Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.217
Peer-review started: July 16, 2021
First decision: August 19, 2021
Revised: September 5, 2021
Accepted: November 28, 2021
Article in press: November 28, 2021
Published online: January 7, 2022
Processing time: 166 Days and 15.1 Hours
Superior mesenteric venous thrombosis (SMVT) is a rare but fatal condition that is typically treated initially with anticoagulation therapy, and if this fails, with endovascular interventions. However, due to its rarity, there are not many studies that have explored the effectiveness of anticoagulation and endovascular therapies in treating SMVT.
To evaluate patients diagnosed with SMVT who received endovascular therapy in addition to anticoagulation and report technical and clinical outcomes.
A retrospective analysis of the patients who underwent endovascular treatment for SMVT at Mayo Clinic from 2000-2019 was performed. Technical success was defined as angiographic improvement in SMV flow after intervention. Primary patency was defined as the interval from reestablishing mesenteric venous flow until the first repeat thrombotic event or need for additional intervention. Secondary patency was defined as successful restoration of flow after repeat intervention until rethrombosis or last follow-up. The adverse events were reported through Clavien-Dindo classification.
Twenty-four patients were included for analysis. The median age at intervention was 60 years (35-74 years) and 16 (67%) were men. Nineteen patients presented with acute thrombosis (79.2%) and 5 with chronic thrombosis with acute manifestations (20.8%). The most commonly used endovascular modalities were thrombectomy in 12 patients (50.0%) and catheter-directed thrombolysis in 10 patients (41.7%). Technical success was achieved in 18 patients (75%). The 14-d and 30-d primary patency rates were 88.9% and 83.3%, respectively. Adverse events were reported in two patients (8.3%), one marked as grade IIIB, and 1 death marked as grade V. Five-year overall survival rate was 82% (58%-100%).
Endovascular intervention with anticoagulation appears to be effective for managing SMVT. This treatment combination may be considered as first-line therapy for SMVT management in select patients.
Core Tip: Superior mesenteric venous thrombosis (SMVT) is a rare condition with a high mortality rate of up to 23%. In our retrospective study, we investigated the use of endovascular treatments after anticoagulation therapies had failed on patients diagnosed with SMVT. By looking at the technical success and patency rates after intervention, we evaluated the effectiveness of these endovascular interventions.
- Citation: Alnahhal K, Toskich BB, Nussbaum S, Li Z, Erben Y, Hakaim AG, Farres H. Superior mesenteric venous thrombosis: Endovascular management and outcomes. World J Clin Cases 2022; 10(1): 217-226
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/217.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.217
Acute mesenteric venous thrombosis accounts for 6% to 9% of mesenteric ischemia cases and 1 in 5000 to 15000 hospital admissions[1-3]. Superior mesenteric venous thrombosis (SMVT) is a rare condition but portends a mortality rate of up to 23%[4]. Risk factors for SMVT include hypercoagulable states, intra-abdominal surgery, infection, inflammation, and malignancy.
Systemic anticoagulation in addition to laparotomy for presentations with peritonitis represent conventional approaches to treating SMVT[3,5,6]. Endovascular therapy, such as thrombolysis and/or thrombectomy, is typically used as adjunctive treatment when medical management has failed. To date, most studies that explore endovascular treatment of SMVT have been case reports and small case series. More information regarding this treatment is needed.
This study aims to report on this rare condition to expand the literature base and evaluate the endovascular therapies effects on technical and clinical outcomes on 24 patients diagnosed with SMVT from a large tri-center institution.
A retrospective search of the Mayo Clinic Health System electronic medical record and imaging report archive was performed. All patients age 18 years and older who were diagnosed with isolated SMVT or SMVT combined with portal vein thrombosis as an inpatient and underwent both medical and endovascular treatment between January 2000 and December 2019 were included for analysis. Patients were excluded if they did not have sufficient follow-up data of at least 1 mo. Patients with peritonitis were not included in this study as these patients are taken to an operating room and would not undergo endovascular treatment. Twenty-four patients were identified and met our criteria. Patient demographics, presenting symptoms, date of symptom onset, risk factors for SMVT admission and endovascular intervention, endovascular modality(s), technical and clinical outcomes, patency rates, and length of stay. The adverse events were evaluated and reported per Clavien-Dindo classification. The follow-up was until last clinical note recorded in the patients’ medical record.
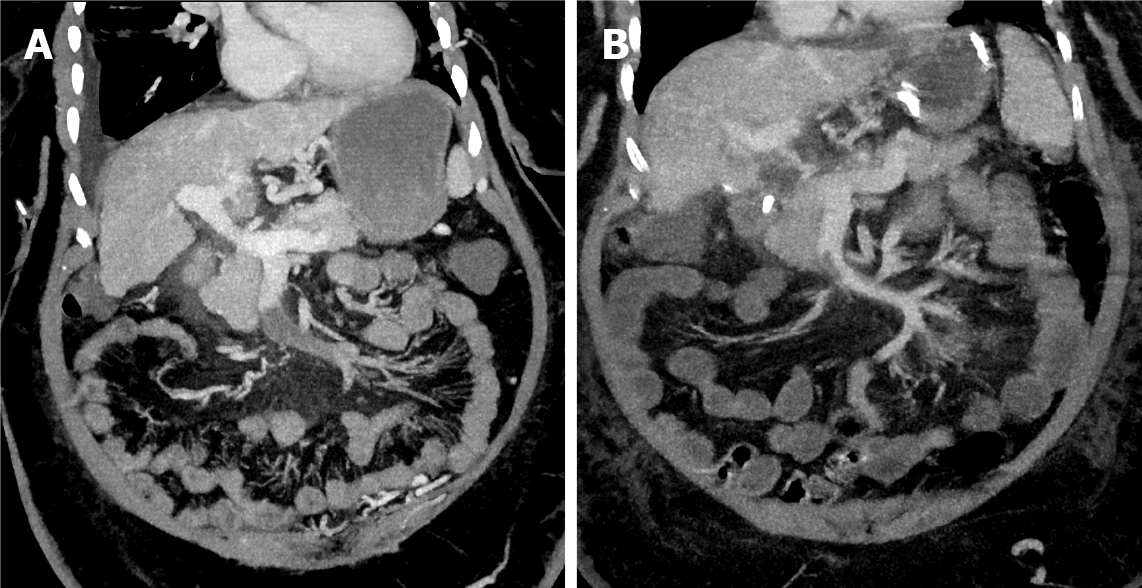
Endovascular therapy was initiated after failure of systemic anticoagulation, determined by a lack of clinical improvement (Figure 1). The anticoagulation management was unfractionated heparin or low-molecular-weight heparin started at the time of diagnosis. Percutaneous transhepatic and/or transjugular approaches were used for all interventions. The selection between approaches was operator dependent.
Pharmacomechanical thrombectomy was performed in most patients who underwent thrombectomy using either rheolysis (AngioJet, Boston Scientific Corporation) or aspiration thrombectomy (Penumbra CAT 8 device, Penumbra Inc.). Catheter-directed thrombolysis (CDT) was performed using a multiple side-hole infusion catheter that extended into the thrombosed SMV. Tissue plasminogen activator was used as thrombolytic agent that initiated at an infusion rate of 0.5-1 mg/h in most patients who underwent thrombolysis for a duration ranging from 24 to 72 h with a low-dose heparin infusion at a standard rate of 500 units/h. Other endovascular techniques were employed when lysis and thrombectomy alone were insufficient including transjugular intrahepatic portosystemic shunt creation (TIPS), which was used if there was significant residual underlying portal hypertension after thrombectomy (portal pressure gradient > 12 mmHg) or if there was evidence of rethrombosis related to poor intrahepatic portal perfusion; balloon angioplasty, and self-expanding or balloon-expandable stents were deployed when a persistent flow-limiting lesion (> 50% stenosis) was present after angioplasty.
The end points of the study were to determine both the short- and long-term technical success, and the clinical outcomes of using the endovascular therapeutic options in treating patients diagnosed with SMVT. Short-term technical success was measured as any improvement in SMV flow per completion angiography at the time of intervention. The long-term technical success was represented by the primary and secondary patency rates. Primary patency was defined as the interval from reestablishing initial mesenteric venous flow until the first thrombotic event, seen through ultrasound imaging follow up, or additional intervention. Secondary patency was defined as successful restoration of flow after thrombosis post reintervention until rethrombosis or last follow-up. Portal involvement defined as any thrombus extension into the main portal vein. The clinical outcomes were assessed by reporting the clinical status of the patient at 1 to 2 d following the procedure, reporting the adverse events via Clavien-Dindo classification, and showing the 5-year survival rate.
Continuous variables were summarized as median (range) and quartiles while categorical variables were reported as frequency (percentage). The Kaplan-Meier method was used to estimate primary patency and overall survival using R version 3.6.2 (https://www.r-project.org). P values were considered statistically significant if P < 0.05. The statistical methods of this study were reviewed by Li Z, MS from Mayo Clinic.
The study population consisted of 24 patients (Table 1). The median age at intervention was 60 years (35-74 years) and 67% (n = 16) were men. The median time between presentation to hospital and intervention was 3 d (0-15 d), while the median time from symptom onset to intervention was 8 d (2-35 d). Of 79.2% (n = 19) patients presented with acute symptoms while acute on top of chronic symptoms occurred in 20.8% (n = 5) of the patients. The most common SMVT risk factors were history of intra-abdominal surgery (n = 11, 45.8%), liver cirrhosis (n = 11, 45.8%), and malignancy (n = 11, 45.8%). Patients presented primarily with abdominal pain 70.8% (n = 17), and nausea and vomiting 50% (n = 12).
| Measure | Value1 |
| Age (yr) | 60 (35-74) |
| Sex | |
| Male | 16 (66.7) |
| Female | 8 (33.3) |
| Symptoms2,3 | |
| Abdominal pain | 17 (70.8) |
| Nausea and vomiting | 12 (50.0) |
| Abdominal distention | 9 (37.5) |
| Constipation | 5 (20.8) |
| Diarrhea (bloody or non-bloody) | 3 (12.5) |
| Hematemesis | 1 (4.2) |
| Signs2,3 | |
| Ascites | 7 (29.2) |
| Abdominal tenderness | 6 (25.0) |
| Fever | 3 (12.5) |
| Hypotension (SBP < 90) | 1 (4.2) |
| Jaundice | 1 (4.2) |
| Risk factors2,3 | |
| Liver cirrhosis | 11 (45.8) |
| Intra-abdominal surgery | 11 (45.8) |
| Pancreatic cancer | 6 (25.0) |
| Intra-abdominal infection | 4 (16.7) |
| Hepatocellular carcinoma | 3 (12.5) |
| Others | 9 (37.5) |
Only 54.2% (n = 13) patients had a reported lactate level at presentation, of them, 23.1% (n = 3) have hyperlactatemia with a median level of 1.4 mmol/L (range, 0.8-4.8 mg/dL). While 91.7% (n = 22) had a reported white blood cell count, where 13.6% (n = 3) have leukocytosis with a median count of 9.2 (× 109/L; range, 3.5-21.9 × 109/L). Computed tomography was the most frequently used imaging modality [62.5% (n = 15)] followed by the magnetic resonance angiography used in 37.5% (n = 9). Among 24 patients, only 2 had isolated SMVT while the portal vein was involved in the other 91.7% (n = 22). The distal part of the SMV (defined as the part of the SMV near the tributaries) was involved in 66.7% (n = 16), the proximal end (defined as the portion of the SMV proper without tributaries) was involved in 12.5% (n = 3), and 20.8% (n = 5) had both proximal and distal involvement.
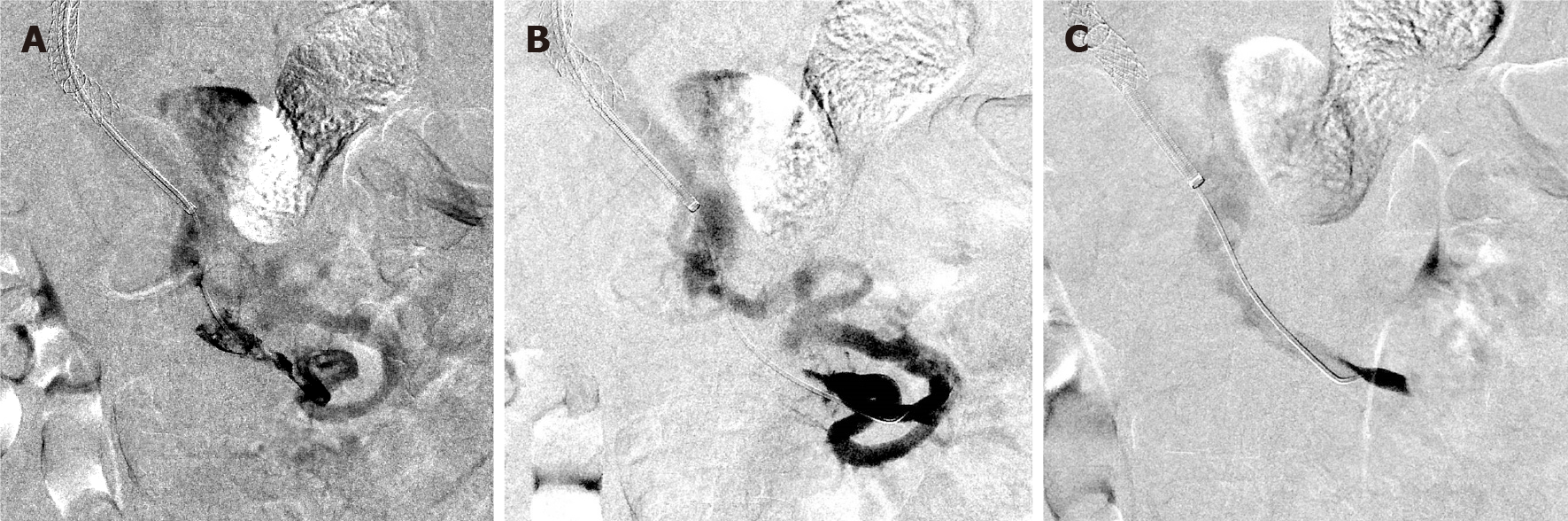
All 24 patients received at least one endovascular modality, while 13 (54.2%) received a combination of treatments (Table 2). Thrombectomy was the most commonly used, which was attempted in 50% (n = 12), Out of 12 patients, a pharmacomechanical system (AngioJet device; Boston Scientific Corporation) was performed in 75% (n = 9) and aspiration thrombectomy was employed in 25% (n = 3) (Figure 2). CDT was attempted in 41.7% (n = 10) of the patients, where CDT alone was in 4 patients and in combination with other modalities in 6 patients. In patients who received CDT, eight were treated with tissue plasminogen activator with an infusion rate of 0.5 to 1.0 mg/h over 24 to 72 h and 2 patients received fixed doses of recombinant tissue plasminogen activator (Table 3).
| Measure | Value1 |
| Endovascular modality2 | |
| Thrombectomy | 12 (50.0) |
| Thrombolysis | 10 (41.7) |
| Stent placement | 9 (37.5) |
| TIPS | 6 (25.0) |
| Balloon angioplasty | 4 (16.7) |
| Technical success outcome3 | 18 (75.0) |
| Complete | 11 (61.1) |
| Partial | 7 (38.9) |
| Clinical outcome | |
| Resolution | 11 (45.8) |
| Partial improvement | 9 (37.5) |
| Not changed | 3 (12.5) |
| Got worse | 1 (4.2) |
| Clavien-Dindo classification4 | |
| Grade I | 4 (16.7) |
| Grade II | 5 (20.8) |
| Grade III | 1 (4.2) |
| Grade IV | 0 (0.0) |
| Grade V | 1 (4.2) |
| Intestinal resection | 1 (4.2) |
| In-hospital time, d | 12 (1-68) |
| Readmission | 1 (4.2) |
| Follow-up duration, mo | 23 (1-145) |
| Measure | n (%) |
| Access (n = 24) | |
| Transhepatic | 13 (54.2) |
| Transjugular | 11 (45.8) |
| Thrombectomy (n = 12) | |
| Pharmacomechanical | 9 (75.0) |
| Aspiration | 3 (25.0) |
| Thrombolysis (n = 10) | |
| Thrombolytic agent | |
| tPA | 9 (90.0) |
| rtPA | 1 (10.0) |
| Infusion rate | |
| 1.0 mg/h | 5 (50.0) |
| 0.5 mg/h | 3 (30.0) |
| Fixed dose | 2 (20.0) |
| Infusion duration1 (n = 8) | |
| 24 h | 2 (25.0) |
| 48 h | 4 (50.0) |
| 72 h | 2 (25.0) |
Other treatment modalities were also employed, including stents placed in 37.5% (n = 9), TIPS created in 25.0% (n = 6), and balloon angioplasty alone performed in 16.7% (n = 4). All patients that had a TIPS creation received an intrahepatic stent.
Technical success was achieved in 18 of 24 patients. In these patients, 61.1% (n = 11) achieved complete recanalization, and 38.9% (n = 7) were established partial recanalization. Three patients underwent more than 1 endovascular intervention: 2 of them with technical success. Out of the 11 patients that had liver cirrhosis, 9 achieved technical success; with only one patient having primary patency failure, and three patients died by the end of the study. An 83.3% (n = 20) of the patients demonstrated clinical improvement 48 h following the procedure (Table 2). A single patient experienced symptom progression after intervention.
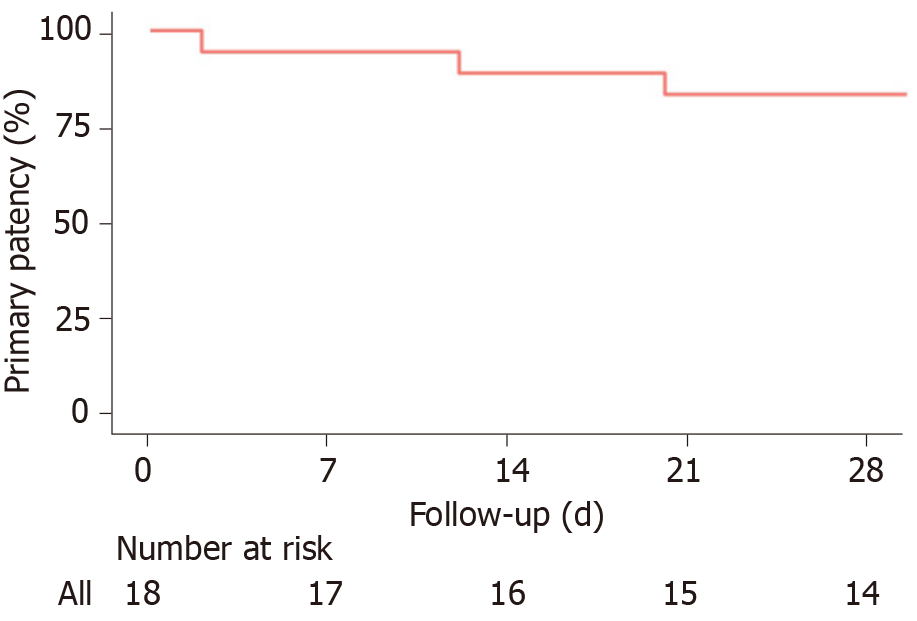
Median follow-up of the patients was 23 mo (range of 1-145). The median length of stay was 12 d (range of 1-68 d). Primary patency at 14 and 30 d were 88.9% (95%CI: 76%-100%) and 83.3% (95%CI: 68%-100%), respectively (Figure 3). Adverse events were identified in 8.3% (n = 2) of patients, one marked as grade IIIB, and 1 death marked as grade V. One patient underwent partial small bowel resection, and the second patient had a subcapsular hematoma and intraperitoneal bleeding and died after 6 d.
A total of 6 patients (25%) died at some point during the study, four of which (16.7%) died before the median follow up time of 23 mo. Five-year overall survival rate was 82% (58%-100%).
SMVT is associated with high rates of morbidity and mortality[1]. Our study described and evaluated the outcomes of endovascular treatment of SMVT in 24 patients, with either acute thrombosis or chronic thrombosis with acute symptoms. The acute onset usually presents with crampy upper, middle, or lower-middle abdominal pain in addition to nausea, anorexia, vomiting, diarrhea, and/or bloody stool. Features of portal hypertension are usually reported in the chronic onset[7,8].
Since the presenting symptoms of SMVT are not specific (e.g., abdominal pain and nausea and/or vomiting), early detection and diagnosis of the condition is challenging, leading to a delay in initiating management; thus, more dangerous consequences can emerge, such as intestinal and bowel infarction. The delay of management in these patients is also due to the failure of anticoagulation which is always the first line of treatment before moving to endovascular therapy and explains the 3-d median time of presentation-to-intervention that was found. Endovascular therapies are becoming increasingly used, however, the data available regarding their use in literature is insufficient[9-12].
In our study, percutaneous thrombectomy was used to treat 14 patients, most commonly using pharmacomechanical thrombectomy through rheolysis and less commonly using aspiration thrombectomy, either in combination with other modalities, especially CDT, or alone, in case CDT was contraindicated or not indicated. Rabuffi et al[10] evaluated the outcomes of 8 patients who were treated by a pharmacomechanical thrombectomy, and showed survival rate of 87.5% and a 12.5% major complication rate at mean follow up of 37 mo, compared to 78.6% and 7.1% in our study at median follow-up of 23 mo.
Many studies investigating the endovascular thrombolytic therapies have been reported. Andraska et al[13] exhibited that thrombolysis achieved a rate of complete or partial recanalization of 91.6% in patients who received this treatment modality alone. Another study[14] evaluated outcomes of performing CDT in 20 patients, demon
Yang et al[14] found among 13 cases who are treated with transcatheter thrombolysis, 4 cases required small bowel resection due to localized bowel infarction and secondary stricture. In our study, only 1 patient, who was still symptomatic for abdominal pain despite the thrombolysis for 72 h, underwent resection of proximal one-third of the jejunum and an ostomy placement after the thrombolysis therapy, which denotes the early detection and diagnosis of the condition and the rapid intervention as well.
TIPS was performed in 9 patients, can be helpful through creating a low-pressure system that assists in the removal of the clots in combination with other treatment modalities such as CDT[10]. Neither hepatic encephalopathy nor pulmonary embolism developed in any of these patients, although TIPS can increase the risk of these complications[10,15].
In a study that included 43 patients who were treated for their ASMVT using multidisciplinary stepwise management, endovascular CDT was performed in 83% (n = 36) patients with or without adjunctive procedures, 20 as an initial procedure and 16 were postoperatively, their recanalization rate was 94.44% (vs 75% in this study). Bowel resection was required in 18 patients, with 30-d mortality and overall in-hospital mortality rates of 11.63% and 16.28%, respectively. The overall 1-year survival was 83.72% (vs 82% 5-year survival rate in our study)[16].
In our study, primary and secondary patencies were reported and those outcomes are not measured in other studies as far as we know. The high rates of the 30-d primary and secondary patencies of 83.3% and 88.9%, respectively, signify the safety and efficacy of using the endovascular interventions in treating those patients; however, the scale used in showing the patency rates and drawing the Kaplan-Meier curve for the primary patency was in days due to rapid occurrence of the rethrombosis events of those 3 patients.
Limitations of our study included its retrospective design. Conducting a large scale randomized controlled trial is not feasible because SMVT is a rare condition and the mechanism for SMVT is variable along with the ethics of trial design in withholding therapy to patients not responding to anticoagulation. Indeed, a study of 24 patients who were diagnosed with an uncommon condition is not considered a small study compared to the studies found in the literature, which are mostly case reports and very small case series. However, with this number of patients (n = 24), conducting a statistical analysis was not possible. Overall, despite the aforementioned limitations, our study’s findings show that endovascular management for SMVT was associated with high thrombus resolution rates and improvement in patients’ clinical outcomes. Further large prospective study is still warranted to provide more significant data about the outcomes of endovascular therapies.
Endovascular intervention with anticoagulation appears to be effective at managing SMVT and may be considered as first-line therapy along with systemic anticoagulation in select patients.
Superior mesenteric venous thrombosis (SMVT) is a rare but deadly condition with mortality rates of up to 23% that is typically treated with anticoagulation therapy and endovascular treatment if this fails.
Most existing studies looking into endovascular of SMVT are small case series and case reports, and as such more information is needed on treatments for this rare condition.
This study aimed to evaluate patients diagnosed with SMVT who received endovascular therapy in addition to anticoagulation and report technical and clinical outcomes.
A retrospective analysis of the patients who underwent endovascular treatment for SMVT at Mayo Clinic from 2000-2019 was performed. We explored the success of these endovascular treatments by determining technical success in each case, defined as any improvement in SMV flow following recanalization, and by assessing the primary/secondary patency rates.
Twenty-four patients were included for analysis. Of these patients, 19 presented with acute thrombosis, while 5 presented with chronic thrombosis with acute manifestations. The most commonly used endovascular modalities were thrombectomy in 12 patients and catheter-directed thrombolysis in 10 patients. Technical success was achieved in 75% of patients, and the 14-d and 30-d patency rates were 88.9% and 83.3% respectively. The 5-year overall survival rate was 83%.
Endovascular interventions in addition to anticoagulation therapy appears to be effective in treating SMVT and may be considered a first-line therapy in select patients.
Our research helps build the base of literature on the rare condition of SMVT by providing an evaluation of the technical and clinical endovascular management outcomes. Larger prospective studies looking into the long-term outcomes of these endovascular interventions and anticoagulation therapy may be a future research direction.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dias E, Han IW, Wang X S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Rhee RY, Gloviczki P. Mesenteric venous thrombosis. Surg Clin North Am. 1997;77:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Brunaud L, Antunes L, Collinet-Adler S, Marchal F, Ayav A, Bresler L, Boissel P. Acute mesenteric venous thrombosis: case for nonoperative management. J Vasc Surg. 2001;34:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15:407-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Kim HK, Hwang D, Park S, Lee JM, Huh S. Treatment outcomes and risk factors for bowel infarction in patients with acute superior mesenteric venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2017;5:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Bergqvist D, Svensson PJ. Treatment of mesenteric vein thrombosis. Semin Vasc Surg. 2010;23:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 339] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 7. | Hmoud B, Singal AK, Kamath PS. Mesenteric venous thrombosis. J Clin Exp Hepatol. 2014;4:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Shibahara K, Tatsuta K, Orita H, Yonemura T, Kohno H. Superior mesenteric and portal vein thrombosis caused by congenital antithrombin III deficiency: report of a case. Surg Today. 2007;37:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yang S, Zhang L, Liu K, Fan X, Ding W, He C, Wu X, Li J. Postoperative Catheter-Directed Thrombolysis Versus Systemic Anticoagulation for Acute Superior Mesenteric Venous Thrombosis. Ann Vasc Surg. 2016;35:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Rabuffi P, Vagnarelli S, Bruni A, Antonuccio G, Ambrogi C. Percutaneous Pharmaco-Mechanical Thrombectomy of Acute Symptomatic Superior Mesenteric Vein Thrombosis. Cardiovasc Intervent Radiol. 2020;43:46-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Liu K, Liu S, Li L, Wang S, Fan X, Wu X, Shi G, Zong H. Evaluation of Endovascular Therapy Combined with Bowel Resection Treatment on Patients with Acute Mesenteric Venous Thrombosis. Ann Vasc Surg. 2020;65:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Hollingshead M, Burke CT, Mauro MA, Weeks SM, Dixon RG, Jaques PF. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:651-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Andraska E, Haga L, Li X, Avgerinos E, Singh M, Chaer R, Madigan M, Eslami MH. Retrograde open mesenteric stenting should be considered as the initial approach to acute mesenteric ischemia. J Vasc Surg. 2020;72:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Yang SF, Liu BC, Ding WW, He CS, Wu XJ, Li JS. Initial transcatheter thrombolysis for acute superior mesenteric venous thrombosis. World J Gastroenterol. 2014;20:5483-5492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Cao G, Ko GY, Sung KB, Yoon HK, Gwon DI, Kim JH. Treatment of postoperative main portal vein and superior mesenteric vein thrombosis with balloon angioplasty and/or stent placement. Acta Radiol. 2013;54:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yang S, Fan X, Ding W, Liu B, Meng J, Xu D, He C, Yu W, Wu X, Li J. Multidisciplinary stepwise management strategy for acute superior mesenteric venous thrombosis: an intestinal stroke center experience. Thromb Res. 2015;135:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |