Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.289
Peer-review started: May 10, 2021
First decision: October 16, 2021
Revised: November 9, 2021
Accepted: November 29, 2021
Article in press: November 29, 2021
Published online: January 7, 2022
Processing time: 233 Days and 18.2 Hours
Hemorrhage lesions may lead to bilateral hypertrophic olivary degeneration (HOD) through interruption of the dentato-rubral-olivary pathway. The pathological features of HOD are unusual neuronal trans-synaptic degenerative changes.
A 56-year-old female was admitted to our hospital because her lower extremities and left upper ones were unable to move for 3 mo, and the swelling of her right lower extremities became worse 3 days ago. She had a hypertension history. Her characteristic clinical manifestations are palatal myoclonus and nystagmus. The patient’s magnetic resonance imaging (MRI) results showed that she had bilateral HOD after an acute pontine hemorrhage. She was given symptomatic and supportive treatment. The gabapentin, the memantine and the trihexyphenidyl were taken twice a day each. The rehabilitation and psychotherapy were implemented. After 3 months of treatment, her eye symptoms improved.
Bilateral HOD is a rare phenomenon after pontine hemorrhage. The key to diagnosis lies in the clinical manifestations and MRI results.
Core Tip: Bilateral hypertrophic olivary degeneration (HOD) after pontine hemorrhage is rare. The clinical manifestations and MRI results of HOD are varied, which make it important to monitor the patient and identify the changes in MRI results. Generally speaking, the mechanism leading to HOD is still unclear, and the effect of symptomatic treatment for some patients is not satisfactory.
- Citation: Zheng B, Wang J, Huang XQ, Chen Z, Gu GF, Luo XJ. Bilateral Hypertrophic Olivary Degeneration after Pontine Hemorrhage: A Case Report. World J Clin Cases 2022; 10(1): 289-295
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/289.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.289
Hypertrophic olivary degeneration (HOD) is an unusual neuronal trans-synaptic degenerative disease because the olive initially becomes hypertrophic rather than atrophic. It is the result of the imbalance of the Guillain-Mollaret triangle, which is the dentato-rubral-olivary pathway (DROP)[1]. HOD diagnosis rate could be increased through the hyperintensity and enlargement of the inferior olivary nuclei on T2-weighted magnetic resonance imaging (MRI), and through the pathognomonic symptoms including palatal myoclonus and nystagmus[2]. In general, the incidence of bilateral HOD is low, so here we report a patient suffering from pontine hemorrhage with palatal myoclonus and pendular nystagmus, whose MRI showed bilateral HOD.
A 56-year-old female patient admitted to our hospital complained that her lower extremities and left upper ones could not move for more than 3 mo and the swelling of her lower right extremities got worse 3 days ago.
The patient’s symptoms lasted for more than 3 mo, and swelling of her right lower extremities had worsened over 3 days. There were no obvious symptoms of dyspnea, headache, dizziness, disturbance of consciousness and movement disturbance of other parts.
This patient had a history of hypertension for more than 15 years. She took oral compound reserpine tablets intermittently. Three months ago, she was diagnosed with pontine hemorrhage by a local community hospital and had been rehabilitated there. She had no history of liver or kidney disease or malignant tumor.
The patient lost 6 kg (baseline body weight: 58 kg) of body weight within the last 3 mo. Her family had no other previous medical history.
The general condition of the patient was fair, but she could not walk. She was conscious, a bit thin and her vital signs were stable. The thyroid appearance and palpation were normal. No obvious vascular murmur was heard in the neck. She had a sinus rhythm, and his heart sounds were normal. The respiratory movement was in the normal range. On auscultation, the breath sounds in both lungs were slightly coarse. Both breasts were symmetrical. The abdomen was felt flat and soft, and there was no tenderness, no muscle tension and rebound pain. We palpated the lower abdomen without obvious lumps and other abnormalities. The spinal movement was normal right, and the upper extremities could move normally. The other extremities did not move actively, and the right lower extremity was mild edema. There were no clinical symptoms of enlarged superficial lymph nodes. The neurological examination showed that her pupils were of equal circle size and sensitive to light reflection. Her pendular nystagmus had some horizontal and torsional movements under a prominently vertical component (three cycles per second). The patient also had rhythmic involuntary contractions of the soft palate and pharyngopalatine arch (two to three cycles per second). Her bilateral frontal lines and nasolabial were shallow, and her tongue was slightly leftward. The other cranial nerve examinations were normal. She could feel the sense of touch and temperature change, but her response was slightly slow to painful stimuli. Her examinations in sense of entity, positioning, and figure were all normal. Both her superficial and deep nerve reflections existed. The pathological signs were positive, including the Babinski sign, Chaddock sign and Oppenheim sign, etc. Her neck muscles had no resistance. The skin scratch test was negative. The Patient did not undergo the other nerve function examinations due to inconvenience. The neuromuscular examination displayed that her muscle tone increased. The left upper extremities muscle tone was level 1, the right upper one was level 3 and the lower one was level 2. The lower and left upper extremities muscle strength was level 0, and the right upper one was level 2. She had dysarthria, and the finger-nose test was negative. She refused to do the other coordination movement examinations due to inconvenience.
Blood test, biochemical test, urine test, stool test, myocardial enzyme test and coagulation test were conducted, and the results of the examinations were listed in Table 1.
| Measurement level | Normal level | |
| Blood test | ||
| WBC (×109/L) | 5.47 | 4.0-10.0 |
| RBC (×1012/L) | 3.32 | 3.5-5.0 × 10.0 |
| Hb (g/L) | 117 | 110-155 |
| PLT (×109/L) | 443 | 100-350 |
| NEUT (%) | 65.40 | 50.0-70.0 |
| Biochemical test | ||
| K+ (mmol/L) | 3.84 | 3.50-5.50 |
| Na+ (mmol/L) | 140.20 | 132.0-150.0 |
| BUN (mmol/L) | 2.90 | 1.80-7.10 |
| Cr (μmol/L) | 31.0 | 44.0-97.0 |
| UA (μmol/L) | 192.60 | 150.0-450.0 |
| hs-CRP (mg/L) | 13.85 | 0.68-0.88 |
| GLU (mmol/L) | 6.21 | 3.90-6.10 |
| TP (g/L) | 60.30 | 60.0-85.0 |
| ALB (g/L) | 35.0 | 32.0-55.0 |
| TB (μmol/L) | 7.20 | 1.70-17.10 |
| DB (umol/L) | 2.50 | 0-7.0 |
| IB (μmol/L) | 4.70 | 1.70-10.20 |
| AST (IU/L) | 90.60 | 0-45.0 |
| ALT (IU/L) | 107.10 | 0-40.0 |
| TC (mmol/L) | 5.28 | 2.84-5.68 |
| TG (mmol/L) | 1.55 | 0.60-2.20 |
| HDL-C (mmol/L) | 0.82 | 1.14-1.91 |
| LDL-C (mmol/L) | 3.78 | 2.0-3.10 |
| HCY (μmol/L) | 8.61 | 5.0-15.0 |
| Urine test | ||
| SG | 1.025 | 1.01-1.03 |
| PH | 7 | 5-8 |
| PRO (g/L) | Negative | Negative |
| GLU (mmol/L) | Negative | Negative |
| KET (mg/L) | Negative | Negative |
| BIL (μmol/L) | Negative | Negative |
| Stool test | ||
| RBC (piece/HP) | 0 | 0 |
| OBT | Negative | Negative |
| WBC (piece/HP) | 0 | 0-1 |
| Myocardial enzyme test | ||
| CK-MB (μg/L) | < 0.001 | 0-18.0 |
| Mb (μg/L) | 18.55 | 10.0-70.0 |
| cTnI (μg/L) | 0.009 | < 0.04 |
| Coagulation test | ||
| PT (s) | 10.20 | 10.50-14.0 |
| APTT (s) | 22.90 | 21.0-34.0 |
| TT (s) | 18.0 | 14.0-20.0 |
| FIB (g/L) | 2.50 | 2.0-4.0 |
| INR | 0.94 | 0.80-1.20 |
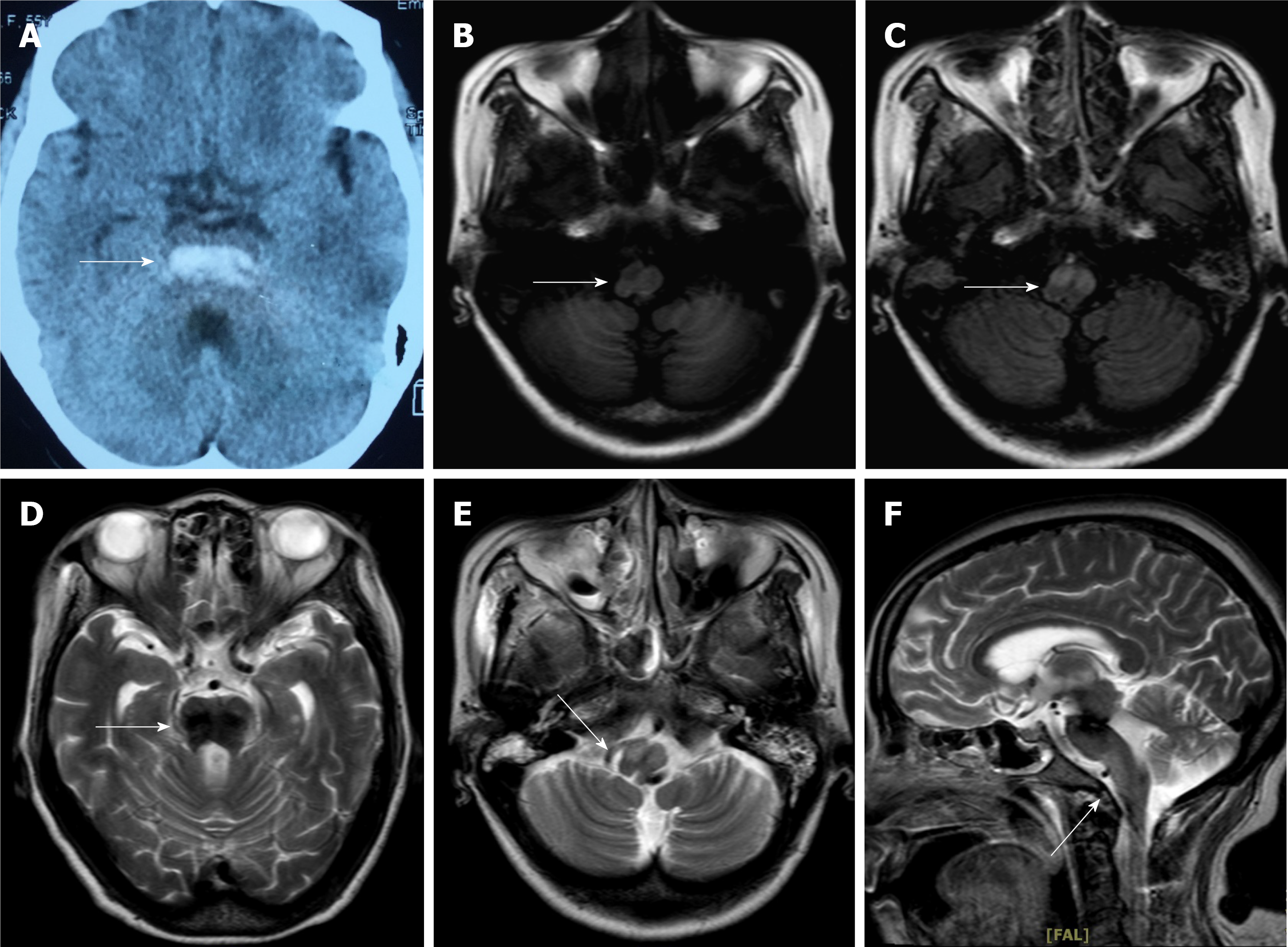
Figure 1A showed that hemorrhage was covered throughout the length of the pontine tegmentum by non-contrast computed tomography 3 mo ago. The results of brain MRI upon admission showed residual hemosiderin in the area of the hemorrhage (Figure 1B) and bilateral symmetrical hypertrophy in the bilateral inferior olivary nuclei. On a T1-weighted MRI, signal intensity was hypointense in these lesions (Figure 1C), which increased on a T2-weighted and a fluid-attenuated inversion recovery sequence MRI (Figure 1D-1F). No obvious thrombus was observed on vascular ultrasound.
Pontine hemorrhage; Bilateral HOD; Hypertension.
We consulted with doctors from departments of neurosurgery, vascular surgery, nutrition, rehabilitation and pharmacy. The patient was given symptomatic and supportive treatment. She was treated with enteral nutrition by nasal feeding, gabapentin (300 mg, two times per day), memantine (10 mg, two times per day) and trihexyphenidyl (10 mg, two times per day) orally. We asked the nurses to turn the patient over and pat on her back to prevent complications. By consultation with physiatrists, rehabilitation physiotherapy, such as the joint movement by the passive aids machine, body massage for 3 times a day, was added to the treatment plan. At the same time, we worked with the patient’s family to carry out psychotherapy and were concerned about her changes. She had established confidence in treatment.
The patient was discharged from the hospital when her symptoms improved, and the edema of the right lower limb had disappeared. Hereafter, we maintained telephone communication with her, and we asked her to take medication and rehabilitation on time. Despite therapy for three months, the patient’s tremor only improved a little, while her extremities symptoms remained unchanged. Four months later, she had difficulty reading and felt dizzy. Since then, she felt depressed and useless, responded slowly, and had no interest in what she used to like. We told her to go to a hospital for follow-up treatment immediately. Later, the patient couldn't be reached by phone anymore.
Bilateral HOD after pontine hemorrhage is rarely observed in clinics. The causes of HOD can be hypertension, vascular malformation, heavy surgery, brain trauma, brainstem tumor, hemorrhagic or ischemic stroke. In addition, ischemia and demyelination can lead to the development of the disease[3-5]. According to neuroanatomical analysis, HOD is trans-synaptic degeneration at the site of DROP. Changes in neuroanatomy have played an important role in the manifestations of the disease. The DROP is composed of three structures, which are the ipsilateral inferior olivary nucleus (ION), the ipsilateral red nucleus, and the contralateral dentate nucleus. The ION is connected to the contralateral dentate nucleus via the inferior cerebellar peduncle, the ipsilateral red nucleus is connected to the ION via the central tegmental tract, and the contralateral red nucleus and the dentate nucleus are connected by the superior cerebellar peduncle[1]. Many of the nerve fibers from dentate nucleus to inferior olive are primarily inhibitory. The results in loss of inhibitory control with consequent hyperactivity of the olivary neurons lead to abnormal involuntary movements. Furthermore, the main pathological features of HOD are often cytoplasmic vacuolation and the neural cell body enlargement, while the ischemia and demyelination could be occasional, which were first described by Oppenheim in 1887[6]. A study by Dogan et al[7] showed that an unusual symptomatic tremor, which is the Holmes Tremor (HT), is characterized by the combination of rest and intention tremor. The pontine-midbrain hemorrhage may be considered to cause the HOD and HT to spread to the upper and lower extremities by influencing anatomy structure in the Guillain-Mollaret triangle. When damage occurs in the red nucleus or the central tegmental tract, the ipsilateral ION would degenerate. On the contrary, when the dentate nucleus or the superior cerebellar peduncle is affected, degeneration would happen in the contralateral ION. If the damage occurs in both the central tegmental tracts and the bilateral superior cerebellar peduncle, bilateral HOD happens[8]. In some cases, the lesion is located in the dorsal pons of the midline and the left DROP, which also causes bilateral HOD[9-11]. In general, these structures are interconnected and may influence each other functions.
Another study confirmed that a 31-year-old female developed HOD after pontine cavernoma surgery. She had the most classic symptom and MRI results of HOD[12]. Our case had a similar situation. The patient had the pontine hemorrhage, and her lower extremities dyskinesia and the swelling of the right leg were complications caused by the pontine hemorrhage. The characteristic clinical manifestations of HOD include palatal myoclonus and nystagmus, which may be the reason for a loss of inhibitory control of DROP. The hyperactivity of the olivary neurons then leads to rhythmic involuntary movements. We found these positive signs through careful physical examination in this case. Patients with HOD may develop ataxia, diplopia, dysarthria, and diplopia denta torubral tremor in the upper extremities, but not all patients have these symptoms[2,7]. Palatal myoclonus as a typical sign of this disease does not always exist[9,13]. Some patients’ tremors may only be observed in physical examinations, which makes it critical to monitor the occurrence of tremor phenomenology, even months after the initial insult[14]. An analysis by Suner et al[15] confirmed that brief weekly measurements with an eye-tracker may allow early detection of HOD. To sum up, when encountered with these abovementioned clinical manifestations, we need to consider HOD. In the meantime, the key to the diagnosis of HOD is the recognition of the results of the head MRI. Research has demonstrated that HOD was detected following primary neurologic insult, but no change in clinical symptoms was observed within a mean of 7.2 mo[16]. The feature is the enlargement of the ION without any contrast enhancement and T2 hyperintensity. In addition, another study has clarified that pontine-midbrain hemorrhage may cause delayed onset of HT thus delaying the appearance of HOD. In such cases, MRI should be referred to[7]. Some researchers have analyzed the results of MRI and offered three phases. Phase 1: the signal of the ION in T2-weighted does not change in 6 mo, but it increases after 3 wk in other regions; Phase 2: the signal of ION hypertrophy and hyperintensity persists for approximately 3–4 years on T2-weighted; and phase 3: the signal increases on T2-weighted for a long time with the disappearance of ION hypertrophy[1]. All in all, the diagnosis of HOD is difficult sometimes, which makes it important to monitor the clinical manifestations and identify the changes in MRI results. In our case, the patient’s MRI result conformed to the first phase of HOD, and her ION had hypertrophy degeneration bilaterally.
The treatment of HOD is generally the use of gabapentin, memantine, botulinum toxin injections, and deep brain stimulation. Psychotherapy is also a good treatment option for people with long-term illnesses. However, some patients showed self-limiting recovery, and excessive treatments are not necessary in such cases. Suffering from palatal or oculopalatal tremor usually last for life, but a few patients may have improved symptoms after many years[5]. Because of the uncertainty of the pathological mechanism of HOD, the treatment effect is not satisfactory. Our patient underwent pontine hemorrhage rehabilitation at the same time with the treatment of HOD, which turned out to be beneficial for the HOD treatment. So far, the pathological mechanism of HOD is still unclear, and more studies need to be done about bilateral HOD after pontine hemorrhage.
Overall, bilateral HOD is a rare phenomenon after pontine hemorrhage. In order to diagnose HOD as soon as possible, it is critical to monitor the patient’s clinical manifestations and the MRI results. The comprehensive treatment of HOD is based on the symptoms. The drugs, rehabilitation and psychotherapy are most commonly used. Further research is required to clarify the pathological mechanism of HOD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Ozair A S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, Montanera W, Willinsky R, Mikulis D. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol. 2000;21:1073-1077. [PubMed] |
| 2. | Carr CM, Hunt CH, Kaufmann TJ, Kotsenas AL, Krecke KN, Wood CP. Frequency of bilateral hypertrophic olivary degeneration in a large retrospective cohort. J Neuroimaging. 2015;25:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Marden FA. Hypertrophic olivary degeneration due to pontine hemorrhage. JAMA Neurol. 2013;70:1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Menezes Cordeiro I, Tavares JB, Reimão S, Geraldes R, Ferro JM. Hypertrophic olivary degeneration after pontine hemorrhage: a cause of delayed neurological deterioration. Cerebrovasc Dis. 2013;36:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Tilikete C, Desestret V. Hypertrophic Olivary Degeneration and Palatal or Oculopalatal Tremor. Front Neurol. 2017;8:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Pandey P, Westbroek EM, Gooderham PA, Steinberg GK. Cavernous malformation of brainstem, thalamus, and basal ganglia: a series of 176 patients. Neurosurgery. 2013;72:573-89; discussion 588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Dogan SN. Hypertrophic Olivary Degeneration and Holmes Tremor: Case Report and Review of the Literature. World Neurosurg. 2020;137:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Gatlin JL, Wineman R, Schlakman B, Buciuc R, Khan M. Hypertrophic olivary degeneration after resection of a pontine cavernous malformation: a case report. J Radiol Case Rep. 2011;5:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Akar S, Drappatz J, Hsu L, Blinder RA, Black PM, Kesari S. Hypertrophic olivary degeneration after resection of a cerebellar tumor. J Neurooncol. 2008;87:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Macht S, Hänggi D, Turowski B. Hypertrophic olivary degeneration following pontine cavernoma hemorrhage: a typical change accompanying lesions in the Guillain-Mollaret triangle. Klin Neuroradiol. 2009;19:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Gerace C, Fele MR, Luna R, Piazza G. Neurological picture. Bilateral hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry. 2006;77:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Yang J, Yang J, Li B, Bao L. Transient Palatal Tremor and Action Induced Foot and Leg Dystonia due to Hypertrophic Olivary Degeneration: a Case Report. J Stroke Cerebrovasc Dis. 2020;29:105147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Lapresle J. Rhythmic palatal myoclonus and the dentato-olivary pathway. J Neurol. 1979;220:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Bird K, Saint-Hilaire M, Curiale G, O'Shea SA. Development of Hypertrophic Olivary Degeneration following Pontine Hemorrhage. Ann Neurol. 2020;87:809-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Suner M, Prusky GT, Carmel JB, Hill NJ. Longitudinal Quantification of Eye-Movement Impairments after Pontine Hemorrhage. Front Neurol. 2017;8:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Onen MR, Moore K, Cikla U, Ucer M, Schmidt B, Field AS, Baskaya MK. Hypertrophic Olivary Degeneration: Neurosurgical Perspective and Literature Review. World Neurosurg. 2018;112:e763-e771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |