Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.338
Peer-review started: July 12, 2021
First decision: July 26, 2021
Revised: July 28, 2021
Accepted: November 28, 2021
Article in press: November 28, 2021
Published online: January 7, 2022
Processing time: 170 Days and 18.3 Hours
Ullrich congenital muscular dystrophy (UCMD) is one of the collagen-VI-related myopathies caused by mutations of COL6A1, COL6A2, and COL6A3 genes. Affected individuals are characterized by muscle weakness, proximal joint contracture, distal joint hyperlaxity, and progressive respiratory failure. There is currently no cure for UCMD. Here, we report the clinical manifestations and prenatal diagnosis of compound heterozygous mutations of the COL6A2 gene in a Chinese family with UCMD.
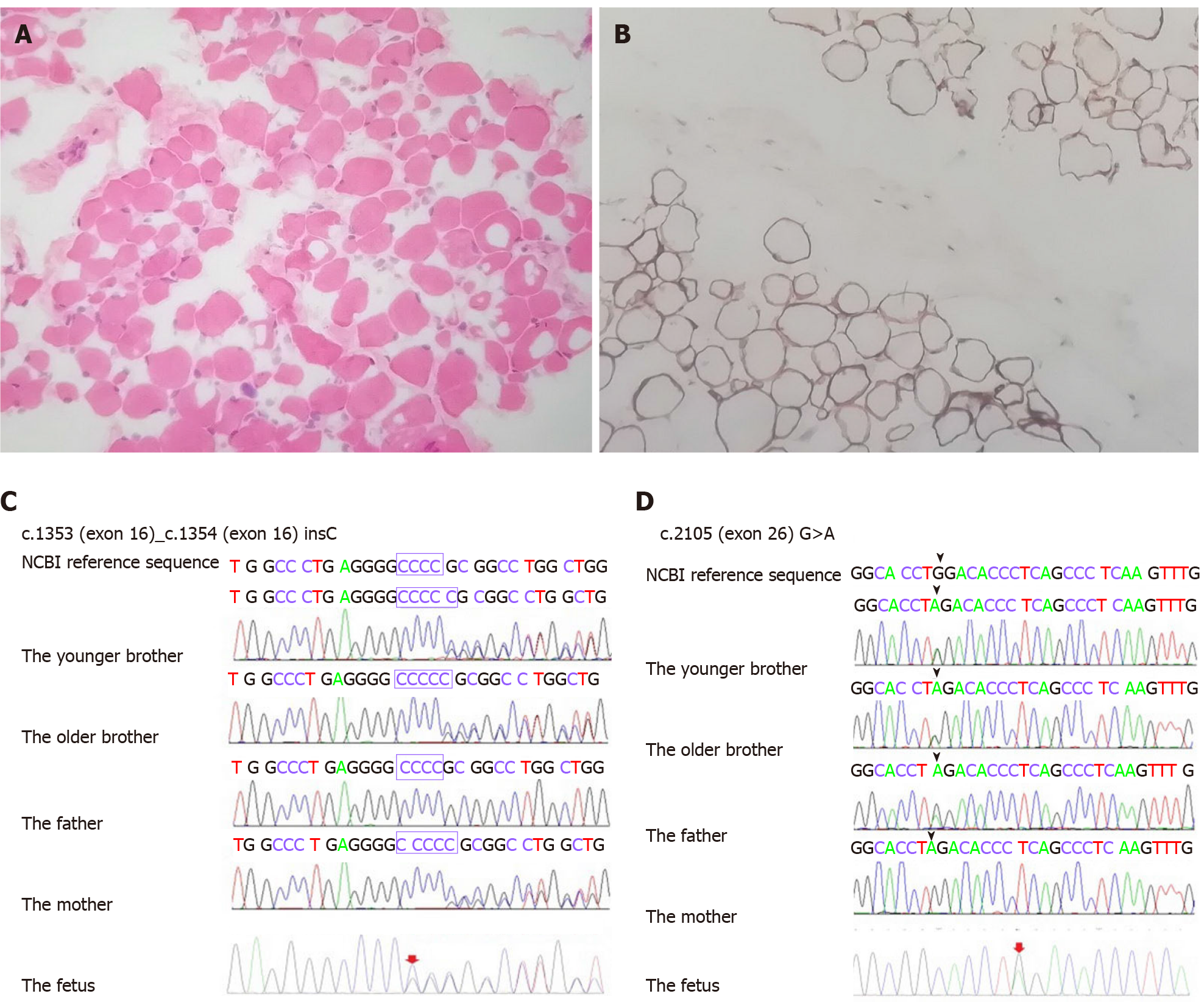
A 3-year-old boy, his 4-year-old brother, their parents, and a 20-wk-old fetus in the mother’s womb were included in the study. The brothers had the typical manifestations of the early-severe subtype: A delayed motor milestone (never walking independently), torticollis, scoliosis, proximal joint contracture, distal joint hyperextension, right hip joint dislocation, and calcaneal protuberance. Both brothers were found by whole-exome sequencing and Sanger sequencing to carry two mutations of the COL6A2 gene (c.1353_c.1354insC, p.Arg453Profs
We report a Chinese family suffering from UCMD. By clarifying the COL6A2 mutations in the probands, the parents had the opportunity to opt for voluntary interruption of the third UCMD pregnancy.
Core tip: We report the clinical manifestations and prenatal diagnosis of compound heterozygous mutations of the COL6A2 gene in a Chinese family with Ullrich congenital muscular dystrophy (UCMD). A 3-year-old boy and his 4-year-old brother had typical UCMD manifestations of the early-severe subtype. They carried two mutations of the COL6A2 gene (c.1353_c.1354 ins C, p.Arg453ProfsTer42/c.2105 G>A, p.Trp702Ter). The absence of collagen VI staining in the younger brother’s muscle was identified accurately. A 20-wk-old fetus in their mother’s womb underwent prenatal diagnosis and carried the same two mutations. After a painful psychological struggle, their parents decided to terminate the pregnancy.
- Citation: Hu J, Chen YH, Fang X, Zhou Y, Chen F. Clinical manifestations and prenatal diagnosis of Ullrich congenital muscular dystrophy: A case report. World J Clin Cases 2022; 10(1): 338-344
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/338.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.338
Ullrich congenital muscular dystrophy (UCMD, OMIM: 254090) is one of the collagen-VI-related myopathies caused by mutations of the COL6A1, COL6A2, and COL6A3 genes. The prevalence of UCMD is not sufficiently known, with an estimated 0.13 per 100000 in Northern England[1], which is higher than that reported in China[2]. Affect
Here, we report the clinical manifestations and prenatal diagnosis of compound heterozygous mutations of the COL6A2 gene in a Chinese family with UCMD. A third UCMD child of the family was prevented from birth through prenatal diagnosis. The study protocol was carried out in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Fujian Medical University (No. 2020KY023). Written informed consent was obtained from the parents.
A 3-year-old boy and his 4-year-old brother presented with their disabilities in walking independently after birth.
The younger brother stood and walked with assistance at the age of 15 mo. He has achieved no further motor milestones since that time. The elder brother could hardly stand, even with help, and could never walk independently.
The brothers were delivered by cesarean section at term after a simple pregnancy. They began to hold up their heads at the age of 3 mo and could sit by themselves at 8 mo. Their language and social communication abilities were normal.
The patients’ parents, a 35-year-old Chinese father and a 32-year-old Vietnamese mother, were unrelated, neither of whom reported a family history of neuromuscular diseases.
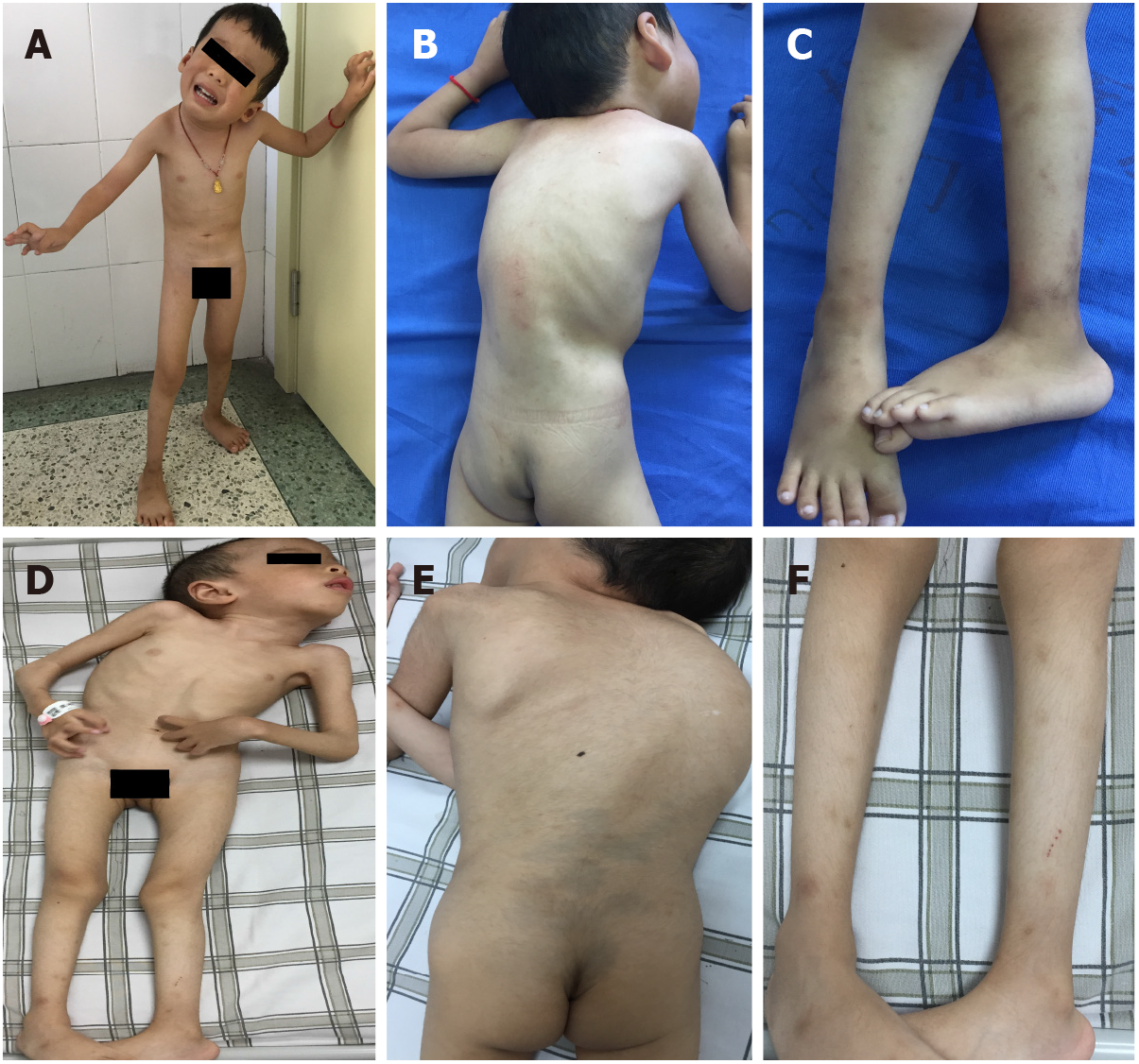
The younger brother had torticollis and scoliosis, could sit by himself, stand and walk with help, but could never walk independently. His muscle tone was generally decreased, and muscle power was graded as 3 or 4 in the extremities. Proximal joint contractures, such as at the shoulders, hips, and knees, were prominent bilaterally. Distal joints, including wrists, fingers, and toes, were markedly hyperextensible. Calcaneal protuberance was remarkable. Muscular atrophy was visible in the whole body. Knee reflexes were present. Pathological signs in the central nervous system were negative (Figures 1A-C). The elder brother had similar clinical manifestations, with the degree more serious than that of the younger brother (Figures 1D-F).
Creatine kinase levels of the brothers were normal. Basic lung tests were normal too. The IQ on the Gesell developmental diagnostic scale was 89.0 and 81.0, respectively.
Genomic DNA extracted from the peripheral blood of both the patients and their parents was analyzed by whole-exome sequencing[5]. High-throughput sequencing was performed on an Illumina NovaSeq 6000 Series Sequencer (PE150). Sanger sequ
Tissue sections obtained by muscle biopsy of the quadriceps femoris in the younger brother were examined after hematoxylin and eosin staining and immunostaining with anti-collagen VI antibodies. Muscle pathology showed the myofibers were of different sizes. They were atrophied, non-necrotic, or broken. There were no intra
Radiography showed cervical thoracolumbar scoliosis and right hip dislocation. Brain magnetic resonance imaging was normal.
Early-severe subtype of UCMD.
There is currently no cure for UCMD. Treatment is mainly supportive against sym
The brothers’ conditions gradually deteriorated. The elder brother died of respiratory failure in October 2020. The younger brother is unable to stand now. His joint contractures and scoliosis worsen than before. The parents opted after prenatal diagnosis for voluntary interruption of the third UCMD pregnancy.
In this study, we report a family suffering from UCMD living in China. In the clinic, patients with UCMD exhibit a wide spectrum of clinical severity with variable motor and respiratory weakness. Three subtypes of UCMD are defined according to walking status: Patients who never achieve ambulation are categorized as early-severe subtype; those who walk but lose this ability (or are about to lose it) are categorized as moderate-progressive subtype; and patients who are still fully ambulatory are categorized as mild subtype[3,4,8-10]. The brothers had the typical manifestations of the early-severe subtype: A delayed motor milestone (never walking independently), torticollis, scoliosis, proximal joint contracture, distal joint hyperextension, right hip joint dislocation, and calcaneal protuberance. Basic lung testing of the brothers was normal, which is consistent with the other reports that describe patients with the early-severe subtype of UCMD as experiencing early respiratory failure at around 10 years old[11].
Based on the clinical features, we initially focused on the collagen VI genes, and finally identified two mutations of the COL6A2 gene (c.1353_c.1354insC, p.Arg
Variable degrees of histological changes can be observed in muscle biopsies of patients with UCMD. The spectrum includes fiber size variation, increased endomysial connective tissue or adipose tissue, and mild necrotic and regenerating process. Collagen VI staining in muscle biopsies of patients with UCMD is variably less or full absent in the extracellular matrix. It is present in the interstitium but is absent or reduced in the sarcolemma[8]. The muscle pathology of the younger brother was consistent with myogenic damage and regarded as collagenopathy. It was in line with the pathological changes of UCMD. Therefore, we considered the brothers as having early-severe subtypes of UCMD.
UCMD seriously affects quality of life and lifespan and no curative care is available to date, which brings a heavy burden to the family and society. Genetic counseling and prenatal diagnosis are crucial for the families at risk, as the autosomal recessive genetic disease affects a quarter of the patient’s siblings. UCMD demonstrates genetic and phenotypic variability. In familial cases, the genetic background must be identified accurately for reliable counseling and prenatal diagnosis[14,15]. The brothers had the typical manifestations of early-severe subtype UCMD and carried compound heterozygous mutations of the COL6A2 gene. The absence of collagen VI staining in the younger brother’s muscle was identified accurately. It was appropriate to offer genetic counseling (including discussion of the potential risks to offspring and reproductive options) to the parents, who were carriers. Prenatal testing for a pregnancy at increased risk and preimplantation genetic testing were feasible. Differences in perspectives may exist among medical professionals, and within families, regarding the use of prenatal testing. While most centers would consider use of prenatal testing to be a personal decision, discussion of these issues can be helpful.
After their mother was pregnant with a third child, the parents requested prenatal diagnosis for the pregnancy. As the brothers had the typical manifestations of the early-severe subtype UCMD and carried compound heterozygous mutations of the COL6A2 gene by whole-exome sequencing, and the absence of collagen VI staining in the younger brother’s muscle was identified accurately, this allowed us to offer MLPA + STRs as a prenatal diagnostic test for this subsequent pregnancy. However, the detection of gene mutations by this approach may not be reliable due to high genetic heterogeneity and detection errors. All the risks, benefits, and limitations of the chosen testing plan were explained to the parents. We proceeded with the fully informed consent from the parents, particularly with respect to the research nature of these tests, and with ethics committee approval. MLPA + STRs confirmed that the fetus carried the same two mutations of the COL6A2 gene in the amniotic fluid. The parents were told that the fetus would likely suffer from UCMD after birth. They needed to decide whether to continue with the pregnancy. After a painful psychological struggle, the parents finally decided to terminate the pregnancy.
We report a Chinese family suffering from UCMD caused by compound heterozygous mutations of the COL6A2 gene (c.1353_c.1354insC/c.2105G>A). By clarifying the type and source of the disease-causing mutations in the probands, the parents had the opportunity to opt for voluntary interruption of the third UCMD pregnancy through prenatal diagnosis.
We thank the boys and their parents who took part in this study. We are also grateful to all the experts involved in the study and support from Fujian Medical University Union Hospital.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Meglio LD S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132:3175-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 2. | Ge L, Zhang C, Wang Z, Chan SHS, Zhu W, Han C, Zhang X, Zheng H, Wu L, Jin B, Shan J, Mao B, Zhong J, Peng X, Cheng Y, Hu J, Sun Y, Lu J, Hua Y, Zhu S, Wei C, Wang S, Jiao H, Yang H, Fu X, Fan Y, Chang X, Bao X, Zhang Y, Wang J, Wu Y, Jiang Y, Yuan Y, Rutkowski A, Bönnemann CG, Wei W, Wu X, Xiong H. Congenital muscular dystrophies in China. Clin Genet. 2019;96:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Mercuri E, Yuva Y, Brown SC, Brockington M, Kinali M, Jungbluth H, Feng L, Sewry CA, Muntoni F. Collagen VI involvement in Ullrich syndrome: a clinical, genetic, and immunohistochemical study. Neurology. 2002;58:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Foley AR, Quijano-Roy S, Collins J, Straub V, McCallum M, Deconinck N, Mercuri E, Pane M, D'Amico A, Bertini E, North K, Ryan MM, Richard P, Allamand V, Hicks D, Lamandé S, Hu Y, Gualandi F, Auh S, Muntoni F, Bönnemann CG. Natural history of pulmonary function in collagen VI-related myopathies. Brain. 2013;136:3625-3633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Marakhonov AV, Tabakov VY, Zernov NV, Dadali EL, Sharkova IV, Skoblov MY. Two novel COL6A3 mutations disrupt extracellular matrix formation and lead to myopathy from Ullrich congenital muscular dystrophy and Bethlem myopathy spectrum. Gene. 2018;672:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Wang D, Gao M, Zhang K, Jin R, Lv Y, Liu Y, Ma J, Wan Y, Gai Z. Molecular Genetics Analysis of 70 Chinese Families With Muscular Dystrophy Using Multiplex Ligation-Dependent Probe Amplification and Next-Generation Sequencing. Front Pharmacol. 2019;10:814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Wu Y, Yin G, Fu K, Wu D, Zhai Q, Du H, Huang Z, Niu Y. Gene diagnosis for nine Chinese patients with DMD/BMD by multiplex ligation-dependent probe amplification and prenatal diagnosis for one of them. J Clin Lab Anal. 2009;23:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Yonekawa T, Nishino I. Ullrich congenital muscular dystrophy: clinicopathological features, natural history and pathomechanism(s). J Neurol Neurosurg Psychiatry. 2015;86:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Briñas L, Richard P, Quijano-Roy S, Gartioux C, Ledeuil C, Lacène E, Makri S, Ferreiro A, Maugenre S, Topaloglu H, Haliloglu G, Pénisson-Besnier I, Jeannet PY, Merlini L, Navarro C, Toutain A, Chaigne D, Desguerre I, de Die-Smulders C, Dunand M, Echenne B, Eymard B, Kuntzer T, Maincent K, Mayer M, Plessis G, Rivier F, Roelens F, Stojkovic T, Taratuto AL, Lubieniecki F, Monges S, Tranchant C, Viollet L, Romero NB, Estournet B, Guicheney P, Allamand V. Early onset collagen VI myopathies: Genetic and clinical correlations. Ann Neurol. 2010;68:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Bönnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011;7:379-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Yonekawa T, Komaki H, Okada M, Hayashi YK, Nonaka I, Sugai K, Sasaki M, Nishino I. Rapidly progressive scoliosis and respiratory deterioration in Ullrich congenital muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22051] [Article Influence: 2205.1] [Reference Citation Analysis (0)] |
| 13. | Camacho Vanegas O, Bertini E, Zhang RZ, Petrini S, Minosse C, Sabatelli P, Giusti B, Chu ML, Pepe G. Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc Natl Acad Sci U S A. 2001;98:7516-7521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Brockington M, Brown SC, Lampe A, Yuva Y, Feng L, Jimenez-Mallebrera C, Sewry CA, Flanigan KM, Bushby K, Muntoni F. Prenatal diagnosis of Ullrich congenital muscular dystrophy using haplotype analysis and collagen VI immunocytochemistry. Prenat Diagn. 2004;24:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Peat RA, Baker NL, Jones KJ, North KN, Lamandé SR. Variable penetrance of COL6A1 null mutations: implications for prenatal diagnosis and genetic counselling in Ullrich congenital muscular dystrophy families. Neuromuscul Disord. 2007;17:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |