Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.166
Peer-review started: April 26, 2021
First decision: October 18, 2021
Revised: October 29, 2021
Accepted: November 28, 2021
Article in press: November 28, 2021
Published online: January 7, 2022
Processing time: 248 Days and 0.4 Hours
Tissue resident memory T (TRM) cells have been reported to play a significant role in the pathogenesis and relapse of chronic eczema.
To compare the efficacy and safety of the intralesional injection of 5-fluorouracil (5-FU) and triamcinolone (TA) with those associated with TA alone for the treatment of chronic eczema.
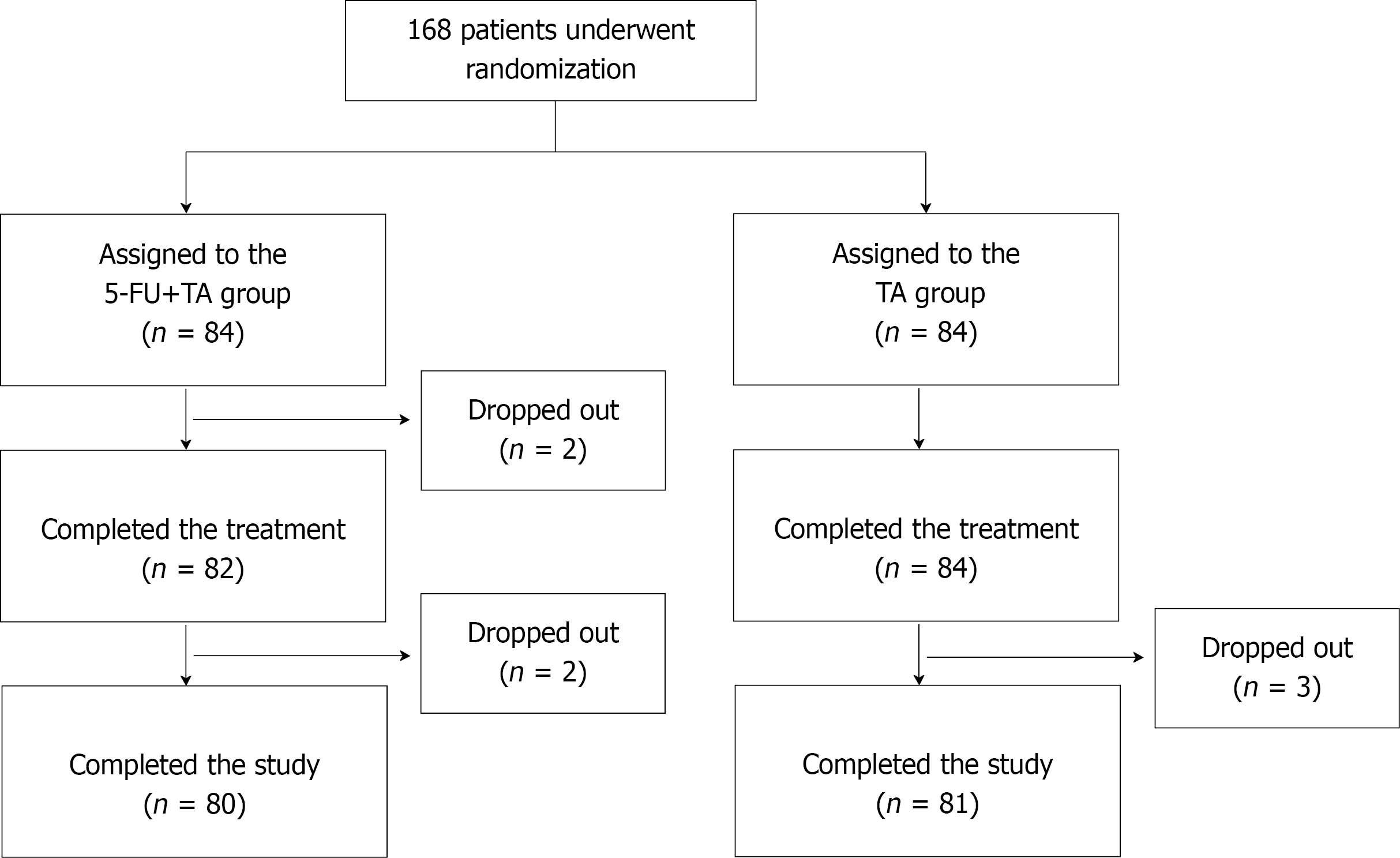
A total of 168 patients were randomized to 5-FU+TA or TA groups and received a one-time intralesional injection of 5-FU+TA or TA only. Biopsies were collected before and 2 wk after treatment for evaluation of histopathological changes. All patients were followed up monthly for up to 1 year.
No serious adverse event was observed in either group. Although the mean atopic dermatitis severity index scores and effective rates were comparable between the two groups after 2 wk of treatment, the relapse rate was significantly lower in the 5-FU+TA group than in the TA group. Histological examination showed significantly fewer CD8+ and CD103+ T cells but not CD4+ T cells in the 5-FU+TA group.
One-time intralesional injection of 5-FU+TA is effective and safe for chronic eczema treatment and can further reduce the retention of TRM cells in the lesional skin and the relapse rate of chronic eczema.
Core Tip: Chronic eczema is characterized by recurrent itchy papules and plaques with lichenification and hyperpigmentation, in which tissue resident memory T (TRM) cells play a significant role. Intralesional injection of 5-fluorouracil (5-FU) and triamcinolone (TA) can effectively reduce local inflammation and recurrence in a mouse model, but no clinical study has been reported. In this study, low-dose intralesional injection of 5-FU+TA was found to effectively and safely treat the localized rash, by significantly reducing the retention of TRM cells in the skin lesion, and to lower the relapse rate of chronic eczema. This combination may provide a new treatment option for chronic eczema patients with hypertrophy and localized rash.
- Citation: Wu Y, Wang GJ, He HQ, Qin HH, Shen WT, Yu Y, Zhang X, Zhou ML, Fei JB. Low-dose intralesional injection of 5-fluorouracil and triamcinolone reduces tissue resident memory T cells in chronic eczema. World J Clin Cases 2022; 10(1): 166-176
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/166.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.166
Chronic eczema is a chronic inflammatory skin condition, characterized by long-course, recurrent itchy papules, and plaques with lichenification and hyperpigmentation[1-3]. Repeated scratching and irritation is the main cause of repeated delay and refractory eczema. In China, 7.5 out of every 100 people suffer from eczema[4]. Recently, tissue resident memory T (TRM) cells have been suggested to play a pivotal role in the pathogenesis of chronic eczema, in addition to Th2-dominant inflammation[5]. It is reported that after 16 wk of treatment with topical corticosteroids (TCS), TRM cells were still present in the tissue[6]. Further studies have confirmed that the skin lesions of dermatitis and eczema patients are dominated by CD8+ TRM cells, and these TRM cells can persist for months and induce recurrent eczema[7,8].
TCS and topical calcineurin inhibitors (TCIs) are the most commonly used topical drugs for eczema treatment, and they can alleviate the localized inflammation[9]. However, some patients still suffer from repeated attacks. Oral corticosteroids and immunosuppressants have been widely used for the treatment of severe eczema. However, their long-term use can cause severe side effects, such as high blood pressure, blood glucose level, electrolyte disorders, decreased immune function, and pathogen infection. So far, Dupilumab has been approved for the treatment of moderate to severe eczema. In a phase 3 clinical trial, more than 65% of patients treated with dupilumab in combination with topical steroids achieved 75% improvement in eczema area severity index from baseline[10]. Other potential treatments for eczema such as mepolizumab and omalizumab are still under evaluation. A recent multicenter, randomized, double-blind, placebo-controlled, phase 2 clinical trial showed that 100 mg mepolizumab administered subcutaneously did not show any clinical improvement in patients with moderate to severe atopic eczema[11]. Omalizumab is a safe and relatively well-tolerated with some clinical benefits in atopic dermatitis patients[12], though its efficacy in adults is still under debate[13]. Moreover, these treatment modalities are expensive and require long-term use, which limits their clinical application.
Local intralesional glucocorticoid injection therapy has been used for decades in the treatment of localized dermatitis. Studies have shown that local injection of no more than 20 mg triamcinolone acetonide (TA) is safe and cost-effective for the treatment of localized dermatitis and other inflammatory skin diseases[14]. 5-Fluorouracil (5-FU), another common drug for intralesional injection, has been used for treating inflammatory hypertrophy in the skin as in scarring[15,16]. Our previous study has demonstrated that compared to the TA group, intralesional injection of 5-FU+TA can effectively reduce local inflammation and significantly reduce the recurrence of eczema in a mouse model[17]. However, there have been no clinical studies investigating the effect of 5-FU+TA for chronic eczema via intralesional injection, let alone with large samples and long-term follow-up.
The aim of this double-blind randomized controlled prospective clinical study was to further evaluate the efficacy and safety of intralesional injection of 5-FU and TA for the treatment of localized rash and management of relapse in chronic eczema patients and to explore the potential underlying mechanism.
This was a double-blind randomized controlled prospective study. The patients and the two dermatologists were both blinded to the group assignment. The diagnosis of chronic localized eczema was established based on the 2011 Guidelines for Eczema Diagnosis and Treatment designated by the Immunology Group, Dermatovenereology Society, Chinese Medical Association[4]. The inclusion criteria were: Age from 28–80 years and a history of chronic localized eczema lasting for > 6 mo. The exclusion criteria were: i) nullipara, pregnant, planning a pregnancy or lactating; ii) known allergies to FU or glucocorticoid; iii) treatment with any systemic corticosteroids/ antibiotics/ immunosuppressants in the previous 6 wk or use of topical dermocorticoids/ antibiotics/ antihistamines or calcineurin-inhibiting type immunosuppressants in the previous 2 wk; and iv) renal failure, liver dysfunction, hematological disease, immune deficiency, systemic or local infection (including chronic obstructive pulmonary disease patients). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Shanghai University of Medicine and Health Science Affiliated Zhoupu Hospital’s Research Ethics Committee (No. 2018-C-014-M01). This study has been registered at http://www.chictr.org.cn/ (No. ChiCTR2100043660).
One hundred sixty-eight patients who visited our hospital from January 2018 to June 2019 and were diagnosed with chronic eczema with localized lesions were recruited for the study. All patients provided signed written consent. Using a random sequence generated by a computer program, patients were randomly assigned to two groups: a 5-FU+TA group (n = 84) and a TA (n = 84) group (Figure 1). Four patients from the 5-FU+TA group and three patients from the TA group discontinued the study for personal reasons.
A one-time injection was performed for both groups. For the 5-FU+TA group, 2 mL normal saline (Shanghai Xudong Haipu Pharmaceutical Co., Ltd.) was mixed well with 1 mL 5-FU solution (Shanghai Xudong Haipu Pharmaceutical Co., Ltd.) (containing 25 mg 5-FU) and 1 mL TA solution (Shanghai General Medicine Industry Co., Ltd.) (containing 10 mg TA). Infiltration and injection were performed to allow 1 mL of the mixture into every 4 cm2 of the skin lesion area. The total injection area should be less than 32 cm2 and protected from contact with water for 3 days after injection to prevent secondary bacterial infection at the injection site. For the TA group, 3 mL of normal saline was mixed with 1 mL of TA solution (containing 10 mg TA). The injection method, total injection area, and course of treatment were the same as those for the 5-FU+TA treatment group.
Follow-up was conducted 2 wk after treatment and monthly thereafter for up to 12 mo. Effectiveness was evaluated, and adverse events were recorded and phot
Before treatment and 2 wk after treatment, two dermatologists graded the skin lesions of each patient independently. The evaluation of clinical efficacy referred to the atopic dermatitis severity index (ADSI) scoring system[18]. Because chronic eczema has no exudation, the remaining four indicators including pruritus, erythema, excoriation, and lichenification were graded on a 4-point scale (range 0–3): none = 0 point; mild = 1 point; moderate = 2 points; and severe = 3 points. The total score for these 4 items was ≤ 12 points. An efficacy score was calculated as:
Efficacy score = (score before treatment – score after treatment)/ score before treatment × 100%
The efficacy criteria were: cure, score > 90%; excellent response, score 61%–90%; good response, score 20%–60%; and poor or nonresponse, score < 20%.
Effectiveness percentage = (number of cured patients + number of patients with excellent response)/ total number of patients × 100%
The patients were followed up for 1 year, and the number of patients who relapsed was recorded.
Relapse rate = number of patients with eczema relapse/ total number of effective patients (cured patients + patients with excellent response) × 100%
Routine blood tests and liver and kidney function tests were performed before and 2 wk after treatment. We also monitored and recorded the events of local infection, skin atrophy, pigmentation, etc. after treatment and during the 1-year follow-up.
To investigate the histopathological changes and the infiltration of TRM cells and other immune cells after treatment of chronic localized eczema, we collected biopsy specimens at the lesion site from 15 patients in each group before and 2 wk after treatment.
For staining of lesional tissue with hematoxylin and eosin (HE), fixed tissues were embedded in paraffin and cut into 5 μm thin sections, followed by staining with HE.
For immunohistochemical staining, the lesional tissue sections were washed in phosphate-buffered saline and then boiled in EDTA for antigen retrieval, followed by 3% hydrogen peroxide incubation and 5% bovine serum albumin blockade. Tissue sections were incubated with primary antibodies against CD4 (1:100, Shanghai Jiehao Biotechnology Co., Ltd., Shanghai, China), CD8 (1:100, Shanghai Jiehao Biotechnology Co., Ltd.,), and CD103 (1:150, Abcam, Cambridge, UK). After incubation with a secondary antibody, staining results were observed under a microscope (Olympus, Tokyo, Japan). The images were recorded for further analysis. Cell counts were performed blindly on randomly selected histological images at ×100 magnification (High power field).
The data were analyzed using PASW software, version 22 (IBM Corporation, Armonk, NY). The graphs were plotted using GraphPad Prism, version 8.00 (GraphPad Software, Inc., San Diego, CA). Disease duration was compared between groups using the Mann–Whitney U test. Independent t-tests were used to compare the patients’ ages, ADSI scores, and T-cell counts. The chi-squared test and Fisher’s exact were used to determine statistical differences between the groups in relation to sex, the anatomical site treated, the effective rate of treatment, and the recurrence rate. A two-tailed P value < 0.05 was considered statistically significant. Unless otherwise specified, data are presented as mean ± SD.
The baseline demographic and clinical characteristics of patients in the two groups are summarized in Table 1. The age, sex, number of anatomical sites treated, and disease duration did not differ significantly between the groups (P > 0.05 for all).
| Patients underwent randomization(n = 168) | Patients completed study(n = 161) | |||||
| 5-FU + TA (n = 84) | TA (n = 84) | P | 5-FU + TA (n = 80) | TA (n = 81) | P | |
| Age (mean ± SD), yr | 54.39 ± 14.31 | 56.12 ± 14.82 | 0.643 | 54.74 ± 14.26 | 55.60 ± 12.56 | 0.683 |
| Range | 28-74 | 28-79 | 28-74 | 29-79 | ||
| 28-45 | 36.17 | 38.48 | 0.120 | 36.27 | 38.16 | 0.236 |
| 46-60 | 53.48 | 53.41 | 0.962 | 53.50 | 53.41 | 0.950 |
| 61- | 67.27 | 66 | 0.258 | 64.56 | 66.13 | 0.416 |
| Sex, n (%) | 0.739 | 0.774 | ||||
| Male | 59 (70.24) | 57 (67.86) | 56 (70) | 55 (67.90) | ||
| Female | 25 (29.76) | 27 (32.14) | 24 (30) | 26 (32.10) | ||
| Anatomical sites treated, n (%) | ||||||
| Trunk | 23 (27.38) | 22 (26.19) | 0.862 | 23 (28.75) | 22 (27.16) | 0.822 |
| Limb | 58 (69.05) | 61 (72.62) | 0.611 | 54 (67.50) | 58 (71.60) | 0.571 |
| Neck | 3 (3.57) | 1 (1.19) | 0.613 | 3 (3.75) | 1 (1.24) | 0.604 |
| ADSI score (mean ± SD) | 7.72 ± 1 | 7.68 ± 1.02 | 0.468 | 7.71 ± 1 | 7.87 ± 0.90 | 0.278 |
| Disease duration, yr (range) | 4 (1-24) | 4 (1-30) | 0.944 | 3 (1-24) | 3 (1-30) | 0.925 |
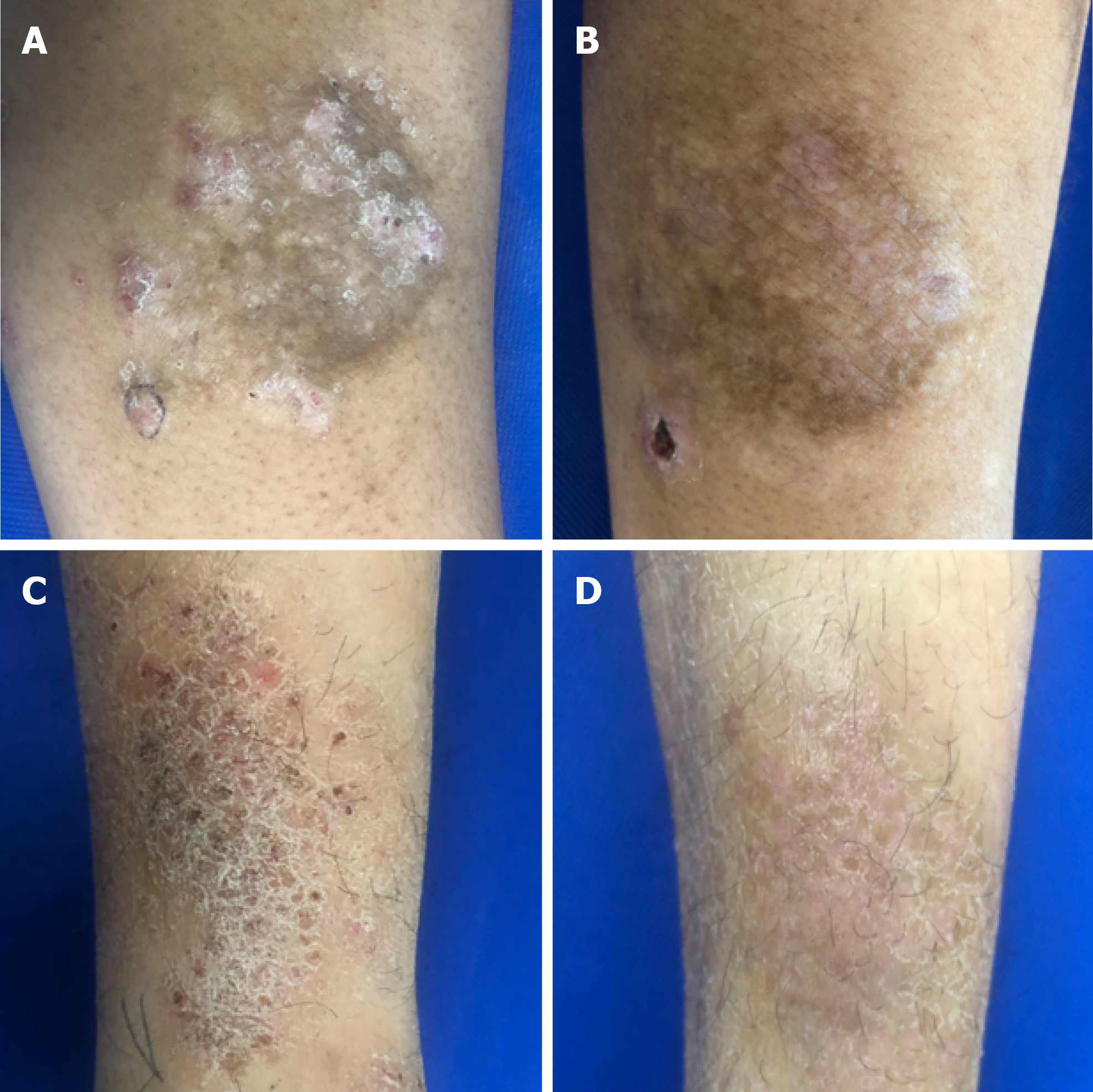
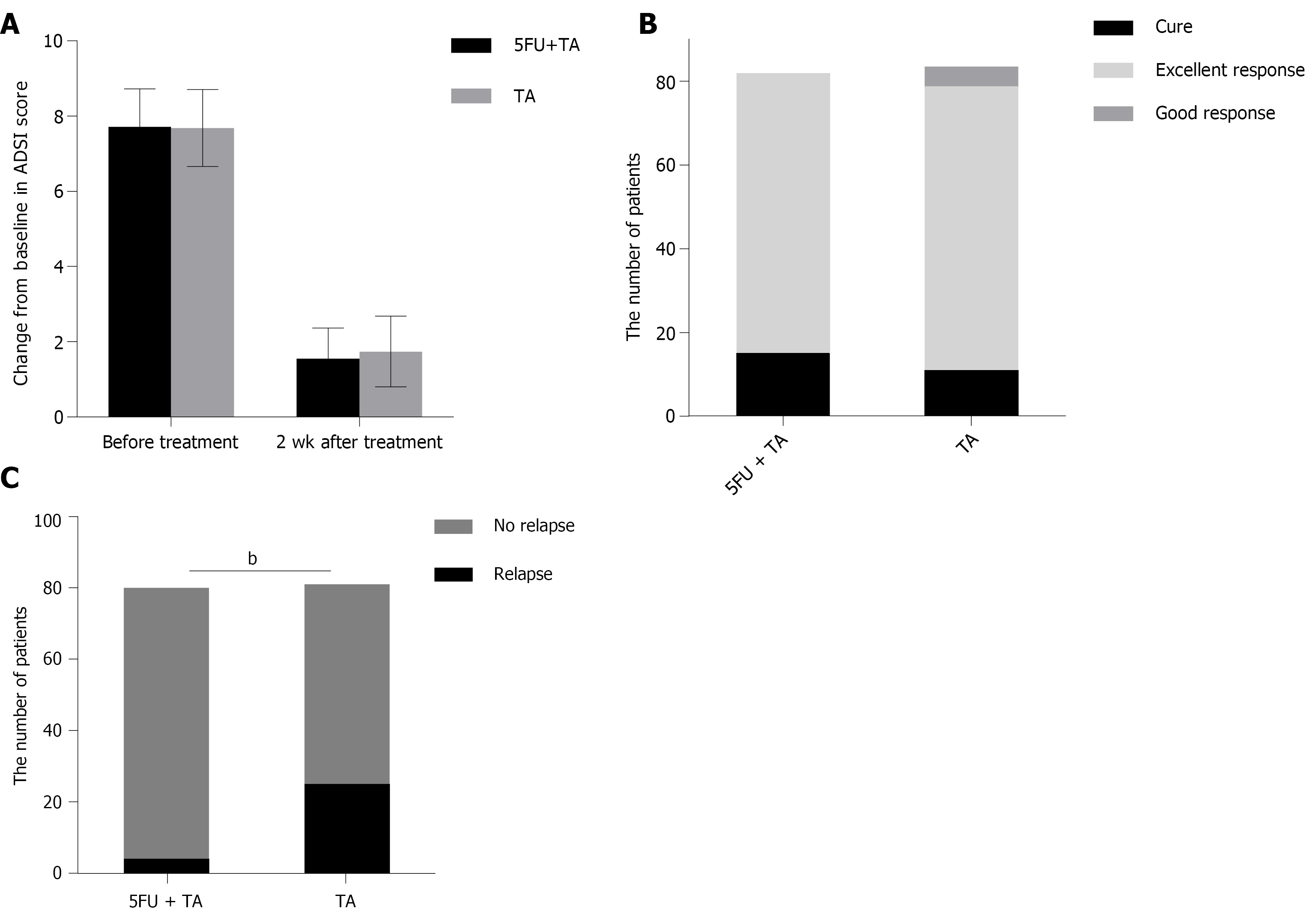
The mean ADSI scores of patients who complete the treatment and evaluation of clinical efficacy before treatment were 7.89 ± 0.89 in the 5-FU+TA group and 7.68 ± 1.02 in the TA group (P = 0.154). Two weeks after treatment, pruritus was alleviated significantly according to the patients, and the color of erythema became lighter in both groups. A small amount of scales was found in 23 out of 84 patients (27.38%) in the TA group (Figure 2). The mean ADSI scores at 2 wk after treatment for the 5-FU+TA group and TA group were 1.55 ± 0.82 and 1.74 ± 0.94, respectively. The ADSI scores for both groups were significantly lower than those before treatment for the same group (P < 0.001 for both), and there was no significant difference between the two groups (t = -1.383, P = 0.168; Figure 3A). The 5-FU+TA group had a cure rate of 18.29% (n = 15) and an excellent response rate of 81.70% (n = 67), and no cases showed a good response or nonresponse. The TA group had a cure rate of 13.10% (n = 11), an excellent response rate of 80.95% (n = 68), a good response rate of 5.95% (n = 5), and no cases of nonresponse. There was no significant difference in the percentage of effectiveness between the two groups (c2 = 3.201, P = 0.074; Figure 3B). Interestingly, at the 1-year follow-up, relapse had occurred in 4 patients (5.00%) in the 5-FU+TA group and 25 patients (30.86%) in the TA group (c2 = 18.232, P < 0.01, Figure 3C).
Six patients in the 5-FU+TA group and five patients in the TA group had local pruritus after injection, which resolved without further treatment. During the follow-up, local pigmentation was observed in both groups, and it gradually improved over time. No obvious skin atrophy, redness, swelling, heat, pain, or other symptoms of infection were noted in either group. There were no adverse reactions such as leukopenia, anemia, and thrombocytopenia in either group during the 1-year follow-up.
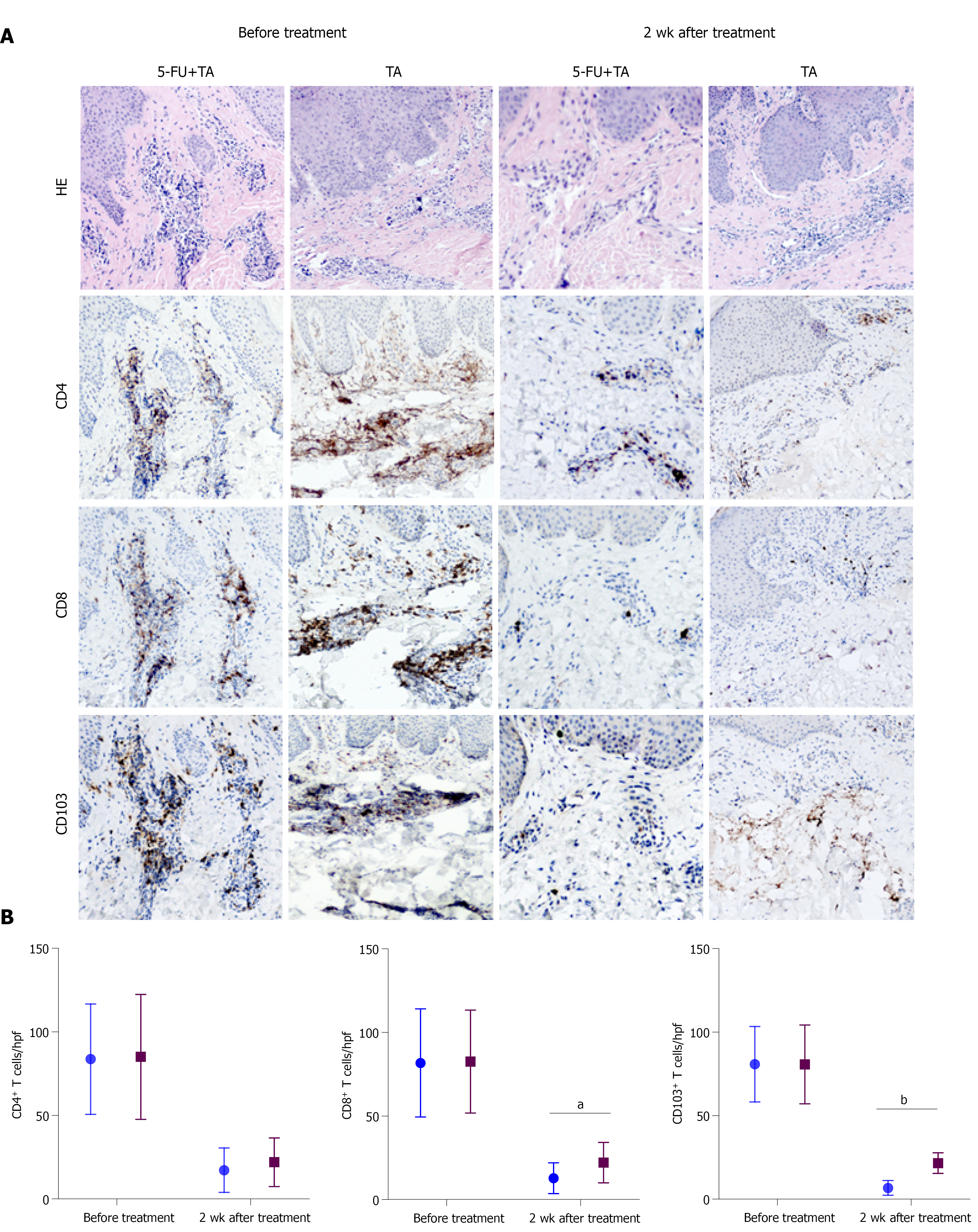
To explore the mechanism by which 5-FU and TA reduced the relapse rate of chronic eczema, we investigated differences in T-cell infiltration in the local skin lesions of patients from both groups before treatment and 2 wk after treatment by HE and immunohistochemical staining. The results showed that the lesional skin was infiltrated with lymphocytes and other inflammatory cells in the epidermis before treatment. A number of CD4+, CD8+, and CD103+ T-cell infiltration was observed in both groups with no significant differences (P = 0.914, 0.945, 0.988, respectively; Figure 4A). Two weeks after treatment, the CD4+, CD8+, and CD103+ T cell counts for the two groups were significantly less than before treatment (all P < 0.001, n = 15 for each group). Although no significant difference was observed in the number of CD4+ T cells between the two groups (P = 0.355) at 2 wk after treatment, the numbers of CD8+ and CD103+ T cells in the skin lesions were significantly less in the 5-FU+TA group than in the TA group (P = 0.025, P < 0.001; Figure 4B). In some skin lesions from the 5-FU+TA group, no inflammatory cell infiltration was observed.
Chronic eczema recurs frequently, and its treatment has always been challenging. Once the skin lesions become hypertrophic, the penetration of conventional topical drugs such as TCIs is decreased. Although intralesional corticosteroid has been proven effective for localized dermatitis, long-term or high-dose application of TCI treatment may cause skin atrophy, telangiectasia, increased vellus hair, systemic absorption, prickling, and burning sensations[3,19]. Intralesional 5-FU has been also used for treating localized inflammatory infection. The current study included 168 patients and showed that the combination therapy of 5-FU and TA was effective and safe for the treatment of chronic eczema. To the best of our knowledge, this prospective study is the first to observe the effect of 5-FU+TA on chronic eczema through intralesional injection.
To the best of our knowledge, there has been no study investigating the effectiveness of corticosteroids for eczema treatment through intralesional injection. A previous study investigated the use of 0.05% fluticasone propionate cream or 0.005% fluticasone propionate ointment given once or twice daily to eczema patients for 1 mo and continued twice weekly thereafter. In that study, the relapse rate at the 16 wk follow-up was 19% for the 0.05% fluticasone propionate cream group and 40% for the 0.005% fluticasone propionate ointment group. For the placebo group, the average relapse time was 6 wk, and the relapse rate was 64%[20]. In the present double-blind randomized study, local injection using 5-FU+TA or TA only was applied, and the patients were followed up for up to 1 year. Our data demonstrate that a one-time intralesional injection of TA in combination with 5-FU could successfully treat the localized rash of chronic eczema. Most importantly, the relapse rate in the 5-FU+TA group was significantly lower than that in the TA group (4.9% vs 31.64%), and also much lower than the rate in the previously mentioned study, demonstrating that 5-FU+TA treatment via intralesional injection can significantly reduce the recurrence of eczema.
The aggravating factors for adult eczema include environmental factors, sweating, physical irritation (including scratching), microbes, stress, and food[21]. Our study was a double-blind randomized controlled prospective study, and all participants were outpatients from our hospital with similar living environments and eating habits. We did not find statistically significant differences in age, sex, anatomical sites treated, or the treatment period among the groups.
There is a common concern of the potential adverse reaction of intralesional TA and 5-FU, such as infertility, skin atrophy, and telangiectasia. For the treatment of keloid scars, it is suggested that 5-FU (maximum 90 mg each injection) + TA treatment resulted in a lower incidence of skin atrophy and telangiectasia than treatment with TA alone (10-40 mg TA single injection)[22,23]. The common dosage range of 5-FU is 45-50 mg/mL, and each injection volume is less than 2 mL, with more than 3 sessions[24]. In this study, 25 mg 5-FU and 10 mg TA were used intralesionally once for the treatment. Compared with the dosage for keloid treatment, our dosage of 5-FU and TA is relatively low. Laboratory tests after 2 wk of treatment showed no abnormal results for blood and liver and renal function. Similarly, no such adverse events were observed during the 1-year follow-up. Moreover, the dosage of TA is half of the maximum safe dose reported in a previous study (20 mg) for the treatment of localized dermatitis[14]. No adverse reactions such as skin atrophy or telangiectasia were observed in this study, suggesting that the combination of 5-FU and TA at these dosages was safe for chronic eczema treatment. 5-FU and TA both have an immune suppressive effect, which requires attention regarding the chance of local infection. Patients were informed about the risk of bacterial contamination. No local infection was observed in either group during the follow-up. In clinical practice, topical antibiotics such as Mupirocin could be prescribed for infection prevention.
TRM cells are involved in the relapse of chronic eczema. TRM cells are distributed in the epidermis and dermis of the skin, and they constantly move around in the local area to quickly recognize a pathogen or an antigen that invades again, and this activity plays an important protective role on the entire skin system. In addition, TRM cells release IL-2, tumor necrosis factor-α, interferon-γ (IFN-γ), and other cytokines to further boost the immune response[25-27]. Results from our histopathological and immunohistochemical examinations suggest that the skin lesions from the 5-FU+TA group had significantly fewer CD103+ TRM cells and CD8+ cells than did those from the TA group at 2 wk after treatment, suggesting that 5-FU may reduce the recurrence of chronic localized eczema by minimizing the number of TRM cells in the lesion. Recent studies have shown that allergens can induce the production of CD8+ TRM cells through IL-17A and IFN-γ[28]. The exact mechanisms by which 5-FU reduces the retention of TRM cells require further investigation.
This study is not without limitations. Occupation may be a cofounding factor that we should have considered. A longer follow-up is warranted for monitoring the relapse rate and confirming the efficacy and safety of 5-FU+TA treatment. Finally, we would like to collect more samples to explore the potential mechanisms, possibly by measuring the cytokines secreted from the lesional site, in our future studies.
In summary, our study demonstrated that intralesional injection of 5-FU+TA can effectively and safely treat the localized rash of chronic eczema and significantly reduced the retention of TRM cells in the skin lesion. This combination may provide a new treatment option for chronic eczema patients with epidermis hypertrophy and localized rash.
Chronic eczema is an itchy, inflamed skin condition that tends to flare periodically. The latest findings suggest that tissue resident memory T (TRM) cells may play an important role in the pathogenesis of chronic eczema.
Intralesional injection of 5-fluorouracil (5-FU) and triamcinolone (TA) can effectively reduce local inflammation and significantly reduce the recurrence of eczema in a mouse model. There have been no clinical studies investigating the effect of 5-FU+TA for chronic eczema via intralesional injection.
To evaluate the efficacy and safety of intralesional injection of 5-FU and TA for the treatment of localized rash and management of relapse in chronic eczema patients and explore the potential underlying mechanism.
In this double-blind randomized controlled prospective study, we used the ADSI score to evaluate the efficacy of treatment and the effect on recurrence, and histopathological changes before and after treatment also were assessed.
The mean ADSI score and effective rates were comparable between the two groups, while the relapse rate was significantly lower in the 5-FU+TA group than in the TA group. Histological examination showed significantly fewer CD8+ and CD103+ T cells but not CD4+ T cells in the 5-FU+TA group.
5-FU+TA can effectively and safely treat the localized rash of chronic eczema and significantly reduce the retention of TRM cells in the skin lesion.
Low-dose intralesional injection of 5-FU+TA may be a new treatment option for chronic eczema patients with epidermis hypertrophy and localized rash.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Gupta SK, Koritala T S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1474] [Cited by in RCA: 1412] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 2. | Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 1057] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 3. | Raveendran R. Tips and Tricks for Controlling Eczema. Immunol Allergy Clin North Am. 2019;39:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Association IGoDSoCM. Guidelines for eczema diagnosis and treatment. Chinese Journal of Dermatology. 2011;1:5-6. |
| 5. | Hale G, Davies E, Grindlay DJC, Rogers NK, Harman KE. What's new in atopic eczema? Clin Exp Dermatol. 2019;44:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Brunner PM, Emerson RO, Tipton C, Garcet S, Khattri S, Coats I, Krueger JG, Guttman-Yassky E. Nonlesional atopic dermatitis skin shares similar T-cell clones with lesional tissues. Allergy. 2017;72:2017-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Gamradt P, Laoubi L, Nosbaum A, Mutez V, Lenief V, Grande S, Redoulès D, Schmitt AM, Nicolas JF, Vocanson M. Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. J Allergy Clin Immunol. 2019;143:2147-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Pan Y, Kupper TS. Metabolic Reprogramming and Longevity of Tissue-Resident Memory T Cells. Front Immunol. 2018;9:1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, Bergman JN, Chamlin SL, Cohen DE, Cooper KD, Cordoro KM, Davis DM, Feldman SR, Hanifin JM, Margolis DJ, Silverman RA, Simpson EL, Williams HC, Elmets CA, Block J, Harrod CG, Smith Begolka W, Sidbury R. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 837] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 10. | Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, Simpson EL, Papp KA, Hong HC, Rubel D, Foley P, Prens E, Griffiths CEM, Etoh T, Pinto PH, Pujol RM, Szepietowski JC, Ettler K, Kemény L, Zhu X, Akinlade B, Hultsch T, Mastey V, Gadkari A, Eckert L, Amin N, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD, Shumel B. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 862] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 11. | Kang EG, Narayana PK, Pouliquen IJ, Lopez MC, Ferreira-Cornwell MC, Getsy JA. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy. 2020;75:950-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Holm JG, Thomsen SF. Omalizumab for atopic dermatitis: evidence for and against its use. G Ital Dermatol Venereol. 2019;154:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Yang N, Chen Z, Zhang X, Shi Y. Novel Targeted Biological Agents for the Treatment of Atopic Dermatitis. BioDrugs. 2021;35:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Wendling J, Marchand A, Mauviel A, Verrecchia F. 5-fluorouracil blocks transforming growth factor-beta-induced alpha 2 type I collagen gene (COL1A2) expression in human fibroblasts via c-Jun NH2-terminal kinase/activator protein-1 activation. Mol Pharmacol. 2003;64:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Huang L, Wong YP, Cai YJ, Lung I, Leung CS, Burd A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br J Dermatol. 2010;163:1181-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Chen X, Wang G, Zeng Q, Zhang H, Hu Y, Yu A, Li T. Intralesional Treatment With 5-Fluorouracil and Steroid Improves Allergic Contact Dermatitis Without Causing Skin Atrophy and Rebound Lesions. Dermatitis. 2017;28:223-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Van Leent EJ, Gräber M, Thurston M, Wagenaar A, Spuls PI, Bos JD. Effectiveness of the ascomycin macrolactam SDZ ASM 981 in the topical treatment of atopic dermatitis. Arch Dermatol. 1998;134:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Papier A, Strowd LC. Atopic dermatitis: a review of topical nonsteroid therapy. Drugs Context. 2018;7:212521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, Parker CA; Multinational Study Group. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ. 2003;326:1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Katayama I, Aihara M, Ohya Y, Saeki H, Shimojo N, Shoji S, Taniguchi M, Yamada H; Japanese Society of Allergology. Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66:230-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Hietanen KE, Järvinen TA, Huhtala H, Tolonen TT, Kuokkanen HO, Kaartinen IS. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections - a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2019;72:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Srivastava S, Patil A, Prakash C, Kumari H. Comparison of Intralesional Triamcinolone Acetonide, 5-Fluorouracil, and Their Combination in Treatment of Keloids. World J Plast Surg. 2018;7:212-219. [PubMed] |
| 24. | Jiang ZY, Liao XC, Liu MZ, Fu ZH, Min DH, Yu XT, Guo GH. Efficacy and Safety of Intralesional Triamcinolone Versus Combination of Triamcinolone with 5-Fluorouracil in the Treatment of Keloids and Hypertrophic Scars: A Systematic Review and Meta-analysis. Aesthetic Plast Surg. 2020;44:1859-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Seidel JA, Vukmanovic-Stejic M, Muller-Durovic B, Patel N, Fuentes-Duculan J, Henson SM, Krueger JG, Rustin MHA, Nestle FO, Lacy KE, Akbar AN. Skin resident memory CD8+ T cells are phenotypically and functionally distinct from circulating populations and lack immediate cytotoxic function. Clin Exp Immunol. 2018;194:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, Mueller SN. Chemokine Receptor-Dependent Control of Skin Tissue-Resident Memory T Cell Formation. J Immunol. 2017;199:2451-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 619] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 28. | Schmidt JD, Ahlström MG, Johansen JD, Dyring-Andersen B, Agerbeck C, Nielsen MM, Poulsen SS, Woetmann A, Ødum N, Thomsen AR, Geisler C, Bonefeld CM. Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8+ T cells. Contact Dermatitis. 2017;76:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |