Published online Dec 16, 2013. doi: 10.12998/wjcc.v1.i9.290
Revised: November 9, 2013
Accepted: December 9, 2013
Published online: December 16, 2013
Processing time: 88 Days and 7 Hours
Tandem internal carotid and middle cerebral artery occlusion after carotid dissection predicts poor outcome after systemic thrombolysis. Current treatments include the use of endovascular carotid stenting, which carries with it a high risk of propagating further embolic events and worsening the dissection. New strategies for avoiding the aforementioned side-effects include recanalization using cross-collaterals for delivery of intra-lesional tissue plasminogen activator (tPA). We present two cases that provide further support for this novel approach. Both patients presented with a National Institute of Health Stroke Scale of 20, received intra-arterial tPA via cross-collateralization, and made full recoveries without the need for stenting.
Core tip: Tandem internal carotid artery and middle cerebral artery occlusions secondary to carotid artery dissections are refractory to stand alone medical management and often result in poor outcomes in patients receiving systemic tissue plasminogen activator (tPA). Cervical carotid stent assisted endovascular thrombolysis is effective, but carries the risk of worsening the dissection and propagating further thromboembolic events. Avoidance of carotid occlusions and recanalization with intra-arterial tPA using cross-collateralization, may be an effective, alternative treatment for patients with tandem internal carotid artery and middle cerebral artery occlusions.
-
Citation: Bulsara KR, Ediriwickrema A, Pepper J, Robertson F, Aruny J, Schindler J. Tissue plasminogen activator
via cross-collateralization for tandem internal carotid and middle cerebral artery occlusion. World J Clin Cases 2013; 1(9): 290-294 - URL: https://www.wjgnet.com/2307-8960/full/v1/i9/290.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i9.290
Dissection of the internal carotid artery (ICA) accounts for a significant proportion of ischemic stroke in young patients, representing up to a quarter of such cases[1,2]. Lucas and colleagues illustrated that the underlying pathophysiology of carotid artery dissection ischemia is most often due to thrombus formation and secondary embolization[3]. In many cases, this embolization can result in a tandem ICA and middle cerebral artery occlusion (TIM)[4]. At presentation, patients with TIM occlusions usually have a similar clinical severity to those with isolated middle cerebral artery (MCA) obstruction. However, they have a lower chance of MCA recanalization after systemic tissue plasminogen activator (tPA) therapy and often result in worse clinical outcomes[4-6]. A review of 221 stroke patients identified TIM occlusions as an independent predictor of poor outcomes after systemic thrombolysis[6].
The current treatment regime involves systemic tPA within 3 h of presentation. However, as previously noted, this may not allow adequate cerebral reperfusion in a TIM occlusion and may, unfortunately, predispose the development of a malignant brain infarct[7]. In order to improve clinical outcomes, this group of patients may need more aggressive intervention to restore cerebral perfusion. However, the ideal treatment for such cases remains elusive[8,9].
Recently, treatment of patients with ICA dissections refractory to medical management has focused on endovascular stenting and angioplasty[10]. A small patient series described stent-assisted thrombolysis in TIM occlusions. Specifically, the proximal ICA was recanalized with stent implantation followed by MCA recanalization via subsequent intra-arterial thrombolysis or thrombectomy[11]. In theory, endovascular therapies in the treatment of carotid dissection occlusions may lead to significant procedural complications, as the lesion pathology requires the interventionist to navigate the true lumen. Failure to do so may result in extending the dissection or vessel perforation, which in turn may lead to worse outcomes.
In certain cases, avoidance of the ICA may be preferred. Treatment of the MCA occlusion in a TIM by bypassing the ICA has only been described in three previous reports[12-14]. We provide two additional cases demonstrating the successful recanalization of an MCA occlusion by administering intra-arterial tPA through a microcatheter guidewire via cross-collateralization.
A 52-year-old male arrived at an outside hospital with global aphasia, right hemiplegia and a National institute of Health Stroke Scale (NIHSS) of 20. He was found to have a left MCA stroke on head computed tomography (CT). Tele Stroke was activated and he was given intravenous (iv) tPA at 1.5 h after symptom onset and transferred to a tertiary hospital. The patient’s exam remained unchanged on arrival, head CT showed no evidence of stroke, and he was subsequently taken to the angiography suite.
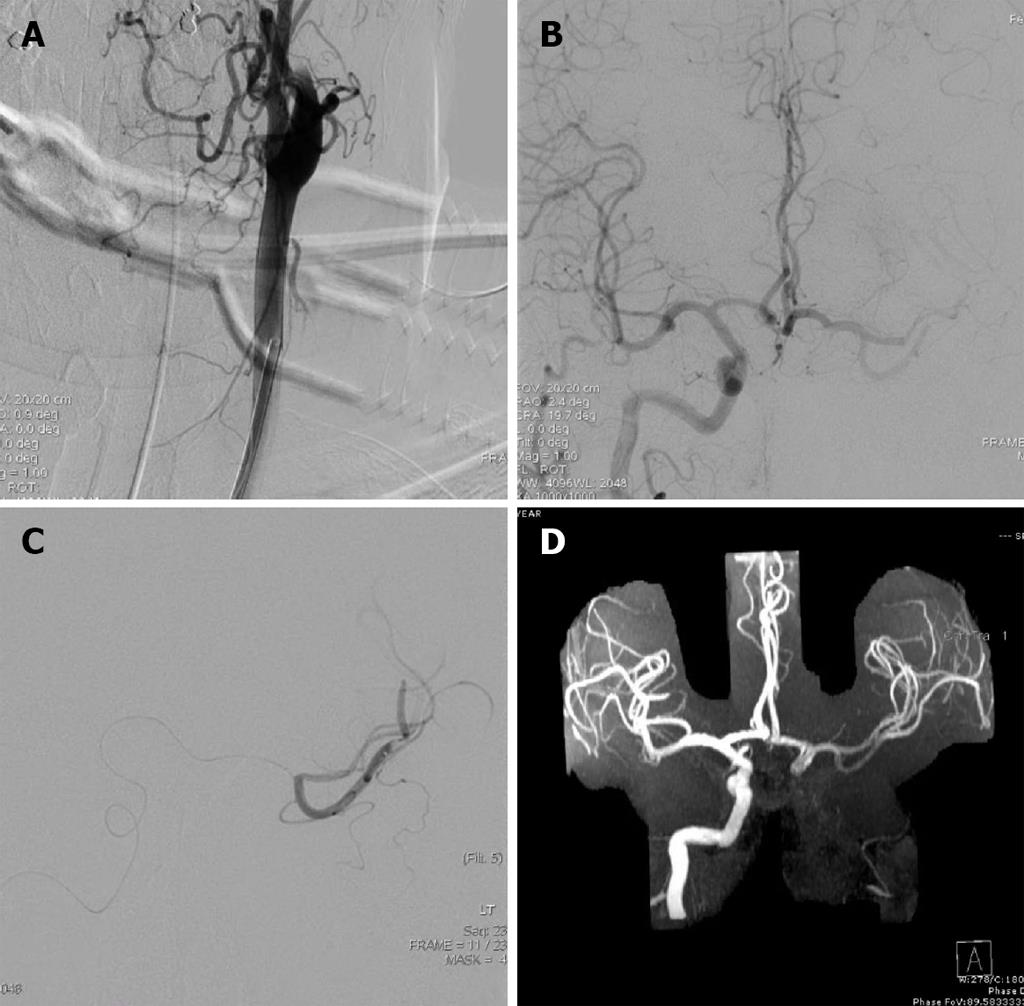
Under general anesthesia, the left common carotid artery was catheterized and images of the cervical carotid arteries were obtained (Figure 1A). He was found to have a left carotid artery dissection (CAD). At this point, the proximal left ICA was partially opened. The true lumen, however, was difficult to identify and further attempts risked propagating the dissection intracranially.
Subsequently, the right ICA was catheterized through the right common carotid artery for angiography. A thrombus was located in the middle Sylvian (M2) position; imaging was obtained during the arterial phase so opacification was not seen. The anterior communicating artery (ACOMM) was slightly greater than 1 mm. At this point, a microcatheter was advanced from the right internal carotid system via the ACOMM into the left MCA. Both the superior and inferior trunks were catheterized and a total of 2 mg of tPA was delivered in each trunk providing an additional 4 mg to the systemic tPA dose (Figure 1B and C).
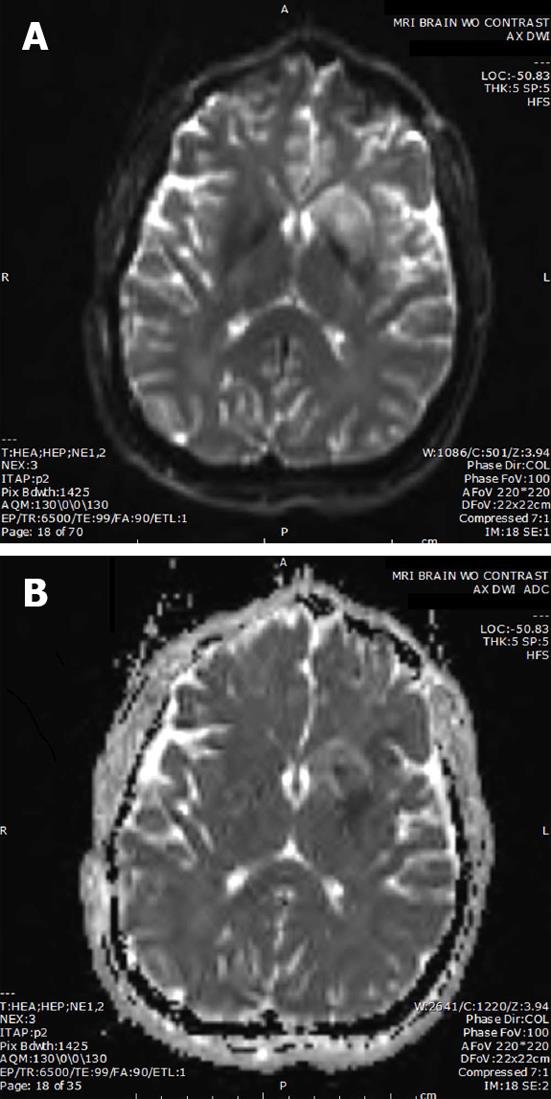
Post-procedure Thrombolysis in Cerebral Infarction (TICI) score and immediate arteriogram were unchanged. The intervention was performed three hours post symptom onset and the procedure duration was two hours. Ten hour magnetic resonance angiography (MRA) confirmed recanalization of the MCA (Figure 1D), and magnetic resonance imaging (MRI) revealed a small stroke (Figure 2). Pre-procedure MRI was not performed. Heparin (iv) was given on the following day. Of note, anticoagulation was not started earlier since the patient received a full dose of iv tPA prior to the intervention. After twenty-four hours, the patient had full strength and minimal word finding with an NIHSS of two. After 3 mo, his NIHSS was zero. Three years later, he has returned to work and continues without neurological deficits.
Our second patient was a 40-year-old male who presented with left hemiplegia, and was diagnosed with CAD after obtaining a cerebral angiogram. He was treated with intra-lesional tPA via cross-collateralization, and recanalization of the right MCA was obtained eight hours post presentation. His pre and post-procedure NIHSS, as well as his NIHSS 3 mo later, were by coincidence the same as the first patient. He continues to have a non-focal neurologic exam three years post treatment.
Ischemic stroke associated with CAD is primarily due to embolic phenomena, which can affect many vessels and commonly targets the ICA, MCA or both in tandem. The latter independently predicts poor clinical outcome and so efficacious and consistent treatments are highly desired[6]. Thrombolysis has typically been the management of choice for CAD, and is thought to prevent subsequent embolic events[15]. However, a definitive treatment of choice for CAD does not exist. Evidence for tPA use in CAD is lacking, and may be associated with certain risks including hematoma extension and subarachnoid hemorrhage. Thrombolytics (iv) have been shown to cause recanalization in approximately 30%-40% of patients. This treatment has not been efficacious in the case of TIM[6]. Of interest, endovascular stenting has successfully been employed to treat medical refractory CAD that resulted in thromboembolic events[11,16].
Lavallée et al[11] analyzed the benefits of stent assisted endovascular thrombolysis with iv tPA involving a small series of ICA dissections. In this study, 10 patients who met the selection criteria were given either systemic tPA (n = 4) or endovascular stenting and intra-lesional tPA (n = 6). Those in the endovascular group had significantly better prognosis, which was linked to recanalization of the occluded vessels, particularly the MCA. The MCA was patent in all endovascular cases and occluded in three of the four cases in the systemic tPA group. Both embolization and in stent thrombosis were side effects of endovascular treatment.
Opening the ICA is the ideal option, however, given the difficulty of observing the true carotid lumen and associated risk of extending the dissection intracranially, bypassing the dissection was considered the optimal strategy. Rahme et al[13] describes 15 cases in the literature of treating MCA thrombosis in TIM occlusions via cross-collateralization, and recanalization was achieved in 54.5%-75.0% of cases. Our report provides further support for the strategy of treating TIM occlusion via delivery of intra-lesional tPA using collateral vessels. In both cases presented, intra-lesional tPA was administered through a microcatheter passed through the ACOMM, and recanalization was observed via angiographic improvement on MRA. At 3 mo, both patients had fully recovered and returned to their daily activities. They continued to have a non-focal neurologic exam three years later.
Our presented cases did not have any complications, however, the risk of the described intervention includes endangering the contralateral carotid circulation, damaging smaller collateral arteries, and compromising collateral flow. Specifically, instrumenting small caliber arteries may result in dissection, occlusion, or distal thrombosis. This approach is ideal for patients in whom it is felt that the cervical carotid artery cannot be safely recanalized or in whom recanalization of the cervical carotid artery may lead to significant reperfusion hemorrhage. The ideal patient for this procedure is directly dependent on the presence of a collateral vessel, like the ACOMM, having an adequate diameter for passage of a microcatheter. Radiographic studies have demonstrated that an intact anterior circulation is present in 74%-90% of the population and an intact posterior circulation is present in 48.5%-63% of the population[17-20]. Of note, a review of anatomic variants in healthy Chinese individuals demonstrated that a complete anterior circulation with incomplete posterior circulation is present in 47.7% of individuals; a complete posterior circulation with an incomplete anterior circulation is only present in 5.2% of individuals[17]. Another risk includes receiving an additional 4 mg of tPA to the systemic dose which increases risk for hemorrhage. It is important to analyze the risks and benefits of any procedure. The presented patients were highly functional with a devastating stroke, and, therefore, the intervention was considered worthwhile.
TIM secondary to CAD are refractory to pure medical management and strongly predict poor outcomes in patients. Stent assisted endovascular thrombolysis is effective when compared against traditional management but carries an additional risk of worsening the dissection and propagating further thromboembolic events. Therefore, avoidance of the occluded carotid artery may be preferred in certain scenarios. In these cases, delivery of intra-lesional tPA using collateral vessels resulted in complete clinical recovery and recanalization of the occluded MCA. Our experience provides further support for utilization of this novel method of treatment when other modalities may not be feasible.
Tandem internal carotid artery and middle cerebral artery occlusions secondary to carotid artery dissections are refractory to stand alone medical management, and recanalization with intra-arterial tissue plasminogen activator (tPA) using cross-collateralization may be an effective alternative for treating these patients.
The patient presented with global aphasia and right hemiplegia suggestive of a left middle cerebral artery stroke.
The differential diagnosis includes left middle cerebral artery thrombosis or dissection.
Cerebral angiography revealed left internal carotid artery dissection and left middle cerebral artery occlusion.
tPA (iv) was first used for recanalization but failed, therefore, catheter guided intra-arterial delivery of tPA was then implemented towards successfully recanalizing the middle cerebral artery occlusion.
There have only been three reports describing the use of intra-arterial tPA delivery via cross-collaterals in the literature, and we provide two more cases supporting its use in treating tandem internal carotid artery and middle cerebral artery occlusions.
Cross-collateralization: Using collateral vessels to navigate endovascular instruments around abnormal or damaged vessels.
Stent assisted endovascular thrombolysis of tandem internal carotid artery and middle cerebral artery occlusions (TIM) is effective but carries the risk of worsening the dissection and propagating further thromboembolic events, and, therefore, avoidance of carotid occlusions and recanalization with intra-arterial tissue plasminogen activator, using collateral vessels may be an effective alternative for treating patients with TIM.
The strengths of this article is that it provides additional cases where utilizing collateral vessels was an effective strategy for treating tandem internal carotid artery and middle cerebral artery occlusions. The report also summarizes important factors to consider when determining whether a patient is a good candidate for this intervention. The study is well-written and interesting.
P- Reviewers: Paraskevas KI, Rossow L S- Editor: Zhai HH L- Editor: A E- Editor: Yan JL
| 1. | Ducrocq X, Lacour JC, Debouverie M, Bracard S, Girard F, Weber M. [Cerebral ischemic accidents in young subjects. A prospective study of 296 patients aged 16 to 45 years]. Rev Neurol (Paris). 1999;155:575-582. [PubMed] |
| 2. | Bogousslavsky J, Pierre P. Ischemic stroke in patients under age 45. Neurol Clin. 1992;10:113-124. [PubMed] |
| 3. | Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | El-Mitwalli A, Saad M, Christou I, Malkoff M, Alexandrov AV. Clinical and sonographic patterns of tandem internal carotid artery/middle cerebral artery occlusion in tissue plasminogen activator-treated patients. Stroke. 2002;33:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Kim YS, Garami Z, Mikulik R, Molina CA, Alexandrov AV. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke. 2005;36:869-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Rubiera M, Ribo M, Delgado-Mederos R, Santamarina E, Delgado P, Montaner J, Alvarez-Sabín J, Molina CA. Tandem internal carotid artery/middle cerebral artery occlusion: an independent predictor of poor outcome after systemic thrombolysis. Stroke. 2006;37:2301-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 7. | Jaramillo A, Góngora-Rivera F, Labreuche J, Hauw JJ, Amarenco P. Predictors for malignant middle cerebral artery infarctions: a postmortem analysis. Neurology. 2006;66:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Brandt T, Caplan L. Spontaneous Arterial Dissection. Curr Treat Options Neurol. 2001;3:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Beletsky V, Norris JW. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;345:467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 971] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 10. | Kadkhodayan Y, Jeck DT, Moran CJ, Derdeyn CP, Cross DT. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328-2335. [PubMed] |
| 11. | Lavallée PC, Mazighi M, Saint-Maurice JP, Meseguer E, Abboud H, Klein IF, Houdart E, Amarenco P. Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke. 2007;38:2270-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Ozdemir O, Bussière M, Leung A, Gulka I, Lee D, Chan R, Spence JD, Pelz D. Intra-arterial thrombolysis of occluded middle cerebral artery by use of collateral pathways in patients with tandem cervical carotid artery/middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2008;29:1596-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Rahme R, Abruzzo TA, Ringer AJ. Acute ischemic stroke in the setting of cervical carotid occlusion: a proposed management strategy. World Neurosurg. 2011;76:S60-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Padalino DJ, Deshaies EM. Tandem middle cerebral artery-internal carotid artery occlusions: reduced occlusion-to-revascularization time using a trans-anterior communicating artery approach with a penumbra device. J Neurosurg. 2012;116:665-671. [PubMed] |
| 15. | Derex L, Nighoghossian N, Turjman F, Hermier M, Honnorat J, Neuschwander P, Froment JC, Trouillas P. Intravenous tPA in acute ischemic stroke related to internal carotid artery dissection. Neurology. 2000;54:2159-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Smith WS, Dowd CF, Halbach VV. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000;21:1280-1292. [PubMed] |
| 17. | He J, Liu H, Huang B, Chi C. [Investigation of morphology and anatomic variations of circle of Willis and measurement of diameter of cerebral arteries by 3D-TOF angiography]. Shengwu Yixue Gongchengxue Zazhi. 2007;24:39-44. [PubMed] |
| 18. | Macchi C, Lova RM, Miniati B, Gulisano M, Pratesi C, Conti AA, Gensini GF. The circle of Willis in healthy older persons. J Cardiovasc Surg (Torino). 2002;43:887-890. [PubMed] |
| 19. | Hartkamp MJ, van Der Grond J, van Everdingen KJ, Hillen B, Mali WP. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke. 1999;30:2671-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |