Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.23
Peer-review started: April 26, 2021
First decision: May 27, 2021
Revised: June 14, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: January 7, 2022
Processing time: 247 Days and 23.4 Hours
Colorectal cancer (CRC) is presently the second most prevalent global mortality-inducing cancer. CRC carcinogenesis is a multifactorial process involving internal genetic mutations and the external environment. In addition, non-neoplastic cell activities within tumor microenvironments for CRC development have been established. However, interleukin (IL)-33, secreted by such cell types, plays a pivotal role in cancer progression due to interaction with cellular constituents within the tumor-inflammation microenvironment. IL-33 belongs to the IL-1 cytokine family and acts as binding attachments for the suppressor of tumorigenicity (ST)2 receptor. Therefore, how to coordinate tumor microenvironment, design and optimize treatment strategies suitable for CRC, based on IL-33/ST2 signal is a challenge. Even though it has established influences upon immunity-linked conditions, IL-33 effects on CRC progression and prevention and related mechanisms are still controversial. Our review depicts controversial activities for IL-33/ST2 within carcinogenesis and cancer prevention. Moreover, IL-33/ST2 signaling is a potential therapeutic target for CRC.
Core tip: Interleukin (IL)-33 belongs to the IL-1 cytokine family and binds to the suppressor of tumorigenicity (ST)2 receptor. IL-33 plays a key role in cancer progression due to interaction with cellular components in the inflammatory microenvironment of tumors. Therefore, it is a challenge to design and optimize treatment strategies suitable for colorectal cancer (CRC) based on IL-33/ST2 signaling to coordinate the tumor microenvironment. IL-33 also has effects on CRC prevention. These findings implicate multifaceted roles of IL-33 in cancer treatment.
- Citation: Huang F, Chen WY, Ma J, He XL, Wang JW. Paradoxical role of interleukin-33/suppressor of tumorigenicity 2 in colorectal carcinogenesis: Progress and therapeutic potential. World J Clin Cases 2022; 10(1): 23-34
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/23.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.23
During 2020 alone, approximately 19.3 million newly diagnosed cancer cases were recorded, together with nearly 10 million global cancer mortalities[1]. Stemming from such statistics, colorectal cancer (CRC) represents the third most prevalent tumor (10%), and the second most prevalent global mortality-inducing cancer (9.4%)[1]. Approximately 10% of all CRC cases are inherited, with over 90% being sporadically randomized. In general, tumor initiation and development are primarily determined by key factors, such as genetic instability, epigenetic changes, antiapoptotic activity, immune-system circumvention, invasiveness, and metastases[2]. Three mechanisms of genetic instability in sporadic CRC have been identified: CpG-island methylation phenotype, chromosomal-based imbalances, and microsatellite instabilities. In particular, it is worth mentioning that several risk factors are related to CRC development, including lack of exercise, smoking, and red meat and alcohol consumption[3]. In addition, obesity, type 2 diabetes and inflammatory bowel disease (IBD) are highly linked to exacerbated CRC development. The parts played by non-neoplastic cells within tumor microenvironments (TMEs) for cancer development have been identified[2,4]. The cytokines, growth factors, and hormones secreted by these non-neoplastic cells are pivotal in cancer progression by interaction with the cellular constituents within tumor inflammation microenvironments[5]. Such cytokines include interleukin (IL)-33, a member of the IL-1 cytokine superfamily, which has been shown to mainly invoke T-helper (Th)2 immune response activities by means of its suppressor of tumorigenicity (ST)2 receptor[6]. IL-33/ST2 signal transduction is involved in IBD, maintenance of tissue homeostasis, and tumor invasion[7,8]. IL-33 can be pro- or antitumorigenic in CRC, with both activities indicating that IL-33 plays vital roles in enrolling immune-system cell types to modulate TMEs. In this review, IL-33/ST2 involvement in colorectal carcinogenesis, progress and therapeutic potential are discussed.
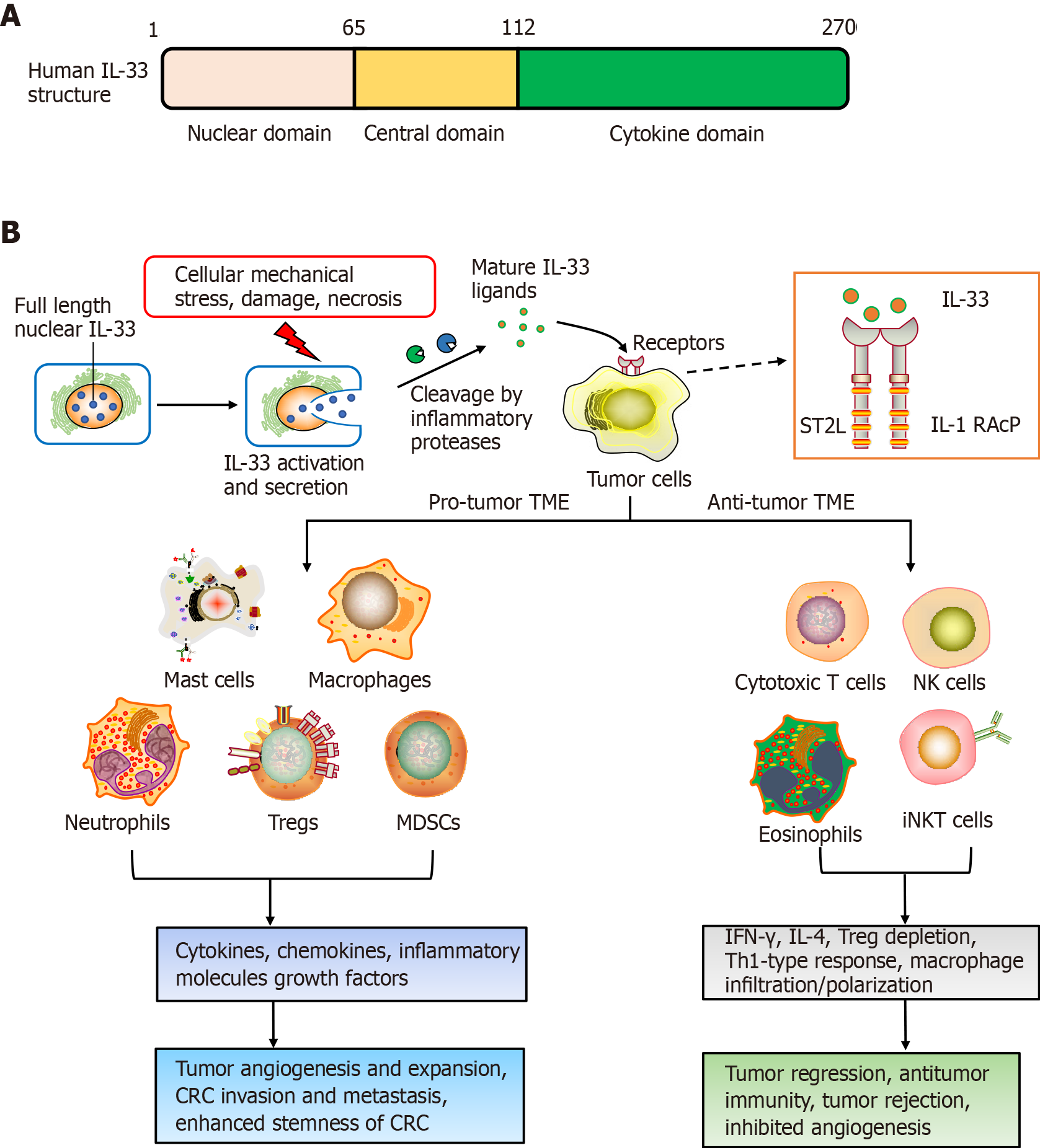
IL-33 was first identified in 2003. It is highly upregulated within hypertrophic veins as a nuclear protein, and given its first name, nuclear factor, from high endothelial venules[9]. Later in 2005, IL-33 was recognized as a member of the IL-1 cytokines family[6]. Meanwhile, IL-33 was recognized to be a ligand for ST2 receptor[6]. The molecular full-length weight for human IL-33 is 30 kDa. This cytokine has 270 amino acid residues, while murine IL-33 has only 266[6]. Human IL-33 consists of three domains: the N-terminal (aa 1–65), which is important for chromatin-binding and nuclear localization; central (aa 66–111), which interacts with nuclear factor-B; and C-terminal IL-1-like cytokine domain (aa 112–270), including the region binding to ST2[10]. After synthesis, IL-33 is passively released following cellular mechanical-stress/damage triggers[11]. Meanwhile, the precursor protein IL-33 is cleaved to produce the 10-fold-active matured version, compared to full-length IL-33, and is segmented to effectively activate group 2 innate lymphoid cells (ILC2s)[12,13].
The ST2 receptor was first recognized as an oncogene in murine fibroblasts[14,15]. It has been investigated for many years before establishment of ligand IL-33, so ST2 was previously considered to be orphan receptor. The ST2 receptor derives from IL-1RL, which is a type-1 transmembrane protein[14]. Four ST2 isoforms are produced by alternative splicing, such as ST2L (ligand), sST2, ST2V (variant), and ST2LV (ligand variant). ST2L is a membrane-anchored receptor similar to IL-1, having three immunoglobulin-like extracellular, transmembrane domains, together with IL-1R1-like intracellular domains[16,17]. sST2 is a soluble-secreted isoform of ST2 that has no transmembrane domain, although it carries an extracellular domain as ST2L, with 5–9 extra amino acids on the C terminus in humans and mice[16,18]. ST2V resembles sST2 and lacks the third extracellular domain, although it has a hydrophobic tail instead of a third immunoglobulin-like domain[19]. ST2LV is an additional soluble isoform with no transmembrane domain[20]. ST2L and sST2 have been thoroughly investigated, altthough knowledge is scarce about ST2V and ST2LV. ST2L is typically expressed on fibroblasts, mast cells, Th2 lymphocytes, dendritic cells and macrophages, and sST2 is mainly present on fibroblasts/epithelial cells[20].
Like most malignant tumors, CRC carcinogenesis involves multiple factors and processes. For most sporadic CRC, the important causes of CRC carcinogenesis are adenomas, intestinal polyp deterioration, tumor suppressor gene APC (adenomatous polyposis coli) mutation and TME formation[21]. Recently, many studies have shown that IL-33/ST2 plays a vital role in CRC occurrence and progression[22]. Cui et al[23] reported that the IL-33/ST2 axis promoted the neoplastic transformation of human colorectal adenoma to CRC, which is closely correlated with increased IL-33 expre
Another study found that IL-33 acts as a mediator of intestinal polyposis and regulator of tumor stromal cell activation in Apc(min/+) mice, a genetic model of intestinal tumorigenesis[24]. In the Apc(min/+) polyps, IL-33 is expressed in tumor epithelial cells, and ST2 is related to two stromal cell types, subepithelial myofibroblasts and mast cells. Stimulation of IL-33 induces stromal cells to express components of the extracellular matrix and growth factors that promote tumor development and growth[24]. He et al[25] reported that epithelial IL-33 promotes intestinal tumorigenesis in Apc(min/+) mice with transgenic expression of IL-33 in intestinal epithelial cells through the expansion of ST2+ T regulatory (Treg) cells, Th2 cytokine production and alternative activation of macrophages. Conversely, loss of IL-33 or ST2 in Apc(min/+) mice inhibits tumorigenesis and tumor angiogenesis, and induces apoptosis in adenomatous polyps[24,25]. This suggests that IL-33 promotes the transition of adenomas and polyposis to CRC through the activation of tumor stromal cells and the formation of a protumorigenic microenvironment.
The TME plays important roles in triggering cancer. The alarm protein IL-33 has been shown to be involved in formation of the early TME and to influence carcinogenesis and progression. Pastille and colleagues used animal models and patient samples to suggest that IL-33/ST2 axis activity restricted effector CD8+ T cell functions in the CRC environment and promoted tumor growth in the colon[26]. In addition, IL-33 downregulates IL-17 and differentiation through forkhead box (FOX)P3, indicating an immunosuppressive environment during CRC tumorigenesis[26]. In murine models for colon cancer, IL-33 within tumor regions can recruit and activate macrophages into the microenvironment, leading to prostaglandin E2 upregulation, consequently exacerbating colon cancer stemness/progression[27]. IL-33/ST2 signaling can activate c-Jun and stem cell genes (NANOG, NOTCH3 and OCT3/4) to induce CRC stemness, eventually to promote carcinogenesis[27]. More importantly, Taniguchi et al[28] reported the potential role of IL-33 in regulating tumor-initiating cells, as well as the impact on stem cell–niche interactions, which is necessary for tumor progression, and highlighted the new role of IL-33 in promoting CRC stemness and carcinogenesis.
A major hallmark for CRC progression is chronic inflammation[29]. IL-33 is upregulated within serum of ulcerative colitis cases, and consequently involved in the development and maintenance of inflammation. Meanwhile, ulcerative colitis is intimately linked to CRC progression, indicating that IL-33 has a pivotal role in triggering colon tumors[30]. Kirsten and colleagues suggested that involvement of the IL-33/ST2 axis was critical for CRC progression using bone marrow chimera investigations. This is partly because activation of the IL-33/ST2 signaling pathway damages intestinal barrier integrity, inducing immune-system cells to express protumorigenic IL-6[31]. Therefore, there is now compelling evidence that IL-6 serum level is linked to late-stage CRC in patients, together with being a predictor for poor prognosis in CRC[32].
It is also reported that epidermal growth factor (EGF) is a powerful signaling molecule, affecting CRC progression and intestinal epithelial cell development[33-35]. IL-33 and ST2 expression profiles can be strongly stimulated by EGF, without increasing the extracellular secretion of IL-33. Consequently, IL-33 upregulation leads to CRC triggering, thus indicating that the EGF/IL-33/ST2 axis components are novel drug targets against CRC[36]. In addition, CRC triggering/progress can be influenced through the immune microenvironment[37-39]. Multiple investigations have indicated that IL-33 thwarts host-based tumor immunity, tumor stroma modulation and exacerbation of angiogenesis, thus contributing to IL-33 receptor ST2-driven CRC[40].
Recently, IL-33/Treg cell interaction has attracted increasing attention. An early study showed that IL-33 can promote Treg cell function in the colorectum, where FOXP3+ Treg cells are abundant[41]. Treg cells can resist dysregulated inflammatory responses, and consequently acquire tissue-specific survival and function. It is well known that TME-resident IL-33 and Treg cells in the TME are individually implicated within CRC progression, albeit this is still in dispute. IL-33/ST2 signaling exacerbates CRC progression through modulation of FOXP3+ Treg cell phenotypic features and curtailing IL-17 differentiation[26]. Furthermore, tumor-derived IL-33 can remodel the TME through the recruitment of CD11b+/GR1+ and CD11b+/F4/80+ myeloid cells and promote CRC growth and liver metastasis in mice, with the potential as a therapeutic target[42]. IL-33 also has a pivotal effect on Treg cell functional stability, with genetic deletion of IL-33 improving the effectiveness of cancer immunotherapies[43].
IL-33 is considered to have a cancer-promoting role because IL-33/ST2 axis induction leads to CRC carcinogenesis/development. However, another concern is that IL-33 has a paradoxical role. Selected studies have indicated that IL-33 has a less-known role of tumor suppressor within many malignant tumors[44,45].
The IL-33/ST2 signaling pathway usually induces Th2-cell-derived expression of IL-4, IL-5 and IL-13. IL-33 is also involved in cellular immunity-related responses through upregulating IL-4 and interferon (IFN)-γ by CD8+ T, invariant natural killer (NK)T cells and NK cells, together with amplifying Th1-oriented immune responses[46-48]. Thus, it shows that IL-33/ST2 signaling plays two roles in tumorigenesis – stimulating tumor growth or inhibiting tumor progression.
In aspects of cancer prevention, tumoral IL-33 overexpression increases antitumoral responses by the immune system, together with tumor rejection through activation of CD8+ T/NK cells[49]. Furthermore, Treg cell depletion synergizes with re-expression of IL-33 to contribute cancer-eliminating Th1-type immunity-related actions, implicating that IL-33 is a promising antitumor cytokine for immunotherapy[49]. Another study implied a protective role for IL-33/ST2 against CRC invasiveness and metastases, resulting in reduced colorectal tumor growth[50]. Malik et al[30] have demonstrated that IL-33-lacking mice are sensitive to colitis-associated cancer (CAC). Meanwhile, this study highlighted that IL-33, IgA, IL-1α and the microbiota are candidate drug targets against IBD/CAC.
Many studies have shed light on IL-33 functions, whereas the literature on ST2 in CRC is scarce. Antitumorigenic functions of the IL-33 receptor have been gradually explained in CRC since 2016. Akimoto and co-workers have reported that soluble sST2 negatively correlated with colon tumor malignant growth in vivo by modifying the TME[51]. They further revealed the mechanisms: sST2 inhibited IL-33-driven angiogenesis, macrophage infiltration/polarization, and Th1 and Th2 activities. Another study by Donnell and colleagues demonstrated that ST2L downregulation in colon cancer, together with elevated tumor grade, led to ST2L downregulation. Colon-tumor-resident ST2 knockdown led to increased tumor expansion in animal studies, with a decrease in IL-33-driven macrophage infiltration and enrollment through antagonizing chemokine CCL2[52]. This indicates that IL-33 has an antitumor function against CRC and the IL-33/ST2 axis exerts protective functions against colon-based tumor-triggering.
Consequently, negative functions for the IL-33/ST2 axis in CRC progression depend on its involvement in the induction of angiogenesis, regulation of anti-tumor-based immunity-related responses and TME modulation[53]. Additional studies are needed to validate the precise functions adopted by IL-33/ST2 signaling in CRC (Figure 1).
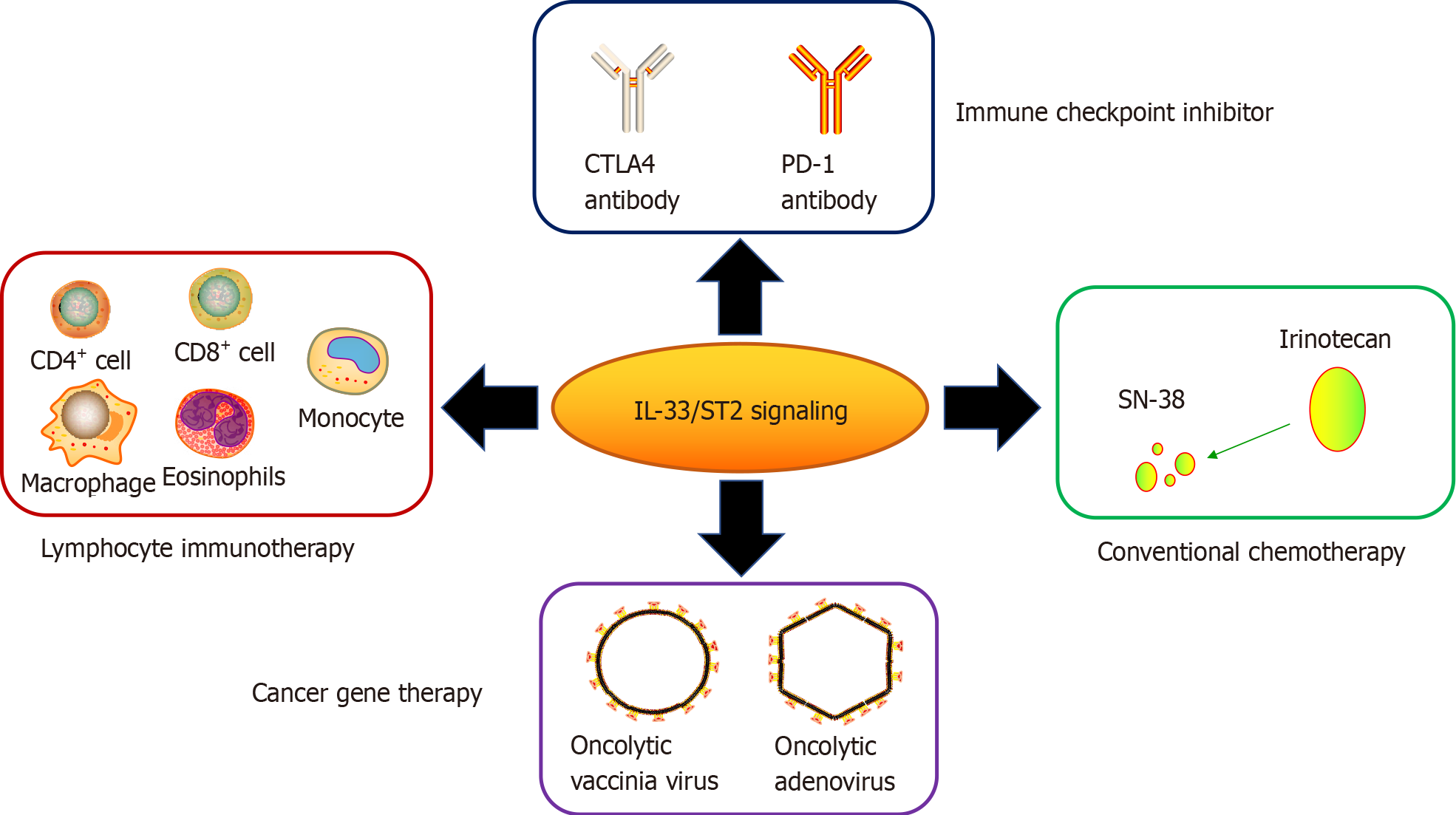
IL-33 is related to carcinogenesis, progression and poor prognosis in some cancers, including CRC[40]. Due to TME-resident IL-33/ST2 variability, their overexpression/ recombinant protein inhibits CRC expansion. This suggests the potential of the IL-33/ST2 axis as a drug target for CRC. Many studies have reported possible strategies for the treatment of CRC based on the IL-33/ST2 axis (Table 1).
| Type of therapeutic strategies | Pattern of IL-33/ST2 signaling involved | Drugs or cells used | Antitumor effects or mechanisms | Ref. |
| Conventional Therapy | Blockade of IL-33/ST2 signaling | Irinotecan, SN-38 | Alleviated mucositis, reduced tumor growth | [55,59,60] |
| Blockade of immune checkpoint | Exogenous IL-33 or its overexpression, ST2 depletion | PD-1 antibody | Activated CD8+ T cell cytotoxicity, tumor regression | [66,67] |
| Lymphocyte immunotherapy | Enhanced IL-33 expression | Tumor-infiltrating CD8+ T, IFN-γ+ CD4+ T cells, eosinophils, ILC2 | Upregulation of IFN-γ, antitumor immunity, inhibition of tumor expansion/metastasis | [69,71,73,76] |
| Cancer gene therapy | Overexpression of IL-33 | Oncolytic adenovirus, vaccinia virus | Oncolysis, inhibition of tumor growth, migration and tumor stem-cell activity | [77,78] |
Intestinal mucositis and severe diarrhea are commonly associated with cancer chemotherapy and are thus dose-limiting adverse effects. Combining radiation with conventional chemotherapy can exacerbate mucositis, leading to chemotherapeutic dose reductions or inevitable cessation of such treatments[54]. Since chemotherapy directly results in DNA damage/apoptosis through reactive oxygen species (ROS) and variation of cytokine production[55], as a proinflammatory factor, IL-33 has a pivotal part in driving inflammation/tumors through its ST2 receptor. One particular investigation highlighted that IL-33, in reduced doses, resisted chemotherapeutic platinum-drug-induced cell death and enhanced cellular invasiveness in selected tumors through JNK pathway triggering[56]. Thus, regulation of the IL-33/ST2 pathway can relieve inflammation/improve chemotherapy function.
Irinotecan (CPT-11) is a topoisomerase I inhibitor, and is an antitumor drug that can be used to treat metastatic CRC[57,58]. The clinical pharmacokinetics of CPT-11 and its metabolites such as SN-38 seem to be the key factor for optimal use of anticancer chemotherapeutic drugs[57]. CPT-11 systemic-based treatment causes intense mucosal disruption and diarrhea, coinciding with small intestinal IL-33 upregulation. However, the symptoms of mucositis were markedly lower within ST2-/-mice. Recombinant IL-33 protein reinforces CPT-11-driven mucositis, and blockade of IL-33 with its complementary antibody (or soluble ST2) significantly alleviates mucositis and reduces tumor growth by CPT-11 in a mouse model of CT26 colon cancer[59]. Such results indicate that thwarting the IL-33/ST2 axis can be exploited as a novel therapy against mucositis, consequently enhancing the beneficial effect of chemotherapy against CRC.
Immunotherapy represents a powerful method in cancer treatment. Stemming from this, immune checkpoint modulation has been broadly applied to treat multiple cancers, following the discovery of cytotoxic T lymphocyte-associated protein 4 and programmed cell death (PD)-1[60,61], which was awarded the 2018 Nobel Prize in Physiology or Medicine. Blockade of immune checkpoint yields promising clinical results in CRC. However, only a subset of cancer patients having an elevated microsatellite instability frequency phenotype develop durable antitumor immune responses due to the complicated TME associated with PD-1 and PD ligand (PD-L)1[62,63]. It can be explained from recent data that IL-33/ST2 can regulate PD-1/PD-L1 signaling within tumors. For example, exogenous IL-33 upregulated PD-1 by CD8+ T cells, together with upregulated PD-L1 within murine acute myeloid leukemia (AML) cells[64]. Thus, combining IL-33 with PD-1 antibody dramatically extends AML murine survival times in a CD8+ T-cell-based fashion, even leading to full regression within 50% of such treated mice. Another study showed that IL-33 triggered CD8+ T cells/ILC2s in pancreatic tumors, and activated ILC2s increased PD-1 expression. Subsequent combined treatment of IL-33 and PD-1 inhibition enhanced immunotherapy outcomes in a murine model[65].
Recent results showed that IL-33/ST2 act as candidate targets of checkpoint inhibitors for CRC immunotherapy, where they are secreted by lymphocytes, stromal cells and tumor cells to recruit immune cells and remodel the tolerogenic TME. A recent study reported that ST2 is specifically expressed in tumor-associated macrophages (TAMs) of CRC, and ST2 upregulation is related to low survival odds and reduced CD8+ T cell cytotoxicity in CRC[66]. They also found that ST2-positive TAMs were enrolled into CRC xenograft model tumors through chemokine receptor CXCR3, promoting an immunosuppressive TME. Thus, the combined effect of ST2 depletion using ST2-knockout mice and treatment with PD-1 antibody had a significant suppressive effect on CRC growth. The use of IL-33 trap fusion protein reduced tumor-infiltrating ST2+ TAMs and thwarted xenograft tumor expansion in CRC preclinical models. Thus, the IL-33/ST2 axis plays a big part in CRC immunotherapy.
Being an alarmin and immune regulation-related factor, IL-33 has a pivotal role in regulating the function of a wide range of immune cells. However, whether IL-33/ST2 signaling-regulated immune lymphocytes exert potential antitumor immunity in CRC is still a question under investigation. Recent research progress seems to suggest the positive reactivity based on Th1 cells (CD8+ T and NK cells) and Th2 cells (CD4+ T, ILC2 and eosinophils etc.).
Several studies indicated that exogenous or endogenous IL-33 is positively related to recruitment and CD8+ T/NK cell triggering within the TME. In melanoma or breast cancer models, exogenous application or transgenic expression of IL-33 recruits and activates (IFN-γ+ CD107+) CD8+ T and NK cells to orchestrate the TME, regulates xenograft tumor expansion and prevents lung metastasis of breast cancer in mice[49,67]. In the CRC model, Xia et al[68] found that overall antitumor responses/IFN-γ expression by tumor-infiltrating CD8+ T cells were impaired in IL-33-deficient mice. Conversely, IL-33 upregulated IFN-γ by activated CD4+/CD8+ T cells, improving CD8+ T cell infiltrative and antitumor responses against protumor effects by Treg cells. These results imply that the balance of CD8+ T cells and Treg cells within the TME is a crucial factor for IL-33-mediated anticancer responses in CRC.
In addition to activating Th1 response, IL-33 additionally modulate Th2 functions, including CD4+ T cells, ILC2s and eosinophils in the TME. IL-33 can directly target conventional and regulatory CD4+ T cells expressing ST2, and promote the immunosuppressive functions of Treg cells, which causes tumor growth and immune evasion[69]. IL-33 preferentially promotes Th2 response to modulate tumor immunity. In murine CT26 or MC38 CRC models, recombinant IL-33 markedly reduced colon tumor expansion/metastatic activity in lungs/liver[70]. IL-33 treatment can augment IFN-γ+ CD4+ T cells, together with upregulating CD40L on TILs. Moreover, IL-33 was found to be adequate for upregulating ST2 on CD4+ T cells, although not in CD8+ T/NK cells, suggesting that IL-33/ST2 signaling activates CD4+ T cells through positive-feedback looping.
Emerging studies have proved the positive role of eosinophils in mediating anticancer immunity-related counteractivity by IL-33 within several cancers, including CRC[71]. A more recent study by Kienzl and colleagues demonstrated that IL-33 can inhibit cancer expansion in CT26 engraftment/colitis-linked CRC mouse models[72]. The IL-33-induced effect was cancelled within eosinophil-lacking dblGATA-1 mice, although it was rescued through adoptive transfer of ex vivo-triggered eosinophils by IL-33[72]. They further found that IL-33 treatment upregulated eosinophil biomarkers associated with triggering and homing (CD11b and Siglec-F), and with degranulation (CD63 and CD107a) in vitro and in vivo. These results implied that eosinophils are a requisite for the antitumor effect of IL-33 in CRC. Moreover, IL-33 stimulation can enrich ILC2s in the TME of many cancers, and ILC2s also constitutively express ST2[73]. Thus, IL-33 targets directly ILC2s and induces ILC2 cell expansion, enrichment and activation in tumors[74]. Thus, it was proved that, in local expression of IL-33 in murine CRC, CT26 enhanced MyD88-based antitumor ILC2 activity[75]. In this study, IL-33 promoted production of CXCL2 from ILC2s, and created a TME with CXCR2-expressing tumor cells through a dysfunctional angiogenesis/hypoxia/ROS axis, which caused tumor cell-specific apoptosis. The finding highlights the vital role of ILC2s in the IL-33-mediated antitumor effect for CRC immunotherapy.
Recently, gene therapy using viral or nonviral vectors to carry therapeutic genes for diseases has attracted increased attention. In particular, breakthroughs have been made in the treatment of genetic diseases. Gene therapy also shows a promising prospect in the field of human cancer treatment. In our group, cancer gene therapy using oncolytic viruses as vectors has achieved encouraging results. We have con
There are at least two anti-IL-33 antibodies (SAR440340 and MEDI3506) being developed to treat chronic obstructive pulmonary disease, moderate-to-severe asthma, and chronic bronchitis in clinical phase I and II trials (NCT03387852, NCT03546907, NCT04751487, NCT04570657, NCT04701983, and NCT04631016). Thus, it suggests that the blockade strategy using anti-IL-33 antibodies has the potential for treatment of human cancer, including CRC, where IL-33 plays the protumorigenesis role.
IL-33 plays a controversial role in carcinogenesis, cancer prevention and cancer immunity, although the specific mechanism is still unclear. In CRC, the divergent roles of IL-33 may depend on the TME. Therefore, how to orchestrate the TME to design and optimize appropriate treatment strategies based on IL-33/ST2 signaling for CRC is an important question. These strategies include how to activate and recruit IFN-γ-secreting CD4+ and CD8+ T cells, NK cells, dendritic cells, M1 macrophages, eosinophils and ILC2s, and how to better combine chemotherapy, immune checkpoint inhibitors and cancer gene therapy to achieve more effective treatments for CRC. Moreover, being an alarmin, IL-33 may take up the role of a potential biomarker for CRC diagnosis, therapy and prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao Y S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Wang LYT
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64637] [Article Influence: 16159.3] [Reference Citation Analysis (176)] |
| 2. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47138] [Article Influence: 3367.0] [Reference Citation Analysis (5)] |
| 3. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2293] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 4. | Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4060] [Cited by in RCA: 5766] [Article Influence: 524.2] [Reference Citation Analysis (0)] |
| 5. | Leibovici J, Itzhaki O, Huszar M, Sinai J. The tumor microenvironment: part 1. Immunotherapy. 2011;3:1367-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2893] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 7. | Nunes T, Bernardazzi C, de Souza HS. Interleukin-33 and inflammatory bowel diseases: lessons from human studies. Mediators Inflamm. 2014;2014:423957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 615] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 9. | Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 790] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 11. | Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941-6948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 12. | Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 13. | Lefrançais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502-15507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 14. | Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 297] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Tominaga S, Yokota T, Yanagisawa K, Tsukamoto T, Takagi T, Tetsuka T. Nucleotide sequence of a complementary DNA for human ST2. Biochim Biophys Acta. 1992;1171:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Yanagisawa K, Takagi T, Tsukamoto T, Tetsuka T, Tominaga S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 1993;318:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 170] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Hardman C, Ogg G. Interleukin-33, friend and foe in type-2 immune responses. Curr Opin Immunol. 2016;42:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994;13:1176-1188. [PubMed] |
| 19. | Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, Tominaga S. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun. 2001;285:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Iwahana H, Hayakawa M, Kuroiwa K, Tago K, Yanagisawa K, Noji S, Tominaga S. Molecular cloning of the chicken ST2 gene and a novel variant form of the ST2 gene product, ST2LV. Biochim Biophys Acta. 2004;1681:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, Wu K, Cao Y, Ng K, Ogino S. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 405] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 22. | Akimoto M, Takenaga K. Role of the IL-33/ST2L axis in colorectal cancer progression. Cell Immunol. 2019;343:103740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Cui G, Qi H, Gundersen MD, Yang H, Christiansen I, Sørbye SW, Goll R, Florholmen J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol Immunother. 2015;64:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Maywald RL, Doerner SK, Pastorelli L, De Salvo C, Benton SM, Dawson EP, Lanza DG, Berger NA, Markowitz SD, Lenz HJ, Nadeau JH, Pizarro TT, Heaney JD. IL-33 activates tumor stroma to promote intestinal polyposis. Proc Natl Acad Sci U S A. 2015;112:E2487-E2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | He Z, Chen L, Souto FO, Canasto-Chibuque C, Bongers G, Deshpande M, Harpaz N, Ko HM, Kelley K, Furtado GC, Lira SA. Epithelial-derived IL-33 promotes intestinal tumorigenesis in Apc Min/+ mice. Sci Rep. 2017;7:5520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Pastille E, Wasmer MH, Adamczyk A, Vu VP, Mager LF, Phuong NNT, Palmieri V, Simillion C, Hansen W, Kasper S, Schuler M, Muggli B, McCoy KD, Buer J, Zlobec I, Westendorf AM, Krebs P. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 2019;12:990-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 27. | Fang M, Li Y, Huang K, Qi S, Zhang J, Zgodzinski W, Majewski M, Wallner G, Gozdz S, Macek P, Kowalik A, Pasiarski M, Grywalska E, Vatan L, Nagarsheth N, Li W, Zhao L, Kryczek I, Wang G, Wang Z, Zou W, Wang L. IL33 Promotes Colon Cancer Cell Stemness via JNK Activation and Macrophage Recruitment. Cancer Res. 2017;77:2735-2745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 28. | Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, Oshimori N. Tumor-initiating cells establish an IL-33-TGF-β niche signaling loop to promote cancer progression. Science. 2020;369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 29. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8181] [Article Influence: 545.4] [Reference Citation Analysis (0)] |
| 30. | Malik A, Sharma D, Zhu Q, Karki R, Guy CS, Vogel P, Kanneganti TD. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469-4481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 31. | Mertz KD, Mager LF, Wasmer MH, Thiesler T, Koelzer VH, Ruzzante G, Joller S, Murdoch JR, Brümmendorf T, Genitsch V, Lugli A, Cathomas G, Moch H, Weber A, Zlobec I, Junt T, Krebs P. The IL-33/ST2 pathway contributes to intestinal tumorigenesis in humans and mice. Oncoimmunology. 2016;5:e1062966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Belluco C, Nitti D, Frantz M, Toppan P, Basso D, Plebani M, Lise M, Jessup JM. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol. 2000;7:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1911] [Cited by in RCA: 1921] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 34. | Abud HE, Watson N, Heath JK. Growth of intestinal epithelium in organ culture is dependent on EGF signalling. Exp Cell Res. 2005;303:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem. 2004;279:44513-44521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Islam MS, Horiguchi K, Iino S, Kaji N, Mikawa S, Hori M, Ozaki H. Epidermal growth factor is a critical regulator of the cytokine IL-33 in intestinal epithelial cells. Br J Pharmacol. 2016;173:2532-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Shi Y, Li Z, Zheng W, Liu X, Sun C, Laugsand JB, Liu Z, Cui G. Changes of immunocytic phenotypes and functions from human colorectal adenomatous stage to cancerous stage: Update. Immunobiology. 2015;220:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | McLean MH, Murray GI, Stewart KN, Norrie G, Mayer C, Hold GL, Thomson J, Fyfe N, Hope M, Mowat NA, Drew JE, El-Omar EM. The inflammatory microenvironment in colorectal neoplasia. PLoS One. 2011;6:e15366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4542] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 40. | Cui G, Yuan A, Pang Z, Zheng W, Li Z, Goll R. Contribution of IL-33 to the Pathogenesis of Colorectal Cancer. Front Oncol. 2018;8:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Löhning M, Belkaid Y, Fallon PG, Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 811] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 42. | Zhang Y, Davis C, Shah S, Hughes D, Ryan JC, Altomare D, Peña MM. IL-33 promotes growth and liver metastasis of colorectal cancer in mice by remodeling the tumor microenvironment and inducing angiogenesis. Mol Carcinog. 2017;56:272-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 43. | Hatzioannou A, Banos A, Sakelaropoulos T, Fedonidis C, Vidali MS, Köhne M, Händler K, Boon L, Henriques A, Koliaraki V, Georgiadis P, Zoidakis J, Termentzi A, Beyer M, Chavakis T, Boumpas D, Tsirigos A, Verginis P. An intrinsic role of IL-33 in Treg cell-mediated tumor immunoevasion. Nat Immunol. 2020;21:75-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 44. | Lu B, Yang M, Wang Q. Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy. J Mol Med (Berl). 2016;94:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Casciaro M, Cardia R, Di Salvo E, Tuccari G, Ieni A, Gangemi S. Interleukin-33 Involvement in Nonsmall Cell Lung Carcinomas: An Update. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 47. | Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 48. | Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 49. | Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, Jiang J, Wu C, Zhang X, Chen X, Turnquist H, Zhu Y, Lu B. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 50. | Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun. 2014;453:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Akimoto M, Maruyama R, Takamaru H, Ochiya T, Takenaga K. Soluble IL-33 receptor sST2 inhibits colorectal cancer malignant growth by modifying the tumour microenvironment. Nat Commun. 2016;7:13589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 52. | O'Donnell C, Mahmoud A, Keane J, Murphy C, White D, Carey S, O'Riordain M, Bennett MW, Brint E, Houston A. An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. Br J Cancer. 2016;114:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Larsen KM, Minaya MK, Vaish V, Peña MMO. The Role of IL-33/ST2 Pathway in Tumorigenesis. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 54. | Lalla RV, Brennan MT, Gordon SM, Sonis ST, Rosenthal DI, Keefe DM. Oral Mucositis Due to High-Dose Chemotherapy and/or Head and Neck Radiation Therapy. J Natl Cancer Inst Monogr. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Paduch R, Kandefer-Szerszeń M, Piersiak T. The importance of release of proinflammatory cytokines, ROS, and NO in different stages of colon carcinoma growth and metastasis after treatment with cytotoxic drugs. Oncol Res. 2010;18:419-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Ye XL, Zhao YR, Weng GB, Chen YC, Wei XN, Shao JP, Ji H. IL-33-induced JNK pathway activation confers gastric cancer chemotherapy resistance. Oncol Rep. 2015;33:2746-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res. 2001;7:2182-2194. [PubMed] |
| 58. | Hebbar M, Ychou M, Ducreux M. Current place of high-dose irinotecan chemotherapy in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. 2009;135:749-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Guabiraba R, Besnard AG, Menezes GB, Secher T, Jabir MS, Amaral SS, Braun H, Lima-Junior RC, Ribeiro RA, Cunha FQ, Teixeira MM, Beyaert R, Graham GJ, Liew FY. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol. 2014;7:1079-1093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 60. | Keilholz U. CTLA-4: negative regulator of the immune response and a target for cancer therapy. J Immunother. 2008;31:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 62. | Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1375] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 63. | Mariotti FR, Quatrini L, Munari E, Vacca P, Moretta L. Innate Lymphoid Cells: Expression of PD-1 and Other Checkpoints in Normal and Pathological Conditions. Front Immunol. 2019;10:910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 64. | Qin L, Dominguez D, Chen S, Fan J, Long A, Zhang M, Fang D, Zhang Y, Kuzel TM, Zhang B. Exogenous IL-33 overcomes T cell tolerance in murine acute myeloid leukemia. Oncotarget. 2016;7:61069-61080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, Ramnarain A, Gasmi B, Gururajan M, Redmond D, Askan G, Bhanot U, Elyada E, Park Y, Tuveson DA, Gönen M, Leach SD, Wolchok JD, DeMatteo RP, Merghoub T, Balachandran VP. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature. 2020;579:130-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 66. | Van der Jeught K, Sun Y, Fang Y, Zhou Z, Jiang H, Yu T, Yang J, Kamocka MM, So KM, Li Y, Eyvani H, Sandusky GE, Frieden M, Braun H, Beyaert R, He X, Zhang X, Zhang C, Paczesny S, Lu X. ST2 as checkpoint target for colorectal cancer immunotherapy. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Qi L, Zhang Q, Miao Y, Kang W, Tian Z, Xu D, Xiao W, Fang F. Interleukin-33 activates and recruits natural killer cells to inhibit pulmonary metastatic cancer development. Int J Cancer. 2020;146:1421-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Xia Y, Ohno T, Nishii N, Bhingare A, Tachinami H, Kashima Y, Nagai S, Saito H, Nakae S, Azuma M. Endogenous IL-33 exerts CD8+ T cell antitumor responses overcoming pro-tumor effects by regulatory T cells in a colon carcinoma model. Biochem Biophys Res Commun. 2019;518:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Komai-Koma M, Wang E, Kurowska-Stolarska M, Li D, McSharry C, Xu D. Interleukin-33 promoting Th1 lymphocyte differentiation dependents on IL-12. Immunobiology. 2016;221:412-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Luo P, Deng S, Ye H, Yu X, Deng Q, Zhang Y, Jiang L, Li J, Yu Y, Han W. The IL-33/ST2 pathway suppresses murine colon cancer growth and metastasis by upregulating CD40 L signaling. Biomed Pharmacother. 2020;127:110232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Andreone S, Gambardella AR, Mancini J, Loffredo S, Marcella S, La Sorsa V, Varricchi G, Schiavoni G, Mattei F. Anti-Tumorigenic Activities of IL-33: A Mechanistic Insight. Front Immunol. 2020;11:571593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Kienzl M, Hasenoehrl C, Valadez-Cosmes P, Maitz K, Sarsembayeva A, Sturm E, Heinemann A, Kargl J, Schicho R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology. 2020;9:1776059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 73. | Ercolano G, Falquet M, Vanoni G, Trabanelli S, Jandus C. ILC2s: New Actors in Tumor Immunity. Front Immunol. 2019;10:2801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Flamar AL, Klose CSN, Moeller JB, Mahlakõiv T, Bessman NJ, Zhang W, Moriyama S, Stokic-Trtica V, Rankin LC, Putzel GG, Rodewald HR, He Z, Chen L, Lira SA, Karsenty G, Artis D. Interleukin-33 Induces the Enzyme Tryptophan Hydroxylase 1 to Promote Inflammatory Group 2 Innate Lymphoid Cell-Mediated Immunity. Immunity. 2020;52:606-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 75. | Kim J, Kim W, Moon UJ, Kim HJ, Choi HJ, Sin JI, Park NH, Cho HR, Kwon B. Intratumorally Establishing Type 2 Innate Lymphoid Cells Blocks Tumor Growth. J Immunol. 2016;196:2410-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Xiao B, Zhang L, Liu H, Fang H, Wang C, Huang B, Liu X, Zhou X, Wang Y. Oncolytic Adenovirus CD55-Smad4 Suppresses Cell Proliferation, Metastasis, and Tumor Stemness in Colorectal Cancer by Regulating Wnt/β-Catenin Signaling Pathway. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Ying C, Xiao BD, Qin Y, Wang BR, Liu XY, Wang RW, Fang L, Yan H, Zhou XM, Wang YG. GOLPH2-regulated oncolytic adenovirus, GD55, exerts strong killing effect on human prostate cancer stem-like cells in vitro and in vivo. Acta Pharmacol Sin. 2018;39:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Zhang X, Meng S, Zhang R, Ma B, Liu T, Yang Y, Xie W, Liu X, Huang F, Zhou X, Wang Y. GP73-regulated oncolytic adenoviruses possess potent killing effect on human liver cancer stem-like cells. Oncotarget. 2016;7:29346-29358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, Zhang L, Cao P, Yan J, Miller D, Zhang HG. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |