Published online Dec 16, 2013. doi: 10.12998/wjcc.v1.i9.276
Revised: October 29, 2013
Accepted: November 2, 2013
Published online: December 16, 2013
Processing time: 172 Days and 4.1 Hours
Acute abdominal pain is a common complaint in pediatric emergency departments. A complete evaluation is the key factor approaching the disease and should include the patient’s age, any trauma history, the onset and chronicity of the pain, the related symptoms and a detailed physical examination. The aim of this review article is to provide some information for physicians in pediatric emergency departments, with the age factors and several causes of non-traumatic acute abdominal pain. The leading causes of acute abdominal pain are divided into four age groups: infants younger than 2 years old, children 2 to 5, children 5 to 12, and children older than 12 years old. We review the information about acute appendicitis, intussusception, Henoch-Schönlein purpura, infection, Meckel’s diverticulum and mesenteric adenitis. In conclusion, the etiologies of acute abdomen in children admitted to the emergency department vary depending on age. A complete history and detailed physical examination, as well as abdominal imaging examinations, could provide useful information for physicians in the emergency department to narrow the differential diagnosis of abdominal emergencies and give a timely treatment.
Core tip: The mini review provides the essential information for physicians in pediatric emergency departments, mainly focused on the clinical diagnosis in different age groups and on several major causes of acute abdominal pain in children.
- Citation: Yang WC, Chen CY, Wu HP. Etiology of non-traumatic acute abdomen in pediatric emergency departments. World J Clin Cases 2013; 1(9): 276-284
- URL: https://www.wjgnet.com/2307-8960/full/v1/i9/276.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i9.276
A common complaint in pediatric emergency departments (ED), abdominal pain is sometimes hard to assess in ill children due to the variation of pain degree, the difficulty in describing it, and being localized to the abdomen. Although most children with abdominal pain have a self-limiting course, some critical medical and surgical emergencies may occur in the ED. The diverse etiologies include acute surgical disease, intra-abdominal medical disorders, extra-abdominal conditions, systemic illness and, commonly, functional abdominal pain. A timely diagnosis is necessary for preventing further complications and, of course, the possible legal problems. This article reviews the non-traumatic causes of acute abdominal pain in pediatric EDs.
Theoretically, abdominal pain results from visceral, somatic and referred pain. Visceral pain results from the distension of a viscus stimulating nerves and generally presents with a dull, poorly localized pain over the epigastric, periumbilical or suprapubic midline area. The somatic pain comes from the stimulation on somatic nerves in the parietal peritoneum, muscle or skin unilateral to the spinal cord level from T6 to L1, presenting as well localized, intense and sharp. Referred pain is felt distant from the diseased organs, characterized by either a sharp, localized sensation or a vague ache. However, all the pains above can present clinically with agonizing pains, making it hard for the pediatrician to take a history and accurate physical examination.
The evaluation should begin with a competent clinical evaluation, including the patient’s age, any trauma history, the onset and chronicity of the pain, the related symptoms and a detailed physical examination. Abdominal imaging is not always required but is sometimes invaluable for narrowing the differential diagnosis or confirming a diagnosis. When assessing the child who develops abdominal pain without a history of trauma in the pediatric ED, the first priority is stabilization if the child is seriously ill. It should be emphasized that abdominal emergency may progress to a shock status and cardiorespiratory shock can present with acute abdominal pain. The second priority is to identify the child who requires immediate or potential surgical intervention, such as acute appendicitis or bowel perforation. The third priority is to diagnose any medical illness from among a large group of acute and chronic abdominal and extra-abdominal inflammatory disorders that require emergency nonoperative management.
During diagnosis, the factors of age, chronicity and any presence of obstruction, peritonitis or a mass should be carefully considered.
Age is a very important factor when assessing abdominal pain in children and the prevalence of each etiology varies greatly in children of different age groups (Table 1).
| Younger than 2 yr | 2 to 5 yr | 5 to 12 yr | Older than 12 yr |
| Infantile colic | Gastroenteritis | Gastroenteritis | Appendicitis |
| Gastroenteritis | Appendicitis | Appendicitis | Gastroenteritis |
| Constipation | Constipation | Constipation | Constipation |
| UTI | UTI | Functional pain | Dysmenorrhea |
| Intussusception | Intussusception | UTI | Mittelschmerz |
| Volvulus | Volvulus | Trauma | PID |
| Incarcerated hernia | Trauma | Pharyngitis | Threatened abortion |
| Hirschsprung’s disease | Pharyngitis | Pneumonia | Ectopic pregnancy |
| Sickle cell crisis | Sickle cell crisis | Ovarian/Testicular torsion | |
| HSP | HSP | ||
| Mesenteric adenitis | Mesenteric adenitis |
The infant with acute abdominal pain in this age group is very difficult for primary clinicians to evaluate because the only symptom may be inconsolable crying and finally lethargy. Bilious emesis is key information and must be taken seriously as a possibility of malrotation with volvulus. A contrast study of the upper gastrointestinal tract is necessary if no other cause is evident. The history taken should include the bowel movement pattern, presence of fever or diarrhea or even currant jelly stool, amount of vomiting and the timing, the sequence of pain and vomiting, and the presence of a productive cough.
Paralytic ileus, manifesting clinically with distension and absent bowel sounds, often accompanies surgical conditions, sepsis and infectious enterocolitis and should be closely followed up. An incarcerated hernia and intussusception are the two most common causes of bowel obstruction in this age range. Abdominal imaging could provide useful evidence of obstruction or perforation signs and any signs of partial or complete obstruction with peritonitis may indicate a perforated viscus from intussusceptions, volvulus or occasionally appendicitis or Hirschsprung’s disease.
Infants with recurrent or chronic abdominal pain in this age group may not have any symptoms. The differential diagnosis includes recurrent intussusceptions, malrotation with intermittent volvulus, milk allergy syndrome and various malabsorptive diseases, such as lactase deficiency. Abdominal sonography should be arranged for any irritably crying infants or infants with lethargy for any evidence of intussusception. Reduction with air or barium is also indicated in cases of suspected intussusceptions which cannot be clearly and directly revealed by ultrasound. Abdominal computed tomography (CT) with contrast is indicated during the process of evaluation when clinicians are suspicious of serious morbidity, such as complex abdominal masses and fluid accumulation.
Common causes of acute abdominal pain in this age group include inflammatory processes such as gastroenteritis and urinary tract infection (UTI). Preschool children may be able to verbally describe the types of abdominal pain and localize the site of pain. Related histories should be taken seriously. Some associated symptoms are helpful in the differential diagnosis, such as that lower gastrointestinal (LGI) bleeding may indicate infectious enterocolitis, intussusceptions, Meckel’s diverticulum or inflammatory bowel disease etc. and extra-abdominal symptoms such as productive cough and pyrexia may be attributed to lobar pneumonia. A diabetic child with acute abdominal pain may be considered as having diabetes ketoacidosis. The important surgical causes of children with abdominal pain in this age group include acute appendicitis, intussusception and malrotation. Clinically ill children with abdominal pain may suffer from life-threatening diseases or some uncommon etiologies. Physical examinations of such patients may appear as jaundice (hepatitis, hemolytic anemia), rash or arthritis (anaphylactoid purpura), or cardiac murmurs (myocarditis). Moreover, chronic constipation starts to increase in frequency in this age group.
Right lower quadrant (RLQ) tenderness, especially persistent pain over here, should be always considered for the probability of acute appendicitis. The most specific finding of an abdominal plain film is the presence of a calcified appendicolith; however, this is only present in a minority of patients (< 10%)[1,2]. Besides, localized bowel obstruction and obliteration of the psoas shadow may be found in cases of acute appendicitis. Abdominal sonography should also be performed over the entire abdomen, including the pelvis area, to exclude any probability of ovarian torsion which is rarely noted but actually presents in female patients of this age group.
In this age group, nonorganic or psychogenic illness, or functional abdominal pain start to increase as causes of acute abdominal pain. The leading organic causes are still inflammatory processes, including gastroenteritis, appendicitis and UTI. If intussusception presents in this age group, a leading point, such as mesenteric adenitis, lymphoma, polyp and anaphylactoid purpura, should be sought for and abdominal ultrasound and CT may be helpful for clinicians to identify the leading points.
The description of abdominal pain is generally reliable in children in this age range. In clinical presentations, the presence of fever may come from infectious causes; diarrhea may be caused by infectious colitis, IBD from an appendiceal abscess irritating the bowel, urinary frequency and dysuria may increase the possibility of UTI. The clinical history of abdominal pain, beginning from the periumbilical area first and migrating to the RLQ of the abdomen after several to 24 h, may commonly present in older children suspected of having acute appendicitis.
Clinical presentations of functional abdominal disorders are generally episodic periumbilical, rarely occur during sleep, have no particular associations with eating and activity and are not of organic causes. Although a worrisome patient and parents may search for definite causes of recurrent abdominal pain, abdominal CT rarely helps. However, the premise is that the diagnosis of functional abdominal pain should be made through complete history, physical examination and even whole abdominal and pelvis ultrasound scan. Of course, if the abdominal pain still presents without improving after a period of light diet or no oral intake, further survey should be carried out, e.g. abdominal CT.
The history and physical examination of acute abdominal pain should be taken carefully, especially for female patients. Complete history taking should involve menstrual history and sexual activity, although it is often hard to ascertain.
Acute pain with peritonitis in adolescents usually results from acute appendicitis. In addition, the differential diagnosis should include testicular or ovary torsion and for females PID and ectopic pregnancy should be considered. Acute abdominal pain without peritoneal signs in males often results from gastroenteritis or a viral syndrome, whereas in females it may often be attributed to UTI or pyelonephritis.
In the ED, postmenarchal girls with abdominal pain should have a pregnancy test when pregnancy cannot be excluded. Ultrasound is often helpful to check the gestational sac and evaluate the condition of adnexa. Especially for the female patient with RLQ pain, sonography with color Doppler is helpful for differentiating acute ovarian torsion from appendicitis and may also be helpful for assessing ovary viability. Besides, some experts have suggested that ovarian cysts greater than 5 centimeters rarely cause ovarian torsion and most cases of ovarian torsion are secondary to adnexal pathology, such as ovarian tumors or cysts, and torsion of normal adnexa is less common.
The diverse etiologies include acute surgical disease, intra-abdominal medical ailments, extra-abdominal conditions, systemic illness and, commonly, functional abdominal pain. We review five non-traumatic causes of acute abdominal pain in the pediatric ED, including acute appendicitis, intussusception, Henoch-Schönlein purpura (HSP), infection, Meckel’s diverticulum and mesenteric adenitis.
Acute appendicitis is the most common condition requiring emergency abdominal surgery in children and is ultimately diagnosed in 1% to 8% of children presenting to the pediatric ED with acute abdominal pain[2,3]. Clinically, the diagnosis of acute appendicitis is often based on a brief history, physical examination and laboratory findings. Common clinical appearances of acute appendicitis in children include migration of abdominal pain, RLQ tenderness, rebound pain, muscle guarding and vomiting. Very young children may reveal diarrhea as a presenting symptom. The classic constellation of symptoms in acute appendicitis is periumbilical pain followed by nausea, right lower quadrant pain, vomiting and fever. Unfortunately, this sequence is present in only less than one-third of all pediatric patients and is less common in children younger than 5 years of age[4]. Appendicitis may be missed at initial clinical examination in 28% to 57% of children aged 12 years or younger and in nearly 100% of children under 2 years old. Because of the difficulty in evaluating young children with abdominal pain, perforation rates for appendicitis are higher than in the general adult population (30% to 65%)[3,5].
No laboratory test is both 100% sensitive and 100% specific for diagnosing appendicitis. White blood cell count may be helpful in the diagnosis of appendicitis and the presence of elevated serum C-reactive protein (CRP) also has been studied as a biomarker for appendicitis. Moreover, the changes after short-term observation in percentage neutrophil counts on the first day of the onset of patients’ symptoms (day 1) and CRP on day 2 and day 3 are relatively specific for diagnosing acute appendicitis and may be used to exclude other inflammatory conditions in the abdomen[6].
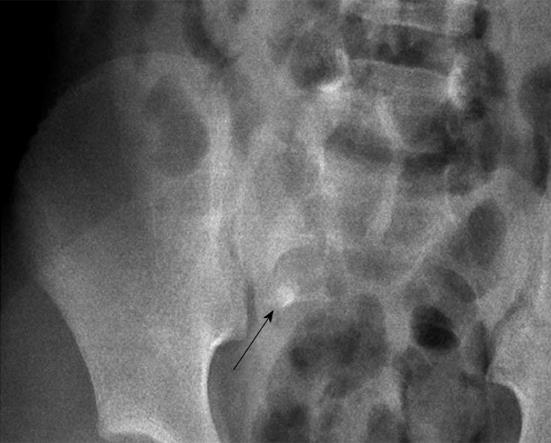
Plain film abdominal series are often obtained in children suspected of having acute appendicitis and the most specific finding is calcified appendicolith. Appendicoliths are present only in approximately 10% of true cases with appendicitis (Figure 1)[1,2,4].
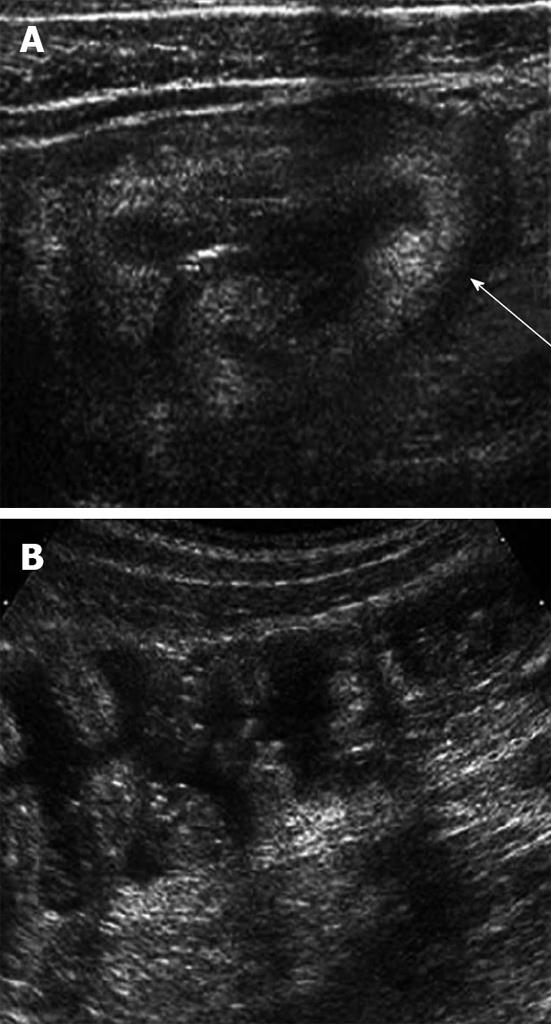
Diagnostic imaging, including graded-compression US and helical CT, play an increasing role in the prompt and accurate diagnosis of acute appendicitis in children. The principal advantages of graded-compression US are its lower cost, lack of ionizing radiation and ability to delineate gynecological disease. However, an important limitation of graded-compression US for the diagnosis of acute appendicitis is that the diagnostic accuracy is highly dependent on the skill of operators, as evidenced by the great variability in its reported diagnostic sensitivity and specificity for this condition. The reported sensitivity of US in children has ranged from 44% to 94% and the specificity has ranged from 47% to 95%[3]. An inflamed appendix is usually aperistaltic, difficult to compress and measures ≥ 6 mm in diameter (Figure 2A). It is important for US performers to visualize the entire appendix to avoid a false-negative reading because sometimes only the distal tip of the appendix is inflamed. A periappendiceal fluid accumulation may indicate an early perforation but may simply result from inflammation.
CT has become the test of choice for surgeons when ultrasonography fails to give a definitive diagnosis. The reported sensitivity of CT for the diagnosis of acute appendicitis in children has ranged from 87% to 100% and the specificity has ranged from 89% to 98%[4,5]. Direct signs of CT for acute appendicitis include an enlarged appendix (> 7-mm transverse diameter), a nonopacified appendiceal lumen and significant wall enhancement with intravenous contrast material administration (Figure 2B). Secondary signs of acute appendicitis include periappendiceal fat stranding or free fluid in the RLQ of the abdomen or pelvis. Focal cecal wall thickening adjacent to an inflamed appendix has been given specific names: focal cecal apical thickening, the so-called arrowhead sign which involves focal thickening of the cecum pointing toward an inflamed appendix, or the so called cecal bar in which an appendicolith is separated from a contrast material-filled cecum by an inflammatory process at the base of the appendix.
CT has dramatically improved our ability to detect appendicitis and its complications. It has led to improved patient outcomes and lessened the number of unnecessary surgeries. Magnetic resonance imaging (MRI) is also superior in its ability to diagnose appendicitis in children but it may not be available or practical.
When the diagnosis of appendicitis is made, preparing the child for the operating room is essential. If there are clinical or radiological signs of perforation, antibiotics with gram negative and anaerobic coverage should be started in the ED.
Intussusception, defined as an invagination of the proximal portion of the bowel into an adjacent distal bowel segment, is the second most common cause of intestinal obstruction in infants. It appears predominantly in males and the most common type is ileocolic invagination. Intussusception is seen most frequently between the ages of 3 mo and 5 years, with 60% of cases occurring in the first year and a peak incidence at 6 to 11 mo of age. In children younger than 2 years of age, a pathological lead point is found in less than 5% to 10% of cases[7,8]. However, pathological lead points are more common in older children, with Meckel’s diverticulum being the most common. Other causes of lead points are submucosal hemorrhage from Henoch-Scholein purpura, lymphomas and intestinal polyp. The classic triad of intussusception, including intermittent colicky abdominal pain, vomiting and bloody mucus stools (currant jelly stools) is encountered in only 20% to 40% of cases[7]. At least two of these findings present in approximately 60% of patients.
A palpable sausage-like abdominal mass in the right upper or lower quadrant may be found but it is difficult to perform in crying infants.
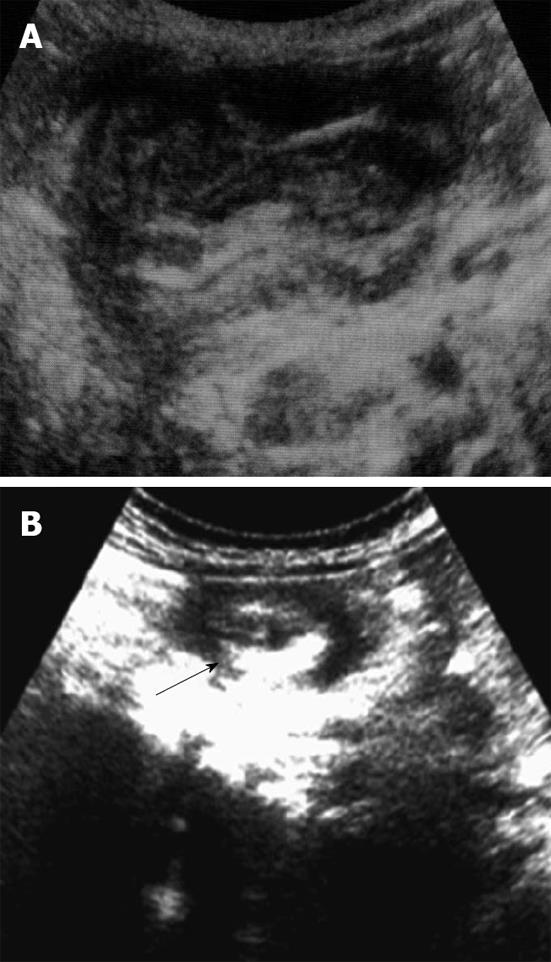
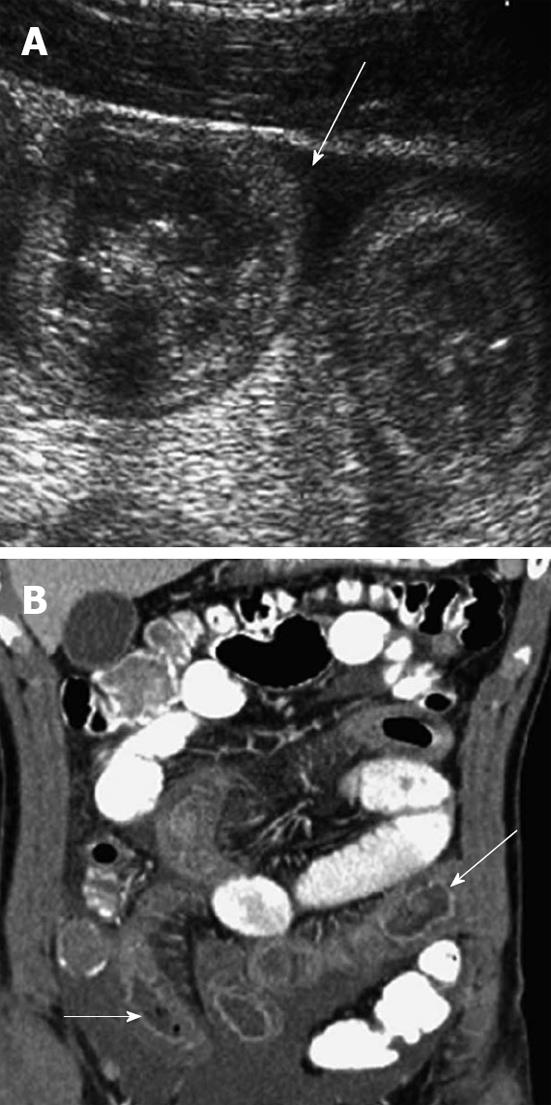
Plain abdominal radiographs are usually the initial studies in children with possible intussusception. Moreover, they are neither sensitive nor specific for intussusception. Plain films may show a variety of abnormalities, including a visible abdominal mass, abnormal distribution of gas and fecal contents, air fluid levels and dilated loops of the small intestine. A ‘‘target sign’’ on plain film consists of concentric circles of fat density similar in appearance to a doughnut visualized to the right of the spine. Ultrasonography is useful for diagnosing intussusception and the classic findings, including the ‘‘target sign’’, a single hypoechoic ring with a hyperechoic center and the ‘‘pseudokidney’’ sign, superimposed hypo and hyperechoic areas representing the edematous walls of the intussusceptum and layers of compressed mucosa (Figure 3)[7].
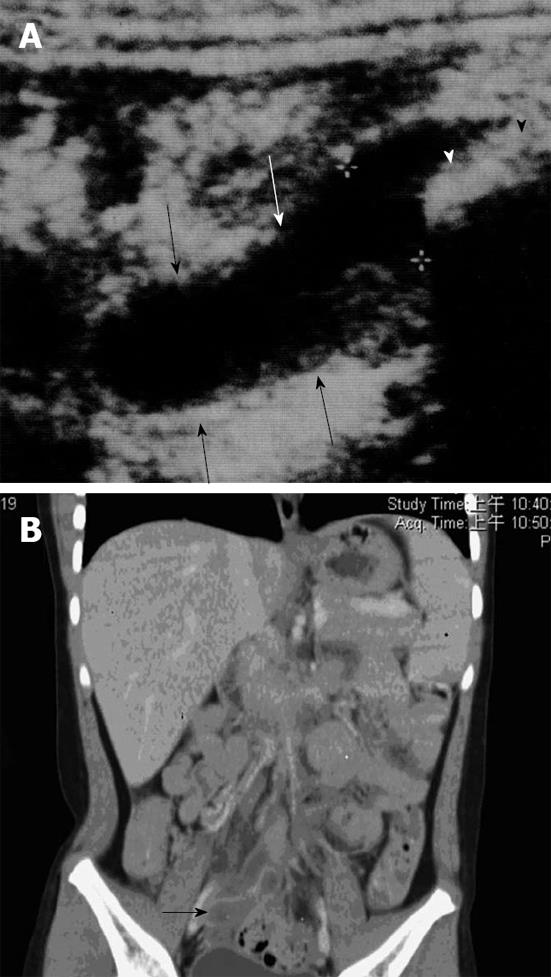
CT may be considered in highly suspected bowel obstruction or surgical emergencies when US cannot determine a definite cause in children. However, CT would not be performed routinely in patients with suspected intussusception. The findings of intussusception on CT reveal bowel loops containing alternating high and low rings of attenuation and a dilated proximal small bowel with air-fluid levels (Figure 4).
Barium enema is the gold standard study for the diagnosis of intussusception and is also a therapy. However, air-contrast enema has been used for many years in some countries and has been shown to be as effective as barium enema for intussusception. Air contrast decreases the risks of chemical peritonitis caused by perforation with barium enema in this disease. An ileo-ileac type of intussusception can be much harder to diagnose and is much harder to be released by air or barium reduction. Not every child with intussusception should undergo bowel reduction by enema. Clinical signs of peritonitis, bowel perforation and hypovolemic shock are clear contraindications to enemas. Relative contraindications to enemas include prolonged symptoms (≥ 24 h), evidence of obstruction, such as air fluid levels on plain abdominal films, and ultrasonography findings of intestinal ischemia or trapped fluid. Even in well-selected patients, enemas may cause the reduction of necrotic bowel, perforation and sepsis. After a successful reduction, the child should be admitted for observation. A small percentage of patients (0.5% to 15%) may have a recurrence of intussusception, usually within 24 h but sometimes after days or weeks. Even after reduction by laparotomy, the recurrence rate is approximately 2% to 5%[8,9].
HSP is the most common vasculitis of childhood and is an acute, systemic, self-limited, small-vessel vasculitis usually seen in otherwise healthy children. The incidence of HSP is reported as ranging from 6 to 22 cases per 100000 children in different populations. Although it most often affects young children, it may develop at any age. Most HSP occurs between 3 and 5 years of age and ninety percent of HSP patients are under 10 years of age. It is thought to occur more often in female children, although some have found a male predominance or both genders to be equally affected[5]. The etiology of HSP remains unknown, although many antigens, such as infective agents, vaccinations, drugs and insect bites are suspected. In 1990, the American College of Rheumatology published diagnostic criteria for HSP, including: (1) palpable purpura, that is slightly raised hemorrhagic skin lesions not related to thrombocytopenia; (2) age, 20 years or younger at onset of first symptoms of the disease; (3) bowel angina, that is diffuse abdominal pain, worse after meals, or the diagnosis of bowel ischemia, usually including bloody diarrhea; and (4) wall granulocytes on biopsy, that is histological changes showing granulocytes in the walls of arterioles or venules. A patient is diagnosed with HSP if at least two of the four criteria are present; this yields a sensitivity of 87.1% and a specificity of 87.7%[10].
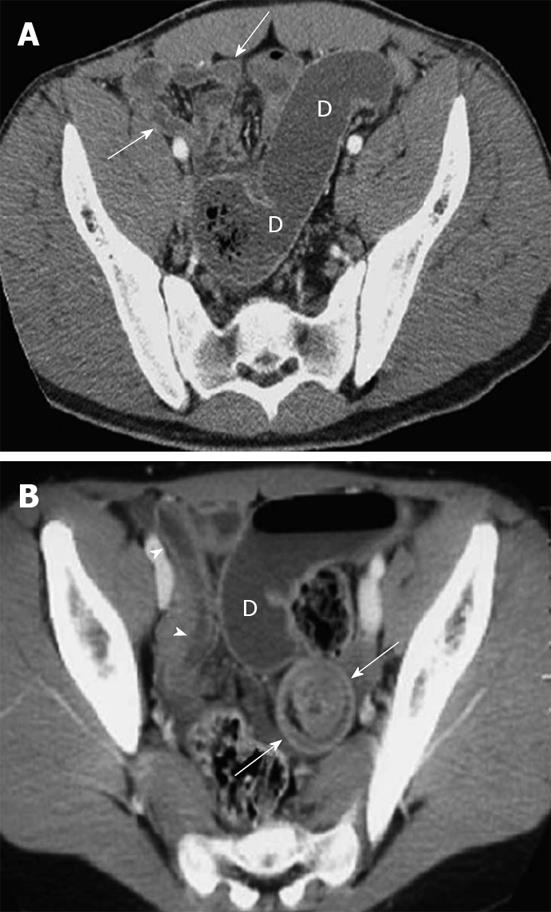
The disease usually lasts for 1 to 4 wk. The predominant cutaneous finding of HSP is painless, palpable purpura. In more than 70% of patients, palpable purpura alone or purpura associated with abdominal and/or joint pain is the first sign. Abdominal pain is the most common gastrointestinal symptom, affecting two-thirds of children. The pain is usually localized to the periumbilical or epigastric region and described as blunt in nature. Bowel wall thickening caused by vasculitis-associated ischemia has a mean thickness of 9 mm and involves longer segments than bowel thickening associated with hematoma (Figure 5)[11].
Treatment for children with HSP is mostly supportive, relieving associated arthralgia and abdominal pain. In patients with normal renal function, therapy should focus on the maintenance of hydration, nutrition and electrolyte balance. Most agree that analgesics and/or nonsteroidal anti-inflammatory agents should be used for the control of arthralgia and inflammation in children. Gastrointestinal involvement has been reported to occur in approximately 50% to 75% of patients with HSP[10,11]. Severe abdominal pain that is unresponsive to conventional treatments may respond dramatically to a trial of intravenous corticosteroids. Systemic steroids, such as oral prednisolone or pulsed intravenous methylprednisolone, may be effective in children with massive gastrointestinal hemorrhage and ischemic bowel.
AGE is the most common gastrointestinal inflammatory process in children. The cause is usually viral and rotavirus is the most common in children. Vomiting usually precedes the diarrhea by as much as 12 to 24 h. A low grade fever may or may not be associated with AGE. Examination of the abdomen usually reveals a nondistended soft abdomen with no localized tenderness (may be diffusely, mildly tender) and usually there is minimal to no guarding. AGE may cause an ileus in severe cases[12]. Viral diarrhea will target the small bowel, resulting in mid-abdominal cramping and large volumes of watery diarrhea. Bacterial diarrhea will target the large bowel, resulting in lower abdominal pain and smaller volumes of bloody mucoid diarrhea. Other diagnoses need to be considered when a child presents with vomiting, such as urinary tract infection, appendicitis, inborn errors of metabolism or volvulus, especially in very young infants, diabetic ketoacidosis and hemolytic uremic syndrome (the appearance of illness in children usually is preceded by diarrhea).
Plain radiographs are often normal but may show mild dilatation of small and large bowel. US is helpful in gastroenteritis to exclude other emergencies such as acute appendicitis or intussusception. US may show fluid-filled hyperperistaltic loops of bowel with little or no wall thickening in patients with gastroenteritis (Figure 6). However, abdominal CT scan is seldom used in patients with AGE except for cases with acute abdomen caused by AGE, such as septic peritonitis caused by bowel perforation.
Meckel’s diverticulum is defined as incomplete closure of the intestinal end of the omphalomesenteric duct that disappears normally by the seventh week of gestation. It is a true diverticulum, containing all layers of the bowel wall, and up to 60% of these diverticula contain heterotopic gastric tissue and heterotopic pancreatic, endometrial and duodenal mucosa[13,14]. It is the most common congenital gastrointestinal tract anomaly and affects 2% of the population. In addition, it is more common in males than females, with a male-to-female ratio of two to four and with the lifetime complication rate of about 4%[4]. The features of Meckel’s diverticulum are commonly described by ‘‘the rule of 2s’’: it is present in approximately 2% of the population, with only 2% of affected patients becoming symptomatic. Forty-five percent of symptomatic patients are under 2 years of age. The most common location is 40 to 100 centimeters from the ileocecal valve and the diverticulum typically is about 5 cm long[15].
The classical presentation of Meckel’s diverticulum is painless or minimally painful rectal bleeding. Such painless bleeding is a result of heterotopic gastric tissue in the diverticulum or the adjacent ileum. Abdominal pain, distension and vomiting may occur if obstruction has occurred and the clinical presentations may mimic appendicitis or diverticulitis. Meckel’s diverticulum may also ulcerate and perforate, presenting as a bowel perforation, or act as a lead point, resulting in intussusception.
Abdominal films may show signs of obstruction, such as dilated loops of bowel or a paucity of bowel gas. A scan of Meckel’s diverticulum can detect the presence of gastric mucosa within the diverticulum with up to 85% accuracy. Meckel’s diverticulum may show one of following patterns of presentations on CT: isolated small-bowel obstruction; intussusception with small-bowel obstruction; or a cystic mass with surrounding inflammatory changes (Figure 7)[15]. However, calcifications are not a common feature of CT for the diagnosis of Meckel’s diverticulum.
A Tc-99m scan is the gold standard but is a less-sensitive tool for making a diagnosis preoperatively. Repeated Tc-99m scans in highly suspected cases and RBC scans in continuous bleeding patients improves the detection rate.
In 1926, Wilensky and Hahn classified mesenteric lymphadenitis into four groups: Group I: Simple mesenteric lymphadenitis; Group II: Suppurative mesenteric lymphadenitis; Group III: Tuberculous mesenteric lymphadenitis; and Group IV: Terminal stage of mesenteric lymphadenitis (calcification). It can, however, cause severe consequences and may at times be fatal. The severe form of this disease with suppuration, abscess formation and peritonitis is rare. Mesenteric adenitis is usually a self-limiting clinical condition characterized by fever, nausea, vomiting, diarrhea, diffuse or right lower quadrant abdominal pain and tenderness, and frequent leukocytosis. The causes of mesenteric adenitis have been reported to be viral, bacterial and mycobacterial infections. Numerous bacteria have been demonstrated to be involved in mesenteric nodes: B. coli, staphylococci, streptococci, pneumococci, typhoid, paratyphoid and tubercle bacilli.
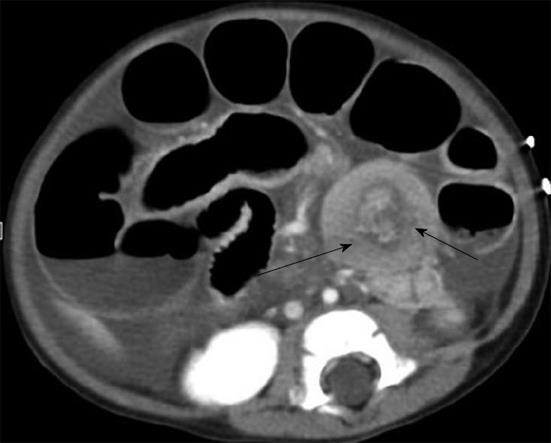
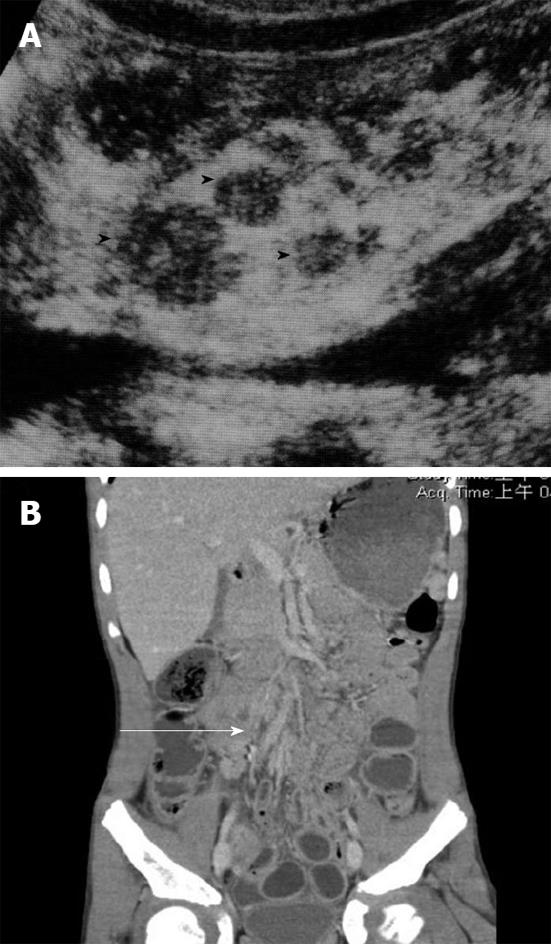
Due to the clinical presentation of the abdomen, it could be difficult to discriminate this condition from other acute abdominal diseases such as acute appendicitis. Imaging examinations, such as an abdominal CT scan, can be a valuable tool in accurately diagnosing mesenteric adenitis and can help to avoid normal appendectomy due to the clinical misdiagnosis of appendicitis. Findings of mesenteric adenitis on CT include: (1) cluster of > 3 lymph nodes in RLQ mesentery with > 5 mm in short axis diameter; (2) normal appendix; (3) ileal wall thickening; and (4) colonic wall thickening (Figure 8)[16,17].
The etiologies of acute abdomen in children admitted to the emergency department vary depending on the age. A complete history and detailed physical examination, as well as abdominal imaging examinations, could provide useful information for physicians in the emergency department in order to narrow the differential diagnosis of abdominal emergencies and give a timely treatment.
P- Reviewers: Pinto A, Sozen S S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Liu XM
| 1. | Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 301] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Andersson RE, Hugander A, Ravn H, Offenbartl K, Ghazi SH, Nyström PO, Olaison G. Repeated clinical and laboratory examinations in patients with an equivocal diagnosis of appendicitis. World J Surg. 2000;24:479-485; discussion 485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Callahan MJ, Rodriguez DP, Taylor GA. CT of appendicitis in children. Radiology. 2002;224:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Lessin MS, Chan M, Catallozzi M, Gilchrist MF, Richards C, Manera L, Wallach MT, Luks FI. Selective use of ultrasonography for acute appendicitis in children. Am J Surg. 1999;177:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Rothrock SG, Pagane J. Acute appendicitis in children: emergency department diagnosis and management. Ann Emerg Med. 2000;36:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 220] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 6. | Wu HP, Yang WC, Wu KH, Chen CY, Fu YC. Diagnosing appendicitis at different time points in children with right lower quadrant pain: comparison between Pediatric Appendicitis Score and the Alvarado score. World J Surg. 2012;36:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Kuppermann N, O’Dea T, Pinckney L, Hoecker C. Predictors of intussusception in young children. Arch Pediatr Adolesc Med. 2000;154:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Lui KW, Wong HF, Cheung YC, See LC, Ng KK, Kong MS, Wan YL. Air enema for diagnosis and reduction of intussusception in children: clinical experience and fluoroscopy time correlation. J Pediatr Surg. 2001;36:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Wang G, Liu XG, Zitsman JL. Nonfluoroscopic reduction of intussusception by air enema. World J Surg. 1995;19:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | González LM, Janniger CK, Schwartz RA. Pediatric Henoch-Schönlein purpura. Int J Dermatol. 2009;48:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Calviño MC, Llorca J, García-Porrúa C, Fernández-Iglesias JL, Rodriguez-Ledo P, González-Gay MA. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Lee CC, Su CP, Chen SY, Chen SC, Chen WJ. Mesenteric adenitis caused by Salmonella enterica serovar Enteritidis. J Formos Med Assoc. 2004;103:463-466. [PubMed] |
| 13. | Chan KW, Lee KH, Mou JW, Cheung ST, Tam YH. Laparoscopic management of complicated Meckel’s diverticulum in children: a 10-year review. Surg Endosc. 2008;22:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Levy AD, Hobbs CM. From the archives of the AFIP. Meckel diverticulum: radiologic features with pathologic Correlation. Radiographics. 2004;24:565-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Olson DE, Kim YW, Donnelly LF. CT findings in children with Meckel diverticulum. Pediatr Radiol. 2009;39:659-663; quiz 766-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Macari M, Hines J, Balthazar E, Megibow A. Mesenteric adenitis: CT diagnosis of primary versus secondary causes, incidence, and clinical significance in pediatric and adult patients. AJR Am J Roentgenol. 2002;178:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |