Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.254
Peer-review started: March 11, 2021
First decision: May 11, 2021
Revised: May 20, 2021
Accepted: July 12, 2021
Article in press: July 12, 2021
Published online: January 7, 2022
Processing time: 294 Days and 4.9 Hours
There has been no report to use camrelizumab with chemotherapy for advanced bladder cancer patients with positive programmed death-ligand 1 (PD-L1) expression and high tumor mutational burden (TMB). More effective predictors of bladder cancer immunotherapy have yet to be explored, and the combination of multiple factors may be more predictive than a single factor.
We report the case of a 74-year-old male patient with recurrent metastatic bladder cancer, which demonstrated positive PD-L1 expression and high TMB. The immune checkpoint inhibitor camrelizumab was administered to the patient in combination with gemcitabine and cisplatin. The patient achieved a partial response with a progression-free survival of 11 mo.
This is the first report to use camrelizumab with chemotherapy for advanced bladder cancer patients with positive PD-L1 expression and high TMB.
Core Tip: Eighty percent of patients with positive programmed death-ligand 1 (PD-L1) expression are unable to benefit from immunotherapy. Herein, we report the case of a 74-year-old male patient with recurrent metastatic bladder cancer, which demonstrated positive PD-L1 expression and high tumor mutational burden. The immune checkpoint inhibitor camrelizumab was administered to the patient in combination with gemcitabine and cisplatin. The patient achieved a partial response with a progression-free survival of 11 mo. More effective predictors of bladder cancer immunotherapy have yet to be explored, and the combination of multiple factors may be more predictive than a single factor.
- Citation: Xie C, Yuan X, Chen SH, Liu ZY, Lu DL, Xu F, Chen ZQ, Zhong XM. Successful response to camrelizumab in metastatic bladder cancer: A case report. World J Clin Cases 2022; 10(1): 254-259
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/254.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.254
Multiple studies have demonstrated that blocking of programmed death 1 (PD-1) or its ligand programmed death-ligand 1 (PD-L1) improved anti-tumor activity in metastatic urothelial cancer patients with disease progression after standard chemotherapy[1-3]. However, only a subset of patients is sensitive to immunotherapy. In addition to microsatellite instability (MSI) status and PD-L1 expression, tumor mutational burden (TMB), the third approved biomarker for immunosuppressant, could be used to predict the efficacy of immunosuppressive agents. Recently, increasing studies have indicated that there were no overlapping effects of PD-L1 expression and TMB on the response rate to PD-1/PD-L1 inhibitors across distinct tumor types, therefore they can be broadly used to categorize the immunologic subtypes of cancer[4].
Camrelizumab, as an anti-PD-1 inhibitor, provided an improved objective response rate (ORR) and disease control rate (DCR) in pre-treated patients with dMMR/MSI-high (MSI-H) and advanced or metastatic solid tumor. Several clinical trials of camrelizumab for advanced urinary system tumors such as NCT03827837 have been launched. It brings more treatment options to patients with an advanced or metastatic solid tumor, including bladder cancer. Herein, we report the case of a recurrent bladder cancer patient with positive PD-L1 expression and high TMB. The patient received camrelizumab in combination with chemotherapy and achieved a partial response (PR).
A patient presented with recurrent metastatic bladder cancer (metastatic urothelial carcinoma, mUC), which demonstrated positive PD-L1 expression and high TMB.
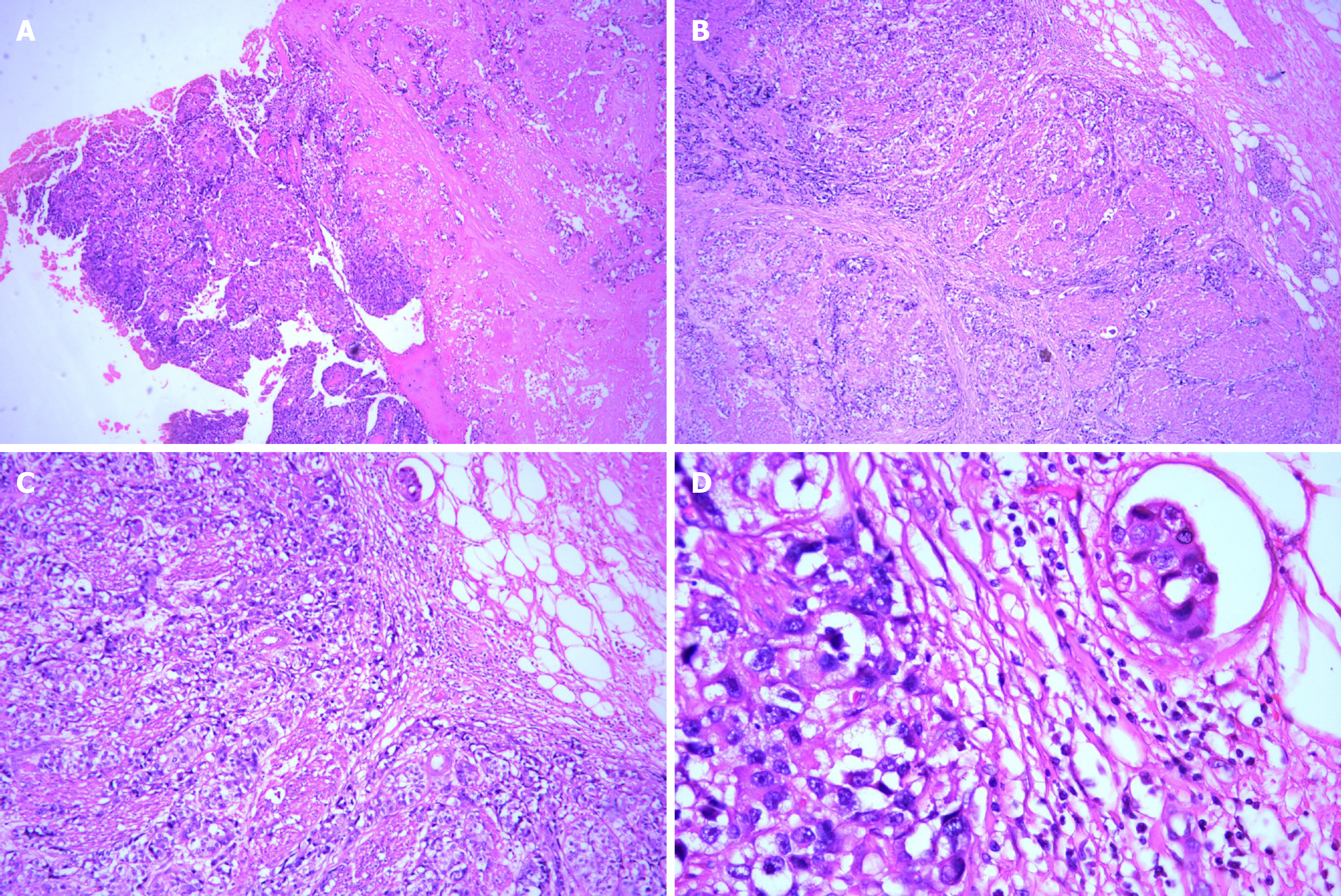
A 74-year-old male patient, with a history of high-grade urothelial carcinoma of the bladder, was initially presented to our hospital in August 2017 due to gross hematuria and dysuria. Recurrence of bladder cancer was declared by computed tomography (CT) imaging. The patient was subjected to radical cystectomy and lysis of pelvic adhesions. The pathological evaluation of the tumor sample indicated infiltrating high-grade urothelial carcinoma of the bladder which invaded the entire layer and surrounding adipose tissue (Figure 1). Two months after surgery, the patient had pain in the right pubic bone while magnetic resonance imaging (MRI) revealed recurrence of stage IV bladder cancer with bone metastases.
The patient had a free previous medical history.
Vital signs and general inspection included the head, neck, anterior torso, posterior torso, anterior chest, abdomen, male genitalia, gait, station, coordination. Our clinical consideration was the recurrence of bladder cancer.
Routine blood tests, routine urine tests and urinary sediment examination, routine fecal tests and occult blood tests, blood biochemistry, immune indexes, infection indexes immunohistochemistry (IHC) test, routine blood test genetic mutation profiling, and TMB evaluation were performed.
The plasma sample of the patient was subjected to genetic mutation profiling and TMB evaluation through next-generation sequencing (NGS) with a panel consisting of 520 cancer-related genes (Burning Rock Biotech, Guangzhou, China). The sequencing identified a total of 19 somatic mutations, including PIK3CA (p.Glu545Lys) andARID1A (p.Ser1948fs) mutations, and RAF1 amplification (CN = 3.6). The TMB level was 19.8 mutations per Mb. Furthermore, IHC on tissue samples indicated positive PD-L1 protein expression with a tumor proportion score (TPS) of 20%.
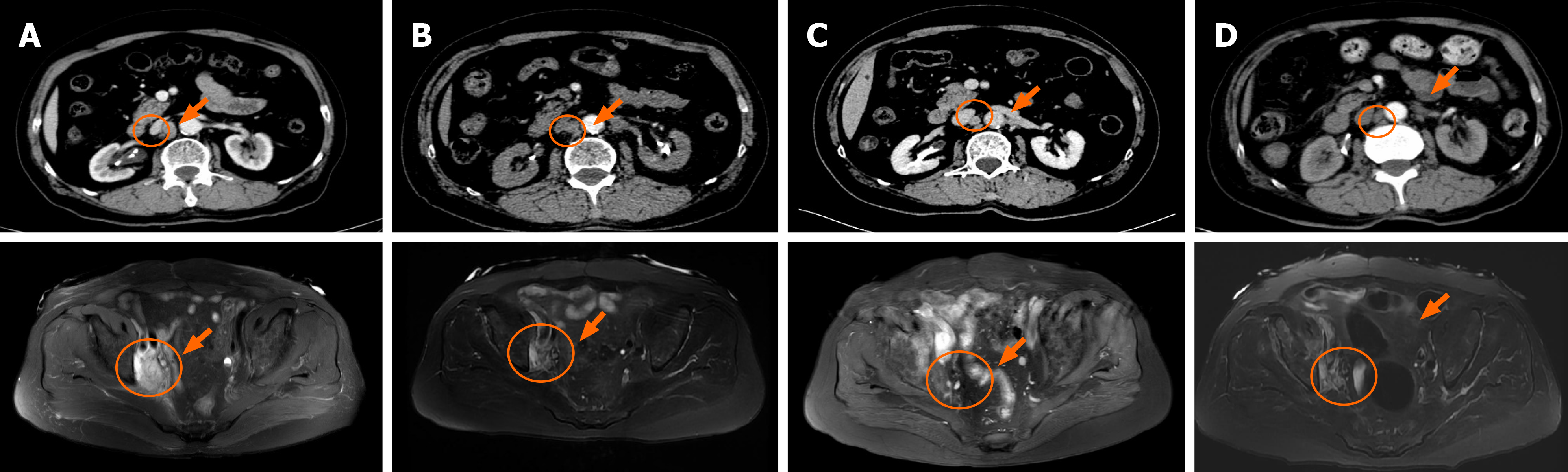
CT imaging and MRI were performed. In September 2019, the reexamination of abdominal CT identified multiple newly appeared swollen retroperitoneal lymph nodes in which the largest node had a diameter of about 1.7 cm (Figure 2A). After three treatment cycles, the patient achieved a PR as the repeated CT scan on December 20, 2019 showed a 53% decrease in the target lesion (Figure 2B). Then, the patient continued camrelizumab (200 mg, D0, q3w) and gemcitabine (1 g, D1, D8, q3w) and maintained the PR (Figure 2C and D).
Recurrence of high-grade urothelial carcinoma of the bladder with bone metastases and multiple newly appearing swollen retroperitoneal lymph nodes.
Palliative radiotherapy in the metastatic site was given to the patient. In May 2018, the patient had pain of the right ilium and the symphysis pubis and received palliative radiotherapy followed by four cycles of chemotherapy with paclitaxel liposome (210 mg Q3W) from July 10, 2018 to September 12, 2018. Based on the high TMB level and positive PD-L1 expression, the patient was administered with immunotherapy of camrelizumab (200 mg, D0, q3w) in combination with chemotherapy including gemcitabine (1 g, D1 and D8, q3w) and cisplatin (40 mg, D1-D3, q3w) since October 2019.
Since the patient had pain in the right pubic bone with bone metastases, palliative radiotherapy in the metastatic site was given to the patient, followed by four cycles of chemotherapy with paclitaxel liposome (210 mg Q3W). Mild gastrointestinal reactions developed during paclitaxel liposome and immunotherapy of camrelizumab (200 mg, D0, q3w) in combination with chemotherapy with gemcitabine (1 g, D1, D8, q3w) and cisplatin (40 mg, D1-D3, q3w). Oppression in the chest, grade 1 myeloid suppression, and abnormal liver and kidney function occurred during the first gemcitabine and cisplatin chemotherapy, and gemcitabine dose was therefore reduced. In addition, abnormal liver function, moderate anemia, and mild gastrointestinal reactions occurred during the immunotherapy with camrelizumab and chemotherapy with gemcitabine and cisplatin. The patient maintained PR. The latest follow-up in October 2020 showed the progression of the tumor. The total progression-free survival (PFS) of the patient since camrelizumab therapy was 11 mo.
The application of immunotherapy has thoroughly promoted the progress of cancer treatment in recent years. However, 80% of patients with positive PD-L1 are unable to benefit from immunotherapy. Hitherto FDA has approved five drugs as immunotherapies for bladder cancer, but the ORRs of these drugs in bladder cancer are 13.4%-21.1%[5]. In clinical trials of camrelizumab in advanced solid tumors, some bladder carcinoma patients got a complete response[6]. Camrelizumab provided improved ORR and DCR in pre-treated patients with dMMR/MSI-H and advanced or metastatic solid tumor[7,8]. With the increase of approved immune checkpoint inhibitors, it is urgent to define patient subgroups that are more likely to have treatment benefits. PD-L1, as the only effective biomarker for prediction, is far from being enough.
High tumor mutation may lead to high neoantigen load and increased immunogenicity, which could promote recognition and killing abilities of the immune system. Multiple studies have shown that TMB is associated with the efficacy of mUC immunotherapy. A study indicated that tumor mutational load predicts survival after immunotherapy in mUC by the TMB cutoff of 17.6% or the cutoff of 25% by quartering[9]. The updated result of the PURE-01 study revealed that TMB may predict the pathological response to pembrolizumab in patients with muscle-invasive bladder cancer while a pretreatment TMB of 15 mutations/Mb was predictive of pathological complete response[10]. Another study showed that higher TMB levels revealed improved overall survival (OS) and lowered tumor recurrence[11]. Based on many relevant clinical studies, On June 16, 2020, the Food and Drug Administration approved pembrolizumab for the treatment of adult and pediatric patients with unresectable or metastatic TMB-high [≥ 10 mutations/megabase (mut/Mb)] solid tumors.
In addition, the NGS results of the patient revealed an inactivated nonsense mutation in ARID1A (p.Ser1948fs) which results in the loss of ARID1A protein expression and indicates potential treatment response to immunotherapy. ARID1A mutation has a high incidence in tumors, and it can increase the efficacy of tumor immunotherapy through multiple mechanisms of action, including MSI-H phenotype, high TMB, upregulation of PD-L1 expression, and immune activated tumor microenvironment. The largest study of 17486 patients with gastrointestinal tumors has demonstrated selective DNA damage repair (DDR) defects and ARID1A defects were associated with high TMB. In general, ARID1A was inactivated with increased TMB and low gene copy number, suggesting that it may be an indicator of immunotherapy[12]. Studies of immunotherapy for advanced urothelial carcinoma have shown that mutations in the DDR gene are associated with improved PFS and OS[13]. The DDR gene is an independent factor associated with the efficacy of immunosuppressive therapy in mUC. In addition to high TMB (19.8 mutations per Mb) and positive PD-L1 expression (TPS 20%), the treatment benefit of camrelizumab observed in our case may also be explained by the change of the DDR gene. Hence, the patient received immunotherapy in combination with chemotherapy and achieved a PR with a PFS of 11 mo.
More effective predictors of bladder cancer immunotherapy have yet to be explored, and the combination of multiple factors may be more predictive than a single factor.
Here, we report a Chinese patient with advanced bladder cancer showing effective treatment response to camrelizumab in combination with chemotherapy. This is the first case report on the treatment effect of camrelizumab for advanced bladder cancer with positive PD-L1 expression and high TMB. Our study suggested that TMB, PD-L1 expression, and DDR gene mutation can be used in combination to predict treatment effects of immunotherapy. More studies are needed to develop effective biomarkers and identify appropriate patient subgroups that are more likely to benefit from cancer immunotherapies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bellini MI, Desai DJ, Sachdeva S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Grayson M. Bladder cancer. Nature. 2017;551:S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Berdik C. Unlocking bladder cancer. Nature. 2017;551:S34-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother. 2020;69:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 4. | Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, Zaidi N, Azad NS, Laheru DA, Frampton GM, Jaffee EM. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 406] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 5. | Yoon HS, Kwak C, Kim HH, Kim HS, Ku JH. Second-Line Systemic Treatment for Metastatic Urothelial Carcinoma: A Network Meta-Analysis of Randomized Phase III Clinical Trials. Front Oncol. 2019;9:679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Zhang H, Chi Y, Yang Q, Xu B. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 7. | Chen J, Quan M, Chen Z, Zeng T, Li Y, Zhou Y, Hai Y, Gao Y. Camrelizumab in advanced or metastatic solid tumour patients with DNA mismatch repair deficient or microsatellite instability high: an open-label prospective pivotal trial. J Cancer Res Clin Oncol. 2020;146:2651-2657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Seront E, Rottey S, Sautois B, Kerger J, D'Hondt LA, Verschaeve V, Canon JL, Dopchie C, Vandenbulcke JM, Whenham N, Goeminne JC, Clausse M, Verhoeven D, Glorieux P, Branders S, Dupont P, Schoonjans J, Feron O, Machiels JP. Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol. 2012;23:2663-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D'Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2239] [Cited by in RCA: 2802] [Article Influence: 467.0] [Reference Citation Analysis (0)] |
| 10. | Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R, Montironi R, Giannatempo P, Farè E, Pederzoli F, Bandini M, Bianchi M, Colombo R, Gandaglia G, Fossati N, Marandino L, Capitanio U, Dehò F, Ali SM, Chung JH, Ross JS, Salonia A, Briganti A, Montorsi F. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol. 2020;77:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 11. | Zhang C, Shen L, Qi F, Wang J, Luo J. Multi-omics analysis of tumor mutation burden combined with immune infiltrates in bladder urothelial carcinoma. J Cell Physiol. 2020;235:3849-3863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Hu G, Tu W, Yang L, Peng G. ARID1A deficiency and immune checkpoint blockade therapy: From mechanisms to clinical application. Cancer Lett. 2020;473:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, Cipolla CK, Bluth MJ, Chaim J, Al-Ahmadie H, Snyder A, Carlo MI, Solit DB, Berger MF, Funt S, Wolchok JD, Iyer G, Bajorin DF, Callahan MK, Rosenberg JE. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol. 2018;36:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 417] [Article Influence: 59.6] [Reference Citation Analysis (0)] |