± 5.236d | 22.017 ± 3.369a,b,c | Tukey HSD test |
| NFD [num/mm2] | 35.0 ± 9.4 | 37.5 ± 10.6 | 35.6 ± 11.3 | 41.3 ± 8.1 | Tukey HSD test |
| NBD [num/mm2] | 46.9 ± 23.1 | 51.3 ± 25.0 | 50.0 ± 23.1 | 60.6 ± 26.3 | Tukey HSD test |
| R1 [ms] | 12.0 ± 0.8b,c,d | 11.2 ± 0.8a | 11.3 ± 1.1a | 10.6 ± 1.0a | Tamhane test |
The R1 Latency was 12.0 ± 0.8 ms in the DEwDM, 11.2 ± 1.1 ms in the DMnDE, 11.2 ± 0.8 ms in the DEnDM, and 10.5 ± 1.0 ms in the nDMnDE groups. The differences in R1 Latency among the four groups were statistically significant (P < 0.001), but only R1 Latency in the DEwDM group showed a significant difference compared with the other three groups (P = 0.008, P = 0.001, P < 0.001, compared with the DMnDE, DEnDM, and nDMnDE groups, respectively).
Patients in the DEwDM and DEnDM groups were divided into two subgroups according to the severity of dry eyes (moderate or severe: Schirmer ≤ 5 mm; mild: Schirmer > 5 mm; Table 4). NBD was significantly higher (P = 0.031) in the DEnDM group compared with the DEwDM group with moderate or severe dry eyes. NFD was significantly lower (P = 0.039) in the DEwDM group compared with the DEnDM group with mild dry eyes. NBD was significantly higher in patients in the DEnDM group with moderate or severe dry eyes than those in the DEnDM group with mild dry eyes (P = 0.019).
| Parameters | DEwDM | P value | DEnDM | P value | DEwDM versus DEnDM P value | |||
| Schirmer test ≤ 5 mm | Schirmer test > 5 mm | Schirmer test ≤ 5 mm | Schirmer test > 5 mm | Schirmer test ≤ 5 mm | Schirmer test > 5 mm | |||
| Number [N] | 39 | 17 | 24 | 9 | ||||
| Age [yr ± SEM] | 57.1 ± 8.5 | 57.9 ± 7.2 | 0.707 | 53.5 ± 11.7 | 49.1 ± 9.0 | 0.323 | 0.164 | 0.011 |
| Gender | 0.686 | 0.122a | 0.632b | 0.026a | ||||
| Male [N (%)] | 23 (59.0) | 11 (64.7) | 8 (33.3) | 6 (66.7) | ||||
| Female [N (%)] | 16 (41.0) | 6 (35.3) | 16 (66.7) | 3 (33.3) | ||||
| Blood glucose [mmol/L ± SEM] | 8.8 ± 3.6 | 8.9 ± 5.0 | 0.893 | NA | NA | NA | ||
| 13.5 ± 6.8 | 9.5 ± 7.4 | 0.054 | NA | NA | NA | |||
| HbA1c [% ± SEM] | 8.1 ± 1.8 | 8.1 ± 2.5 | 0.995 | NA | NA | NA | ||
| BUT [s ± SEM] | 4.3 ± 2.9 | 3.1 ± 1.0 | 0.105 | 5.6 ± 4.3 | 3.2 ± 1.3 | 0.114 | 0.153 | 0.856 |
| Schirmer test [mm ± SEM] | 2.1 ± 1.7 | 9.9 ± 3.1 | 0.000 | 1.9 ± 1.5 | 8.7 ± 2.5 | 0.000 | 0.590 | 0.319 |
| NFL [mm/mm2 ± SEM] | 16.671 ± 4.749 | 16.727 ± 5.006 | 0.968 | 17.036 ± 4.691 | 16.514 ± 5.549 | 0.788 | 0.767 | 0.922 |
| NFD [num/mm2 ± SEM] | 32.4 ± 9.7 | 30.1 ± 11.7 | 0.310 | 35.7 ± 8.3 | 34.7 ± 16.3 | 0.429 | 0.072 | 0.039 |
| NBD [num/mm2 ± SEM] | 52.1 ± 22.9 | 55.5 ± 30.9 | 0.241 | 65.6 ± 23.7 | 49.3 ± 22.2 | 0.019 | 0.031 | 0.104 |
| R1 [ms ± SEM] | 12.1 ± 0.9 | 11.8 ± 0.6 | 0.406 | 11.1 ± 0.8 | 11.5 ± 0.9 | 0.288 | 0.000 | 0.215 |
The difference of R1 Latency between patients in the DEwDM and DEnDM groups with moderate or severe dry eyes was statistically significant (P < 0.001, Table 4). However, such a difference was not observed between patients with mild dry eyes (P = 0.215). No difference in R1 Latency was observed between DEwDM (P = 0.406) and DEnDM (P = 0.288) groups with different degrees of dry eye severity.
In the DMnDE group, the R1 Latency of patients with diabetes for a short duration (≤ 10 years) was statistically different (P = 0.001) from those with diabetes for a long duration (> 10 years). In contrast, no difference (P = 0.083) in R1 Latency was observed between patients with different durations of diabetes in the DEwDM group (Table 1). The difference in R1 Latency was observed between patients with diabetes for a short duration in the DEwDM and DMnDE groups (P < 0.001), but not between patients with diabetes for a long duration in the DEwDM and DMnDE groups (P = 0.183).
No significant difference in the morphology of the central subbasal corneal nerve fibers was observed between patients with different durations of diabetes in the DEwDM group and patients with diabetes for a short or long duration in the DEwDM and DMnDE groups (Table 1). No differences in NBD and NFD were observed between patients with different durations of diabetes in the DMnDE group. In contrast, the NFL of patients with diabetes for a short duration was significantly longer (P = 0.047) than that of patients with diabetes for a long duration in the DMnDE group.
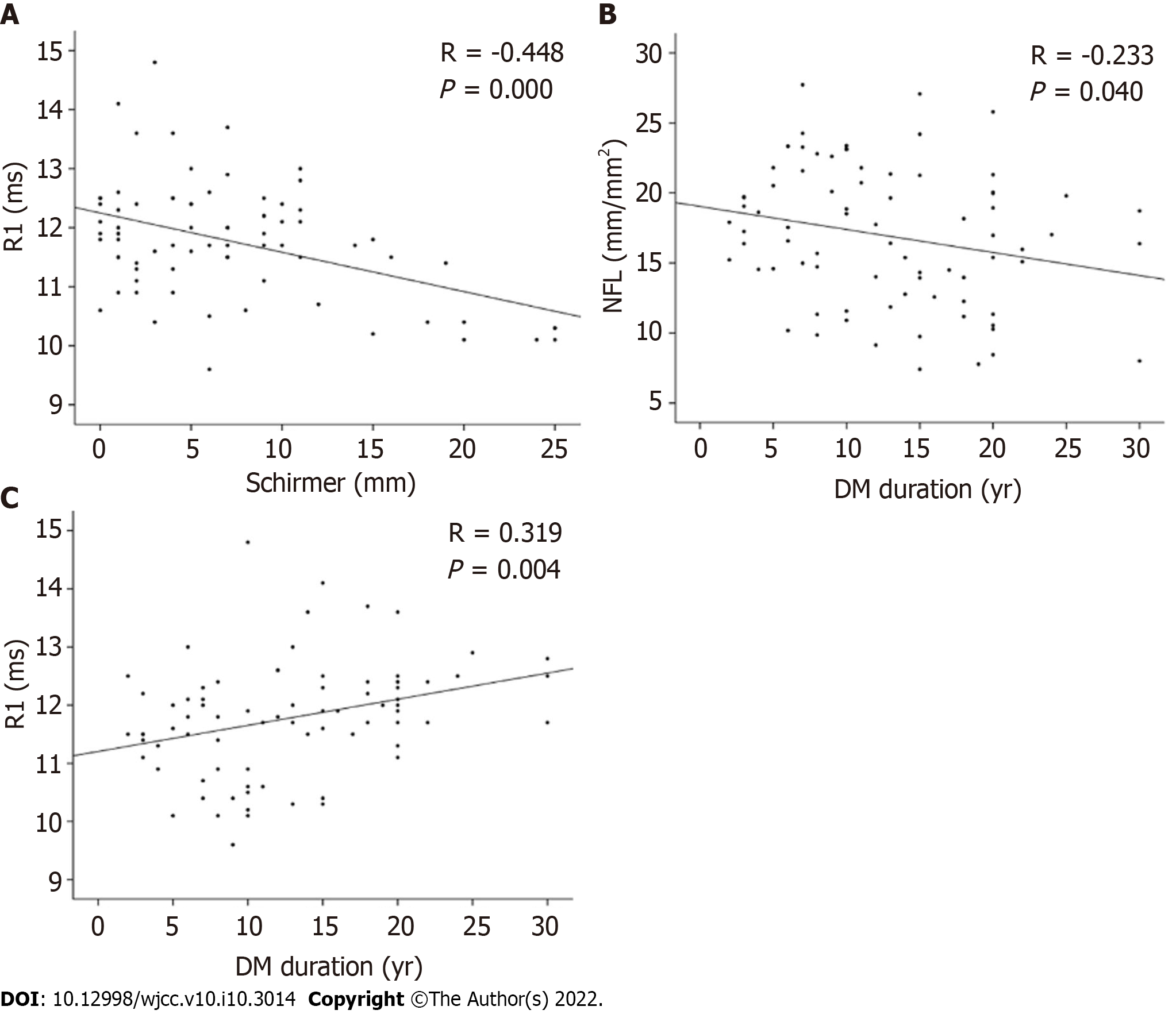
Correlations between age, duration of diabetes, corneal nerve morphology, R1 Latency, Schirmer I score, and BUT were assessed. The Schirmer I score negatively correlated with R1 Latency (r = -0.448, P < 0.001; Schirmer = 42.511 - 3.010 × R1; Figure 2A). The Schirmer I score positively correlated with NBD (r = 0.272, P = 0.016; Schirmer = 3.316 + 0.069 × NBD; Figure 2B). The BUT negatively correlated with age (r = -0.271, P < 0.001; BUT = 14.488 - 0.154 × age; Figure 2C); however, it showed no correlation with corneal nerve morphology and R1 Latency. The statistical analysis on all diabetic patients, including dry eye and non-dry eye, dry eye-diabetes group, and diabetic non-dry eye group, revealed that the three HbA1c were normal distributions. No correlation was found with each observation index (Table 5).
| Group | HbA1c | P value related to R1 | P value related to Schirmer | P value related to BUT | P value related to NFL | P value related to NFD | P value related to NBD |
| Diabetes groups | 8.08 ± 1.89 | 0.072 | 0.280 | 0.796 | 0.621 | 0.794 | 0.952 |
| Diabetic dry eye group | 8.14 ± 2.01 | 0.933 | 0.508 | 0.141 | 0.589 | 0.823 | 0.851 |
| Diabetic non-dry eye group | 7.93 ± 1.57 | 0.419 | 0.376 | 0.140 | 0.910 | 0.421 | 0.832 |
Dry eye has become a common which has a significant association with diabetes. It affects people’s quality of life by causing eye discomfort, decrease in vision, and tear film instability, relating to ocular surface inflammation[31]. Therefore, in this study, we explored the morphology of the corneal subbasal nerve plexus by IVCM and measuring BR. This study revealed that among four groups, the NFL was reduced in three groups, and R1 Latency of BR increased in one group. This suggests that corneal nerve morphology changed in patients with dry eyes and/or diabetes. However, the closed-loop reduction is only in those with dry eyes and diabetes.
Diabetes is a risk factor for dry eyes[18]. This study also found that the Schirmer I score was lower in the group with longer diabetes duration regardless of whether they had dry eyes (Table 1). The corneal subbasal nerve plexus is an essential observation in patients with diabetes having a dry eye condition. A study showed that the number of fibers, beadings, and branching pattern of fibers significantly decreased in patients with diabetes with a significant increase in nerve tortuosity, indicating the progression of corneal neuropathy with peripheral diabetic neuropathy[15]. Corneal nerve density was significantly lower in patients with T1DM with cardiac autonomic neuropathy than in those without[32]. Important correlation has been identified between the tortuosity of nerve and severity of diabetic neuropathy[33]. Corneal NFL, NBD, and NBL showed a progressive and significant reduction in patients with diabetic autonomic neuropathy[34]. In this study, the corneal NFL was lower in the DEwDM and DMnDE groups than in the normal control group, indicating that diabetes could cause a decrease in the corneal NFL, which was similar to what was found in patients with T1DM. In this study, NFL was also associated with diabetes duration (Figure 2).
Also, the corneal NFD was lower in the DEnDM group than in the normal control group. This meant that neuromorphic corneal damage existed in patients with dry eyes not having diabetes, probably due to other disease factors. Studies indicated that corneal sensitivity significantly decreased in patients with dry eyes and glaucoma compared with controls. The density and number of subbasal corneal nerves also significantly decreased in these patients[34]. This indicated that dry eye symptoms coming from other factors might also be associated with corneal nerve fiber damages or other related issues.
A study on patients with T1DM indicated a reduction in long nerve fiber bundles per image in patients with mild-to-moderate neuropathy. However, corneal mechanical sensitivity decreased only in patients with severe neuropathy[10]. However, in this study, no significant variation was found in NFL or NBD between the patients with a severe or mild dry eye condition, irrespective of whether the patients had T2DM. The sensory nerve functions are regulated by morphology changes and the expression of different proteins (e.g., TRPA1, NCS, AISC, and CaBP/calneuron) serving as sensors, such as mechanosensitive channels expressed on corneal nerves[35]. In this study, the measurement of expression of mechanosensitive channels could not be performed.
The trigeminal and facial nerve function in dry eyes and diabetes was unclear earlier. A case report showed bilateral trigeminal dysfunction using BR measurement in a 68-year-old male patient with dry eyes[36]. However, in this study, a statistically significant longer R1 Latency was observed in patients with diabetes having dry eyes compared with those with diabetes not having dry eyes, those with dry eyes not having diabetes, and controls (Table 3). These suggest that this abnormality might occur after morphological changes regarding the NFL changes in patients with dry eye or/and T2DM. Meanwhile, the correlation analysis showed that R1 Latency correlated with the Schirmer I score and diabetes duration time (Figure 2).
Further analysis showed that among patients with severe dry eye, the R1 Latency of patients with diabetes was longer than that of patients without diabetes (Table 4). Also, in patients without a dry eye, the R1 Latency was longer in the group with a longer diabetes course than in a shorter diabetes course group. In patients with diabetes course less than ten years, the R1 Latency was longer in the patients with the dry eye than in those without a dry eye (Table 1). These results suggested that BR function changes were related to T2DM and dry eyes. However, the role of BR changes in dry eye disease with or without T2DM needs further research.
Interestingly, NBD was significantly higher in patients without diabetes having mild dry eye symptoms than in those without diabetes having severe DES symptoms and those with diabetes having similar DES severity (Table 4). Studies reported that the corneal nerve fibers in Sjogren's syndrome had growth cones and might have nerve regeneration[37]. Therefore, it was hypothesized that neurogenesis existed in patients with mild DES symptoms and that diabetes might inhibit this process, the mechanism of which needs to be further explored.
This observational study also had several limitations. (1) Some of the selected patients with diabetes did not have diabetic peripheral neuropathy simultaneously, and it might have impacted the results; (2) As this study was a cross-sectional study, the causal relationship or long-term correlation among diabetes mellitus, corneal nerve injury, and dry eye could not be analyzed; (3) A large difference in the sample size was observed between some subgroups, which might have affected the statistical results. This was because this observation involved ophthalmology, neurology, and laboratory examinations, which could not be completed at one time. The number of patients with non-dry eyes was relatively small. It required patients to come to the hospital multiple times to perform various examinations separately; (4) The patients in the DEwDM group had the core complaint of dry eyes, but it was not clear whether the corneal nerve morphology and function had changed; and (5) We understand that the American diabetes association recommended the oral glucose tolerance test (OGTT) or HBA1C as the gold standard test. However, we used fingertip glucose tests for the diagnostic test in this current study. Thus, we plan to use the OGTT or HBA1C in future studies on this topic.
In conclusion, we analyzed several groups of patients with diabetes and dry eye in this study. We observed that patients with type 2 diabetes and dry eye had impaired corneal neuromorphology and function. In contrast, patients with diabetes alone or dry eye only had abnormal corneal neuromorphology and no impaired neurological function. Further research is needed on corneal morphology and neurological function in patients with dry eye and diabetes in order to provide better treatment. Perhaps in patients with diabetes or dry eyes, the corneal nerve function has been damaged. However, the blink reflex does not detect its subtle changes, and more sophisticated instruments and detection methods are needed to find out.
Dry eye syndrome (DES) decreases the quality of life and is associated with type 2 diabetes mellitus (T2DM). The prevalence of DES increases with multiple complications worldwide. Blink reflex (BR) can be used to assess the corneal nerve closed-loop morphology, but it is unclear whether it could be used in DES. This study helps understand more about corneal nerve morphology in patients with DES.
DES was significantly associated with T2DM, thus with the HBA1c level. However, the underlying mechanism of DES in T2DM is still unclear. This study will help determine the relationship between corneal nerves morphology and DES abnormalities in patients with T2DM.
The BR changes the corneal nerve morphology in patients with DES having T2DM is unclear. Thus, the objective of this study is to investigate corneal nerve fibers, and corneal nerve closed loop function in patients with DES having T2DM or not.
In this study, a total of 131 patients were enrolled from the ophthalmology and endocrinology Operationalized Psychodynamic Diagnosis with or without dry eye complaints. The tear film break-up time, Schirmer I test, in vivo confocal microscopy, and BR have performed accordingly after grouping. Data were presented as mean ± SD, and statistical analysis was performed using SPSS Statistics 16.0. This cross-sectional study is one of the primary studies to uncover the BR changes in these patients.
The findings of this study revealed that among this four DEwDM (n = 56), DMnDE (n = 22), DEnDM (n = 33), and nDMnDE (n = 20) groups, the nerve fiber length was reduced in three groups. The R1 Latency of BR increased in one group, suggesting changes in the corneal nerve morphology in DES patients with or without T2DM. The role of BR changes in DES with or without T2DM needs further assessment through future studies.
Further research is needed on the corneal morphology and neurological function in DES patients having T2DM in the future to provide clinical benefits to these patients. Moreover, OGTT or HBA1c as the diagnostic test should be used in future studies. The number of patients with DES needs to be increased in future studies.
This study observed that T2DM and DES had abnormal corneal morphology and function, whereas T2DM or DES alone had only abnormal corneal morphology. Therefore, future research should focus specifically on T2DM patients and DES and their morphological and functional improvements of corneal nerve.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Herold Z, Hungary; Okechukwu PN, Malaysia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Clayton JA. Dry Eye. N Engl J Med. 2018;378:2212-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 232] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 611] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 3. | Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 1642] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 5. | Liu NN, Liu L, Li J, Sun YZ. Prevalence of and risk factors for dry eye symptom in mainland china: a systematic review and meta-analysis. J Ophthalmol. 2014;2014:748654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Liu ZG, Wang H. [Focusing on the management of chronic dry eye disease]. Zhonghua Yan Ke Za Zhi. 2018;54:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 348] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 8. | Hosam SH, Tseng SCG. The role of amniotic membrane for managing dry eye disease, 1 edn. London: JP MEDICAL; 2013. [DOI] [Full Text] |
| 9. | Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59:263-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Labbé A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926-4931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Malik RA. Corneal sensitivity is reduced and relates to the severity of neuropathy in patients with diabetes. Diabetes Care. 2007;30:1895-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Zou X, Lu L, Xu Y, Zhu J, He J, Zhang B, Zou H. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol. 2018;18:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Meng ID, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res. 2013;117:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond). 2005;19:1276-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Petropoulos IN, Alam U, Fadavi H, Asghar O, Green P, Ponirakis G, Marshall A, Boulton AJ, Tavakoli M, Malik RA. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36:3646-3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Ferdousi M, Petropoulos IN, Kalteniece A, Azmi S, Ponirakis G, Efron N, Soran H, Malik RA. No Relation Between the Severity of Corneal Nerve, Epithelial, and Keratocyte Cell Morphology With Measures of Dry Eye Disease in Type 1 Diabetes. Invest Ophthalmol Vis Sci. 2018;59:5525-5530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Yazdani-Ibn-Taz MK, Han MM, Jonuscheit S, Collier A, Nally JE, Hagan S. Patient-reported severity of dry eye and quality of life in diabetes. Clin Ophthalmol. 2019;13:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. 2014;31:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Wu Z, Begley CG, Situ P, Simpson T. The effects of increasing ocular surface stimulation on blinking and sensation. Invest Ophthalmol Vis Sci. 2014;55:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Herrero-Vanrell R, Peral A. [International Dry Eye Workshop (DEWS). Update of the disease]. Arch Soc Esp Oftalmol. 2007;82:733-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 1164] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 22. | Bron AJ. The Doyne Lecture. Reflections on the tears. Eye (Lond). 1997;11 (Pt 5):583-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Keratology Group; Ophthalmology Branch; Association CM. 2013 Chinese Clinical Diagnosis and Treatment Experts Consensus of Dry Eye. Chin J Ophthalmol. 2013;49:73-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 25. | Ganguly A, Kaza H, Kapoor A, Sheth J, Ali MH, Tripathy D, Rath S. Comparative Evaluation of the Ostium After External and Nonendoscopic Endonasal Dacryocystorhinostomy Using Image Processing (Matlabs and Image J) Softwares. Ophthalmic Plast Reconstr Surg. 2017;33:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017;15:15-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 27. | Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A. In Vivo Confocal Microscopic Evaluation of Corneal Nerve Fibers and Dendritic Cells in Patients With Behçet's Disease. Front Neurol. 2018;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Trujillo-Hernández B, Huerta M, Pérez-Vargas D, Trujillo X, Vásquez C. Blink reflex alterations in recently diagnosed diabetic patients. J Clin Neurosci. 2003;10:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | He H, Deng JG, Luo ZH. The application of blink reflex combined with facial nerve conduction to early idiopathic facial neuritis. Zhongguo Yiyao 2018; 8: 253-256. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Jerath N, Kimura J. F wave, A wave, H reflex, and blink reflex. Handb Clin Neurol. 2019;160:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Uchino M, Schaumberg DA, Dogru M, Uchino Y, Fukagawa K, Shimmura S, Satoh T, Takebayashi T, Tsubota K. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Maddaloni E, Sabatino F, Del Toro R, Crugliano S, Grande S, Lauria Pantano A, Maurizi AR, Palermo A, Bonini S, Pozzilli P, Manfrini S. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet Med. 2015;32:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Su P, Chen T, Xie J, Zheng Y, Qi H, Borroni D, Zhao Y, Liu J. Corneal nerve tortuosity grading via ordered weighted averaging-based feature extraction. Med Phys. 2020;47:4983-4996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. 2015;52:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, Lo TY, Wang F, Li T, Thompson-Peer KL, Gong J, Murthy SE, Coste B, Yakubovich N, Patapoutian A, Xiang Y, Rompolas P, Jan LY, Jan YN. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron. 2019;102:373-389.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 36. | Lin PY, Cheng CY, Wu CC, Yen MY, Wang SJ, Liao KK, Lee SM. Bilateral neurotrophic keratopathy complicating Vidian neurectomy. Am J Ophthalmol. 2001;132:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren's syndrome. Exp Eye Res. 2008;86:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |