Published online Aug 16, 2013. doi: 10.12998/wjcc.v1.i5.176
Revised: April 22, 2013
Accepted: May 7, 2013
Published online: August 16, 2013
Processing time: 143 Days and 5.4 Hours
A 73-year-old Japanese woman was hospitalized for detailed examination of nausea, diarrhea and loss of appetite. Atypical erosion in the ileum was found on endoscopy. Biopsy of this erosion showed proliferation of cells containing numerous Russell bodies. Differential diagnoses considered were Russell body enteritis, crystal-storing histiocytosis, Mott cell tumor, immunoproliferative small intestinal disease (IPSID) and mucosa-associated lymphoid tissue (MALT) lymphoma. The cells containing prominent Russell bodies showed diffuse positivity for CD79a and CD138, but negative results for CD20, CD3, UCHL-1, CD38 and CD68. Russell bodies were diffusely positive for lambda light chain, but negative for kappa light chain, and immunoglobulin (Ig) G, IgA and IgM. Based on these findings, Russell body enteritis, crystal-storing histiocytosis and IPSID were ruled out. As the tumor formed no mass lesions and was restricted to the gastrointestinal tract, MALT lymphoma with extensive plasma cell differentiation was finally diagnosed. The patient showed an unexpectedly aggressive clinical course. The number of atypical lymphocytes in peripheral blood gradually increased and T-prolymphocytic leukemia (T-PLL) emerged. The patient died of T-PLL 7 mo after admission. Autopsy was not permitted.
Core tip: This report describes an extremely rare case of B-cell neoplasm, comprising mucosa-associated lymphoid tissue (MALT) lymphoma of the gastrointestinal tract showing extensive plasma cell differentiation with prominent Russell bodies. The pathological diagnostic strategy is also discussed. The patient died of sequentially emerging T-prolymphocytic leukemia (T-PLL). Concomitant T-PLL and MALT lymphoma has not been reported previously.
- Citation: Kai K, Miyahara M, Tokuda Y, Kido S, Masuda M, Takase Y, Tokunaga O. A case of mucosa-associated lymphoid tissue lymphoma of the gastrointestinal tract showing extensive plasma cell differentiation with prominent Russell bodies. World J Clin Cases 2013; 1(5): 176-180
- URL: https://www.wjgnet.com/2307-8960/full/v1/i5/176.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i5.176
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma is an extranodal lymphoma composed of morphologically heterogeneous small B-cells, including marginal zone cells, cells resembling monocytoid cells, small lymphocytes and scattered immunoblasts and centroblast-like cells. Plasmacytic differentiation is frequently found in cutaneous MALT lymphomas and is a constant and often striking feature in thyroid MALT lymphomas, but is relatively rare in MALT lymphoma of the gastrointestinal tract including gastric lesions[1]. We encountered a rare case of MALT lymphoma of the gastrointestinal tract showing extensive plasma cell differentiation with prominent intracytoplasmic immunoglobulin (Ig) called Russell bodies. Furthermore, T-prolymphocytic leukemia (T-PLL) emerged sequentially. To the best of our knowledge, no such case has been reported previously in the English literature.
A 73-year-old Japanese female was admitted to hospital for detailed examination after she presented with nausea, diarrhea and loss of appetite. She had been taking medication for hypertension and a pacemaker had been implanted for sick sinus syndrome. Laboratory tests on admission revealed mild pancytopenia (red blood cell count, 2.73 × 106/μL; hemoglobin, 8.3 g/dL; hematocrit, 24.0%; white blood cells, 3.3 × 103/μL; platelets, 3.3 × 104 /μL). Serology and coagulation tests showed no abnormality: total protein, 5.1 g/dL (normal range, 8.3-6.7 g/dL); albumin, 2.4 g/dL (normal range, 5.0-3.8 g/dL); and C-reactive protein, 1.4 mg/dL (normal range, < 0.30 mg/dL). Protein compartmentation was almost normal. Serological testing revealed positivity for serum anti-human T-lymphotropic virus (HTLV)-1 antibody.
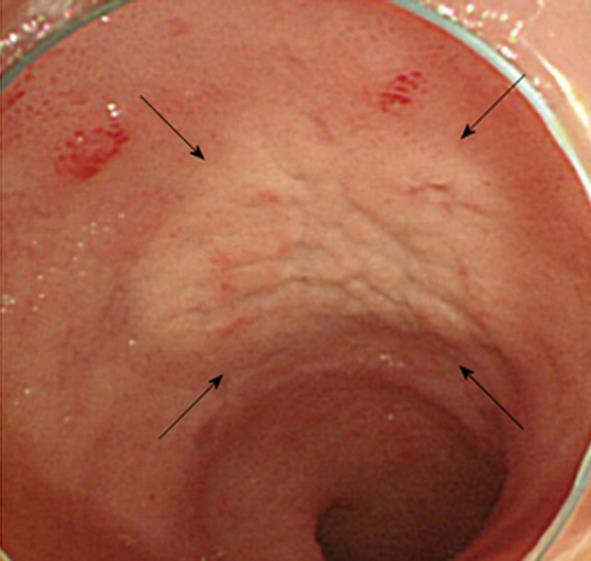
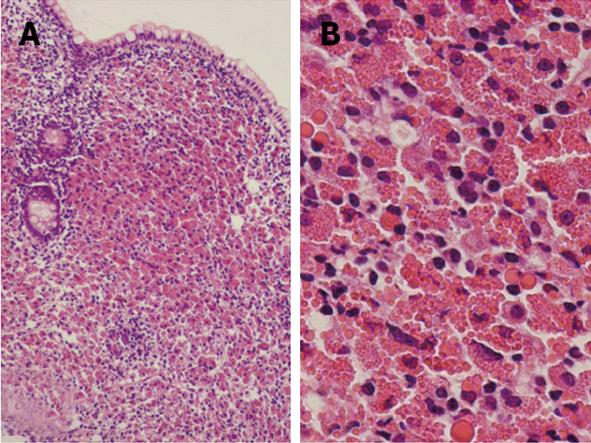
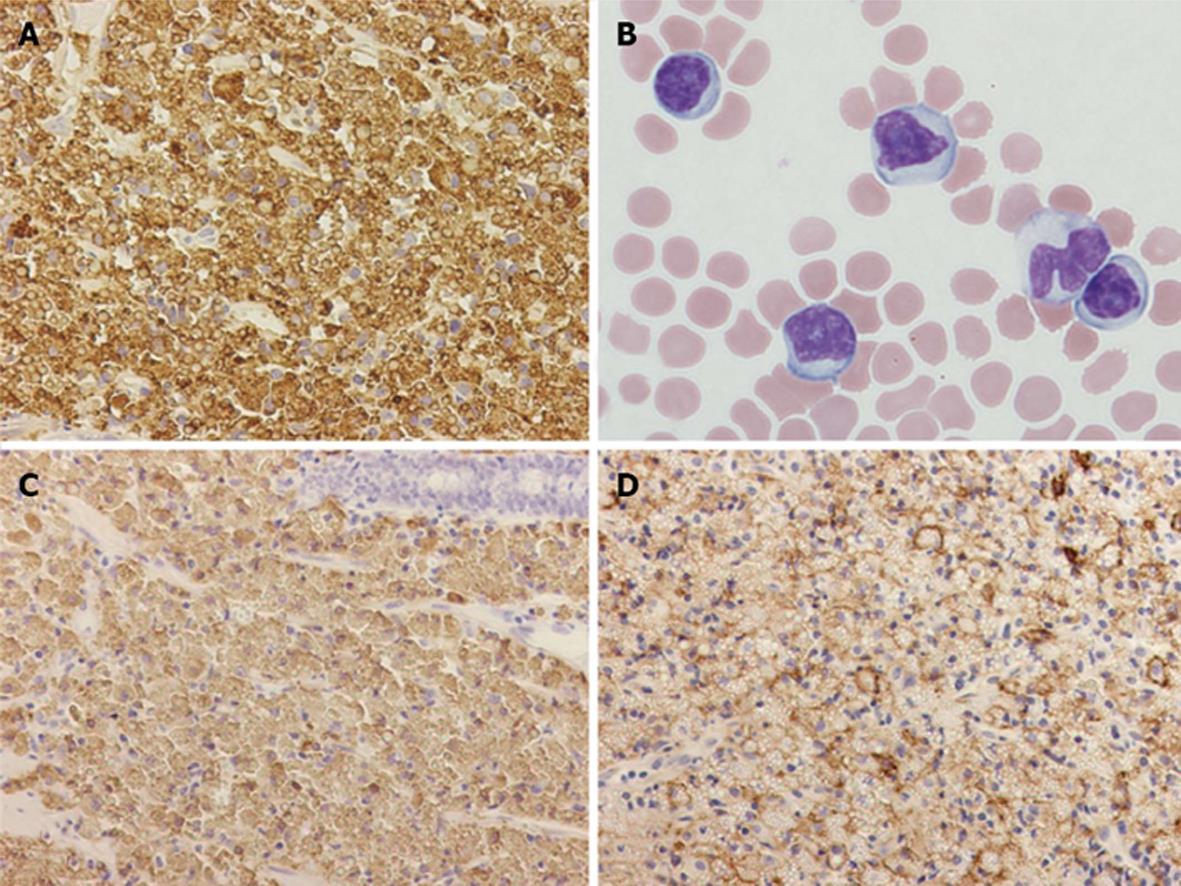
Contrast-enhanced computed tomography of chest, abdomen and pelvic regions showed no specific finding. Endoscopy of the upper gastrointestinal tract showed findings of mild chronic gastritis. Biopsy from the stomach yielded no specific findings such as amyloid or neoplastic lesions. Helicobacter pylori (H. pylori) infection was histologically examined using hematoxylin and eosin, Giemsa and immunohistochemical staining, but no evidence of H. pylori infection was found. Endoscopic study of the colorectum and ileum revealed color-faded cobble-stone like erosion in the ileum (Figure 1). Biopsy of this erosion showed proliferation of mononuclear cells with extensive cytoplasm containing numerous eosinophilic globules in the submucosal layer (Figure 2). Morphologically, the eosinophilic globules were considered to represent Russell bodies. Similar histology was observed in a biopsy specimen obtained from an erosion of the rectum found on colonoscopy 2 mo later. Immunohistochemistry for light chain gamma-globulin revealed monoclonality of the lambda chain (Figure 3A). Initial pathological diagnosis of this lesion was crystal-storing histiocytosis. The possibility of underlying lymphoproliferative or plasma cell disorders that produce monoclonal Ig, such as plasma cell myeloma, lymphoplasmacytic lymphoma, and monoclonal gammopathy of undetermined significance (MGUS) was clinically considered. Additional examination of peripheral blood revealed high levels of soluble interleukin-2 receptor (sIL-2R) (2280 U/mL; normal range, 122-496 U/mL) and Bence-Jones protein (BJP) was detected from first examination of urine. However, no abnormality was observed on examination of bone marrow and BJP was never detected second or subsequent urine examinations. A definite clinical diagnosis of lymphoproliferative or plasma cell disorder thus could not be made.
The patient was clinically observed using intravenous drip infusion for nutrition. However, the number of atypical lymphocytes in peripheral blood (Figure 3B) and the serum level of sIL-2R gradually increased (maximum: white blood cells, 9.8 × 104/μL; atypical lymphocytes, 78%; sIL-2R, 4810 U/mL). Analysis by flow cytometry revealed that atypical lymphocytes in peripheral blood expressed CD2, CD3, CD5 and CD7. As this patient was a HTLV-1 carrier, adult T-cell leukemia/lymphoma was initially considered. However, negative results for proviral DNA of HTLV-1 in tumor cells and the evidence of gene rearrangement of T-cell receptor (TCR)-beta/gamma indicated a diagnosis of T-PLL. The diagnosis of T-PLL was reached 4 mo after admission, but the patient showed complications with several infectious diseases. Although therapies to control infections were performed, chemotherapy for T-PLL could not be initiated because of poor general condition. The patient died of T-PLL 3 months after diagnosis. No autopsy was permitted.
Although the initial pathological diagnosis of the erosion in the ileum was crystal-storing histiocytosis, further pathological investigation and discussion of the ileal lesion was performed. The cells containing prominent Russell bodies were diffusely positive for CD79a and CD138 (Figure 3C and D), but negative for CD20, CD3, UCHL-1, CD38 and CD68. This indicated the neoplastic tumor cells with plasma cell differentiation rather than secondary deposition of Ig produced by plasma cell disorders of another site. The tumor cells were negative for IgG, IgA and IgM. Immunoproliferative small intestinal disease in the form of alpha heavy chain disease was thus ruled out. The remaining difficulty was whether the case should be categorized as MALT lymphoma or plasmacytoma. As the tumor formed no nodules and was restricted solely to the gastrointestinal tract (ileum and rectum), the final pathological diagnosis was MALT lymphoma showing extensive plasma cell differentiation with prominent Russell bodies.
The characteristic feature of ileal lesions in the present case was cells containing prominent Russell bodies. Similar pathology could be observed in Russell body gastritis, crystal-storing histiocytosis and Mott cell tumor. These entities are all rare and therefore not well-known and potentially easily confused.
Crystal-storing histiocytosis is a rare condition in which crystalline material accumulates in the cytoplasm of histiocytes, typically in association with lymphoproliferative or plasma cell disorders such as plasma cell myeloma, lymphoplasmacytic lymphoma, or MGUS[2,3]. In the present case, although abundant cytoplasm containing numerous globules suggested that these cells were macrophages that had phagocytosed Ig, negative expression of the macrophage marker CD68 ruled out the pathological diagnosis of crystal-storing histiocytosis.
The other potentially confusing entity is Russell body enteritis. Tazawa et al[4] described a very peculiar, localized accumulation of plasma cells with Russell bodies in the gastric mucosa, which they named Russell body gastritis. Relationships of H. pylori have been documented in several series[5-7], but H. pylori-negative cases have also been reported[8,9]. Only two cases with duodenal lesions have been reported as Russell body duodenitis[10,11], and lesions of the small intestine, colon and rectum have not previously been described. Russell body gastritis is considered a non-neoplastic, inflammatory lesion and therefore the Ig produced is usually polyclonal. In the present case, Russell bodies were diffusely positive for lambda light chain. This indicates the produced Ig was monoclonal and additional findings of diffuse positivity for CD79a (B-cell marker) and CD138 (plasma cell marker) indicated that this lesion was a B-cell neoplasm showing extensive plasma cell differentiation with production of monoclonal Ig. The diagnosis of Russell body enteritis was thus ruled out.
The remaining problem is whether to categorize this lesion as MALT lymphoma with extensive plasma cell differentiation or plasma cell neoplasm. Extensive plasmacytic differentiation is frequently found in cutaneous MALT lymphomas[12] and thyroid MALT lymphomas[13], but is rare in the gastrointestinal tract. Plasma cell myeloma is a bone marrow-based, multifocal plasma cell neoplasm[1], and thus is not compatible with the present findings. Although distinction between lymphomas that exhibit extreme plasma cell differentiation and extraosseous plasmacytoma is difficult[14,15], the present case was finally diagnosed as MALT lymphoma because plasmacytoma usually forms mass lesions, while no mass lesions were identified in the present case.
A Mott cell (also known as a grape cell) is a specific form of plasma cell that contains multiple Russell bodies. Plasmacytoma involving such cells is called Mott cell tumor or grape cell plasmacytoma[16,17]. Extramedullary Mott cell tumors are rare. Several cases of Mott cell tumor of the gastrointestinal tract including Mott cell tumor-like lesions have been reported[18-22]. These reported cases were all gastric lesions. We consider the diagnosis of Mott cell tumor as more suitable for plasmacytoma consisting of Mott cells than MALT lymphoma with Russell bodies and/or Dutcher bodies. Given this consideration, the term “Mott cells” was avoided in the diagnosis of the present case.
From the site of involved organs, IPSID, as an alpha heavy chain disease, must be included in the differential diagnosis because the small intestine is typically involved in this pathology. IPSID typically occurs in the Middle East[23], the Cape region of South Africa[24] and a variety of other tropical and subtropical locations and plasma cells in IPSID produce IgA. The diagnosis of IPSID did not fit the present case, given the location and absence of IgA production.
The patient died of T-PLL, representing a key aspect of this case. T-PLL is rare, representing approximately 2% of cases of mature lymphocytic leukemia in adults[25]. The diagnosis is made based on peripheral blood films showing a predominance of small to medium-sized lymphoid cells with non-granular basophilic cytoplasm, round oval or markedLy irregular nuclei and a visible nucleolus. TCR genes, TCR beta (TRB@ at 7q34), and gamma (TRG@ at 7p14) are commonly rearranged in T-PLL[1]. Although response to alemtuzumab (anti-CD52) has been reported[26,27], the majority of cases show a clinical course of aggressive, chemotherapy-resistant malignancy with a median survival of less than one year. T-PLL has been associated with the development of second lymphoma. Among the associations reported in the literature are T-PLL and classic Hodgkin disease[28] in which patients diagnosed and treated for T-PLL developed diffuse large B-cell lymphoma (DLBCL)[29-31]. Although DLBCL could occur via MALT lymphoma, no previous reports have described T-PLL accompanying MALT lymphoma.
Because no autopsy was performed, the spread of MALT lymphoma by the terminal stage was unknown. If nodal involvement, bone marrow involvement or mass lesions of B-cell tumor had been found at autopsy, this case might have been categorized as plasmacytoma and might have been reported as Mott cell tumor. It is also unclear whether the patient’s chief complaints involving the digestive tract depended on MALT lymphoma or indolent T-PLL.
In summary, this report describes an extremely rare case of B- and T-cell neoplasms along with the pathological diagnostic strategy. Although unsolved questions remained, we reported this case because of the rarity of the pathology and the educational value of the discussion.
We would like to thank Dr. Morishige Takeshita (Department of Pathology, Fukuoka University) for his valuable comments and advice regarding the pathological diagnosis. This case was presented and discussed at the 329th Kyushu-Okinawa slide conference. We also wish to thank the participants for valuable comments and discussion. We are grateful to Mr. Fumihiro Mutoh for his contribution to the immunohistochemistry for H. pylori.
P- Reviewers Mehdi I, Singh RR, Sugimoto M S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | SwerdLow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stain H, Thiele J, Vardiman JW, editors . WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer 2008; 214-217. |
| 2. | Jones D, Bhatia VK, Krausz T, Pinkus GS. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Dogan S, Barnes L, Cruz-Vetrano WP. Crystal-storing histiocytosis: report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012;6:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Tazawa K, Tsutsumi Y. Localized accumulation of Russell body-containing plasma cells in gastric mucosa with Helicobacter pylori infection: ‘Russell body gastritis’. Pathol Int. 1998;48:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Pizzolitto S, Camilot D, DeMaglio G, Falconieri G. Russell body gastritis: expanding the spectrum of Helicobacter pylori - related diseases? Pathol Res Pract. 2007;203:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Ensari A, Savas B, Okcu Heper A, Kuzu I, Idilman R. An unusual presentation of Helicobacter pylori infection: so-called “Russell body gastritis”. Virchows Arch. 2005;446:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Karabagli P, Gokturk HS. Russell body gastritis: case report and review of the literature. J Gastrointestin Liver Dis. 2012;21:97-100. [PubMed] |
| 8. | Del Gobbo A, Elli L, Braidotti P, Di Nuovo F, Bosari S, Romagnoli S. Helicobacter pylori-negative Russell body gastritis: case report. World J Gastroenterol. 2011;17:1234-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Erbersdobler A, Petri S, Lock G. Russell body gastritis: an unusual, tumor-like lesion of the gastric mucosa. Arch Pathol Lab Med. 2004;128:915-917. [PubMed] |
| 10. | Paniz Mondolfi A, Samuel M, Kikhney J, Moter A, Feldman D, Slova D, Filatov A, Theise N. Russell body duodenitis: a histopathological and molecular approach to a rare clinical entity. Pathol Res Pract. 2012;208:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Savage NM, Fortson T, Schubert M, Chamberlain S, Lee J, Ramalingam P. Isolated Russell body duodenitis. Dig Dis Sci. 2011;56:2202-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Geyer JT, Ferry JA, Longtine JA, Flotte TJ, Harris NL, Zukerberg LR. Characteristics of cutaneous marginal zone lymphomas with marked plasmacytic differentiation and a T cell-rich background. Am J Clin Pathol. 2010;133:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Kojima M, Shimizu K, Shimizu K, Masawa N. Incidental MALT type lymphoma exhibiting prominent plasma cell differentiation associated with Hashimoto’s thyroiditis. A two case report. Head Neck Pathol. 2009;3:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Hussong JW, Perkins SL, Schnitzer B, Hargreaves H, Frizzera G. Extramedullary plasmacytoma. A form of marginal zone cell lymphoma? Am J Clin Pathol. 1999;111:111-116. [PubMed] |
| 15. | Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am. 1999;13:1249-1257. [PubMed] |
| 16. | Woyke M. Case of grape cell plasmacytoma of the IgG type. Acta Haematol Pol. 1973;4:129-132. [PubMed] |
| 17. | Wickramasinghe SN. Normal haemopoiesis: Cellular composition of normal bone marrow. 2. New York: Churchill Livingstone 1986; 41-72. |
| 18. | Matsumoto K, Shikuwa S, Kawase Y, Ooi J, Honda M, Ito M, Sekine I, Kunisaki T, Ifuku M. Gastric plasmacytoma with rod-shaped intracytoplasmic inclusions. Report of a case studied by light and electron microscopy and immunohistochemistry. Acta Pathol Jpn. 1988;38:815-821. [PubMed] |
| 19. | Maitre F, Mourot J, Galian A. Malignant plasmatic gastric lymphoma secreting IgA-kappa. Case report with histo-immunofluorescent study (author’s transl). Arch Anat Cytol Pathol. 1979;27:209-212. [PubMed] |
| 20. | Shinozaki A, Ushiku T, Fukayama M. Prominent Mott cell proliferation in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2010;41:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kokosadze NV, Kovrigina AM, Probatova NA. [Stomach MALT-lymphoma with marked plasmocytic differentiation: a variant of Mott’s cell tumor]. Arkh Patol. 2004;66:40-42. [PubMed] |
| 22. | Fujiyoshi Y, lnagaki H, Tateyama H, Murase T, Eimoto T. Mott cell tumor of the stomach with Helicobacter pylori infection. Pathol Int. 2001;51:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Pinkel D. Differentiating juvenile myelomonocytic leukemia from infectious disease. Blood. 1998;91:365-367. [PubMed] |
| 24. | Price SK. Immunoproliferative small intestinal disease: a study of 13 cases with alpha heavy-chain disease. Histopathology. 1990;17:7-17. [PubMed] |
| 25. | Matutes E, Brito-Babapulle V, Swansbury J, Ellis J, Morilla R, Dearden C, Sempere A, Catovsky D. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78:3269-3274. [PubMed] |
| 26. | Dearden CE, Matutes E, Cazin B, Tjønnfjord GE, Parreira A, Nomdedeu B, Leoni P, Clark FJ, Radia D, Rassam SM. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98:1721-1726. [PubMed] |
| 27. | Keating MJ, Cazin B, Coutré S, Birhiray R, Kovacsovics T, Langer W, Leber B, Maughan T, Rai K, Tjønnfjord G. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol. 2002;20:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Miyata A, Kojima K, Yoshino T, Fujii S, Shinagawa K, Ichimura K. Concurrent Hodgkin’s disease (mixed cellularity type) and T-cell chronic lymphocytic leukemia/prolymphocytic leukemia. Int J Hematol. 2001;73:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Miyata A, Yoshino T, Kojima K, Fujii S, Kikuchi T. [T-cell prolymphocytic leukemia complicated by diffuse large B-cell lymphoma of the stomach]. Rinsho Ketsueki. 2001;42:47-50. [PubMed] |
| 30. | Alduaij A, Treaba DO, Winer ES. CD30-positive EBV-associated diffuse large B-cell lymphoma occurring after immunosuppressive therapy for T-cell prolymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Sohani AR, Ferry JA, Chang PS, Abramson JS. Epstein-barr virus-positive diffuse large B-cell lymphoma during therapy with alemtuzumab for T-cell prolymphocytic leukemia. J Clin Oncol. 2010;28:e69-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |