Published online May 16, 2013. doi: 10.12998/wjcc.v1.i2.87
Revised: April 3, 2013
Accepted: April 10, 2013
Published online: May 16, 2013
Processing time: 94 Days and 18.7 Hours
We report a case of 61-year-old male who had synchronous advanced rectal cancer involving the urinary bladder massively associated with multiple liver metastases, and esophageal cancer successfully treated by neoadjuvant chemotherapy followed by two-stage resection. Although complete resection of each of the lesions was considered possible by performing anterior pelvic exenteration, liver resection, and esophagectomy, it might be impossible for the patient to endure the stress of all of these operative procedures at once. Therefore, we planned to perform staged treatment with prioritizing consideration. First, we instituted chemotherapy with the FOLFOX (oxaliplatin + fluorouracil + leucovorin) plus cetuximab regimen, which could adequately control both rectal and esophageal cancer. After 6 cycles of chemotherapy, high anterior resection combined with cystoprostatectomy and lateral segmentectomy plus partial hepatectomy was performed followed by staged esophagectomy with three-field lymph node dissection. It was possible to use oxaliplatin and cetuximab safely as neoadjuvant therapy not only for advanced rectal cancer but for esophageal cancer, and it was effective.
Core tip: In case with synchronous multiple cancers, it is sometimes difficult to identify the origin especially when liver and/or pulmonary lesions have a possibility of metastases, or to decide on a course or priority of the treatment. FOLFOX + cetuximab (Cet) therapy could provide a favorable control of not only rectal origin accompanied by liver metastases but also esophageal cancer, which made possible to undergo two-stage curative resection. FOLFOX + Cet regimen might be a useful option for such refractory rectal and esophageal cancer.
- Citation: Utsunomiya S, Uehara K, Kurimoto T, Hirose K, Fukaya M, Takahashi Y, Taguchi Y, Itatsu K, Nagino M. Synchronous rectal and esophageal cancer treated with chemotherapy followed by two-stage resection. World J Clin Cases 2013; 1(2): 87-91
- URL: https://www.wjgnet.com/2307-8960/full/v1/i2/87.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v1.i2.87
In the last decade, remarkable advances have been achieved in chemotherapy for colorectal cancer as a result of the advent of the novel anticancer drugs such as irinotecan and oxaliplatin (L-OHP), and the molecularly targeted drugs such as vascular endothelial growth factor inhibitors and epidermal growth factor receptor (EGFR) inhibitors. Cetuximab (Cet), one of the EGFR inhibitors, has a strong cytoreductive effect and survival benefit in KRAS wild-type colorectal cancer, and it has not only been incorporated in treatment strategies together with L-OHP as palliative therapy for unresectable metastatic colorectal cancer, but as perioperative chemotherapy for resectable advanced cancer or conversion chemotherapy for unresectable metastatic diseases[1,2].
Although, more than 50% of esophageal cancers are adenocarcinoma in Western countries, approximately 90% of esophageal cancers are squamous cell carcinoma (SCC) and the half of them are located in the mid-thoracic esophagus in Japan[3]. The gold standard of treatment for esophageal cancer in Japan is radical esophagectomy with three-field lymph node dissection, and neoadjuvant chemotherapy with a 5-fluorouracil (5-FU) plus cisplatin regimen (FP) is recommended for clinical stage II/III patients[4]. In Western countries, some regimens including L-OHP or Cet have been reported to be safe and effective for advanced esophageal cancer[5-8].
We report a case of synchronous advanced cancers of the rectum and the esophagus, successfully treated by neoadjuvant chemotherapy with a FOLFOX (L-OHP + 5-FU + leucovorin) plus Cet regimen followed by two-stage surgical resection.
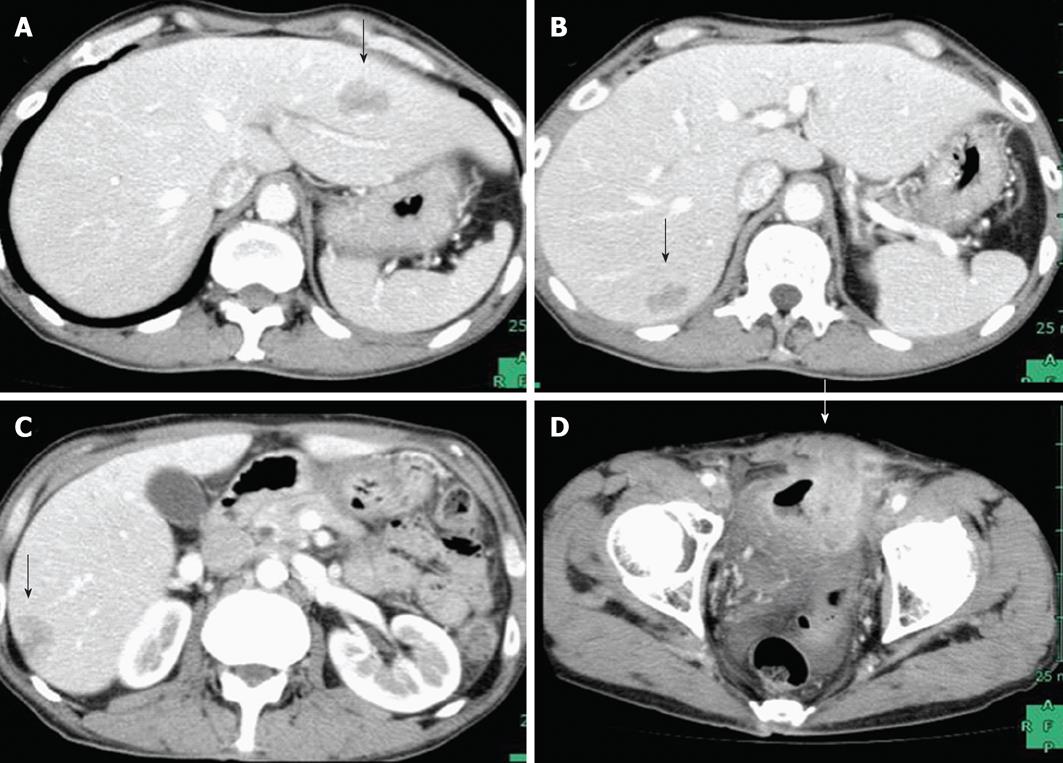
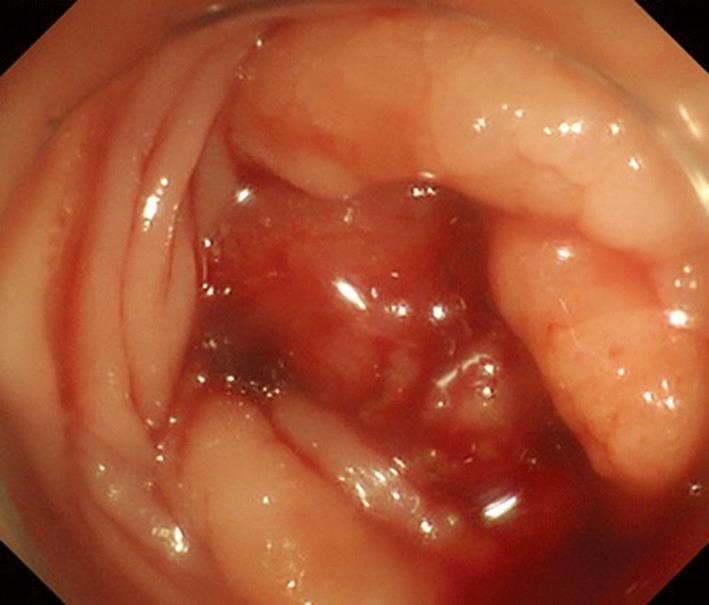
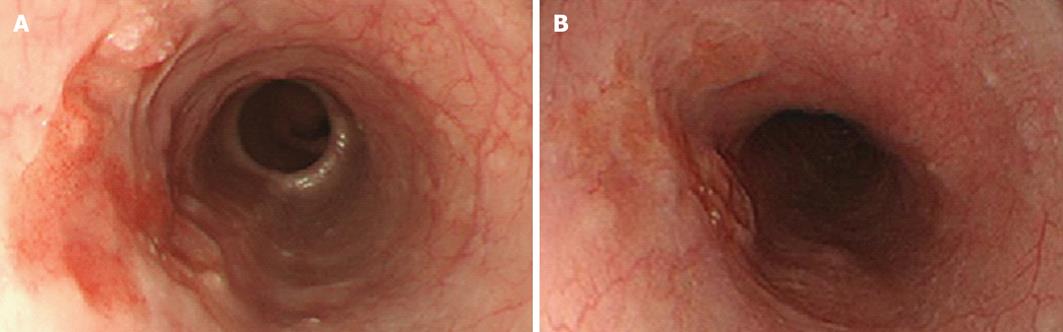
A 61-year-old male patient was admitted for a urinary tract infection. An abdominopelvic computed tomography (CT) revealed an intrapelvic mass that had invaded the bladder and multiple hepatic lesions with weak contrast enhancement (Figure 1). On colonoscopy, a circumferential ulcerative lesion was seen in the upper rectum (Figure 2). Biopsy revealed a moderately differentiated adenocarcinoma, and KRAS genotyping showed that it was the wild type. On esophagogastroscopy, a depressed lesion with a little surrounding elevation was observed in the mid-thoracic esophagus, and biopsy resulted in a diagnosis of SCC (Figure 3). Based on the results of these examinations, a diagnosis of synchronous advanced cancers consisting of a rectal cancer involving the urinary bladder associated with multiple liver metastases (cT4b, cN0, cM1, Stage IV), and an esophageal cancer (cT2, cN0, cM0, Stage IB) were made.
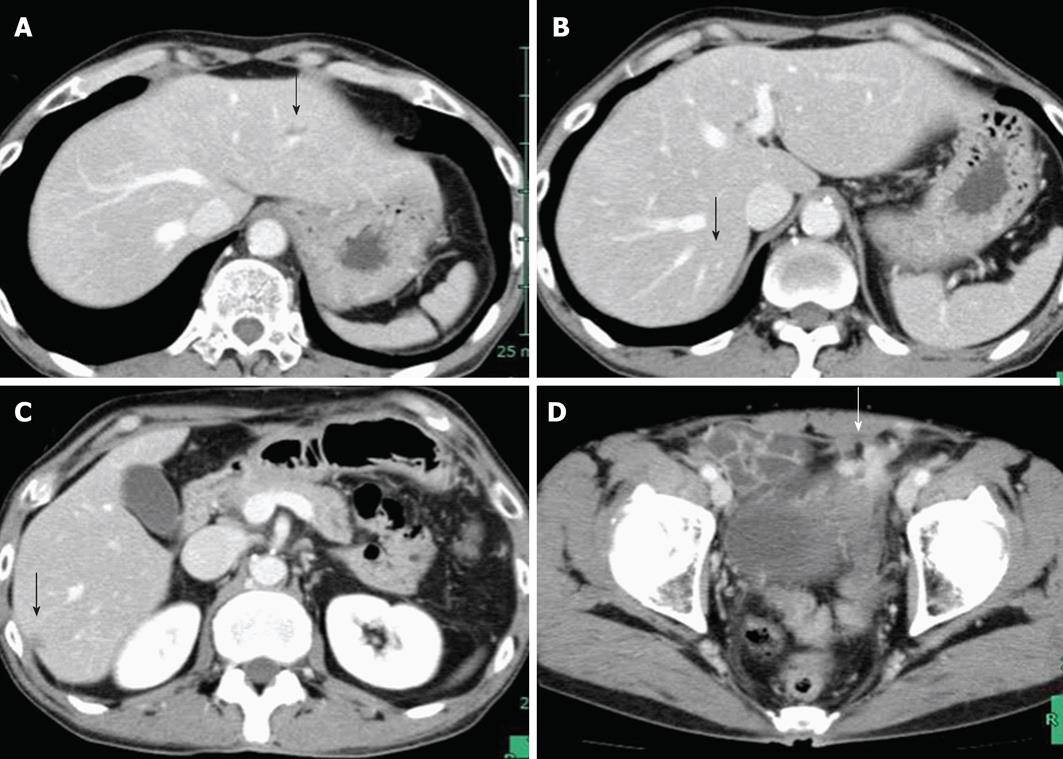
First, after creating a loop colostomy with the sigmoid colon in order to control the urinary tract infection, which had developed as a result of a bladder fistula, and to remove the rectal obstruction, we instituted neoadjuvant chemotherapy with the FOLFOX + Cet regimen. An abdominopelvic CT after 6 cycles of chemotherapy showed marked regression of both the primary lesion in the rectum and the liver metastases (Figure 4). Although no marked change in the size of the esophageal cancer was observed during esophagogastroscopy, flattening of the lesion was noted. Five months after diagnosis, high anterior resection combined with cystoprostatectomy, diverting ileostomy, neobladder reconstruction, and lateral segmentectomy plus partial hepatectomy were performed with 6-wk completion of chemotherapy. The operative time was 981 min, and blood loss was 1555 mL. The pathological diagnosis of the both lesions was moderately differentiated adenocarcinoma with invasion of the urinary bladder (ypT4b, ypN0, ypM1, Stage IV). There was moderate vascular invasion, but the surgical margins were negative. According to the Japanese Classification of Colorectal Carcinoma, the histological effect of the chemotherapy was Grade 1b at the primary site and Grade 2 at the site of the liver metastases. Although wound infection and urinary tract infection occurred as postoperative complications, they treated conservatively, and the patient was discharged on postoperative day 15.
Because recovery of the patient’s general condition was delayed due to postoperative watery diarrhea, ileostomy closure was performed. Even though 5 mo had passed since the completion of chemotherapy, esophagogastroscopy showed no evidence of an increase in size of the esophageal cancer, and it was concluded that the disease had been adequately controlled (Figure 3B). Esophagectomy with three-field lymph node dissection and anterosternal reconstruction with gastric tube were performed 4 mo after rectal resection. The operative time was 584 min, and blood loss was 1027 mL. The pathological diagnosis was poorly differentiated SCC (ypT1b, ypN1, cM0, Stage IIB). The histological effect of chemotherapy was minimal with slight cancer cell degeneration or necrosis. Postoperatively, anastomotic leakage occurred, but it was improved by conservative management, and the patient was discharged on postoperative day 70.
Eleven months after rectal resection, pulmonary node with elevation of carcinoembryonic antigen (CEA) was pointed out and right lower lobectomy was performed. Pathological findings showed it metastasis from rectal adenocarcinoma. The patient is alive without cancer 15 mo after pulmonary resection.
In case with synchronous multiple cancers, it is sometimes difficult to identify the origin especially when liver and/or pulmonary lesions have a possibility of metastases, or to decide on a course or priority of the treatment. When the origin of liver or pulmonary lesions is unclear, there are several solution strategies. Biopsy is the most reliable method for a definite diagnosis. However, biopsy occasionally causes a future disseminated diseases, therefore it might be avoided in cases having possibility of curative resection. The increasing level of tumor markers is sometimes helps us to diagnosis. Another promising option is chemosensibility. The good response to chemotherapy is helpful to make diagnosis. In this case, if the liver tumor came from the esophageal cancer, FOLFOX and Cet might be invalid. However, in general, surgical treatment in curative intent has not indicated for the patients with liver metastases from esophageal squamous carcinoma or with colorectal liver metastases which progress in spite of aggressive chemotherapy. As a result, shrinkage of the liver tumor enabled us to perform curative resection and to confirm the origin to be the rectum. In case of progression in any tumor, palliative treatment should be one of the options. The pulmonary recurrence is also considered to come from the rectal cancer, because of increasing level of CEA. If the level of CEA was within normal range, initial chemotherapy or biopsy might be selected.
R0 resection of each of the lesions in this patient with two cancers was considered possible by performing anterior pelvic exenteration, liver resection, and esophagectomy. However, it might be impossible for the patient to endure the stress of all of these operative procedures at once. Therefore, we planned to perform staged treatment with prioritizing consideration. A 5-year overall survival (OS) rate of approximately 45% had been reported after surgical resection of liver metastases from colorectal cancer[9], as opposed to a rate of 60% after initial surgical treatment of clinical T1/2 SCC of the thoracic esophagus[10]. Additionally, the patient’s complaints are another important part. In this case, the patient had repeated urinary tract infection and no esophageal obstructions. Therefore, we decided to treat the colorectal cancer and liver metastases preferentially.
The only potentially curative treatment for patients with liver metastases from colorectal cancer is surgical resection, but the postoperative recurrence rate has been reported to be approximately 75%, which is too high. To improve the survival rate or reduce the recurrence rate, new surgical strategies combined with developed systemic anticancer agents have recently been evaluated. As a result of EORTC40983 study, reported by Nordlinger et al[1], curative surgery combined with 6-mo perioperative chemotherapy is now generally recommended for patients with resectable liver metastasis, especially for chemotherapy naïve patients. However, the optimal chemotherapy regimen remains uncertain. A phase II trial of the efficacy of Cet in combination with either the FOLFOX regimen or FOLFIRI regimen (CELIM study) was conducted in patients with unresectable liver metastasis. Since the overall response rate (68%) and R0 resection rate (38%) in the Cet plus FOLFOX regimen group were superior to those (57% and 30%, respectively) in the Cet plus FOLFIRI regimen group[2], we treated our patient with the mFOLFOX6 + Cet regimen in anticipation of obtaining the maximum tumor regression effect and survival benefit.
The greatest concern with this treatment strategy was rapid progression of the esophageal cancer as a result of the decreased immune capacity associated with the highly invasive operation, which consist of extended pelvic surgery and liver resection. Therefore, controlling the esophageal disease during this period for rectal cancer treatment was extremely important. A Japanese randomized phase III trial (JCOG 9907) compared preoperative chemotherapy to postoperative chemotherapy for patients with clinical stage II/III SCC of the esophagus, and the results showed that the 5-year OS of the preoperative chemotherapy group was significantly superior to that in the postoperative chemotherapy group (55% vs 43%, P = 0.04)[4]. Based on the results of this trial, the standard treatment for advanced thoracic esophageal cancer has changed from surgery followed by postoperative chemotherapy to preoperative chemotherapy followed by surgery in Japan. In Western countries, modern cytotoxic agents and targeted drugs have been reported to be effective and safe in patients with advanced esophageal cancer. L-OHP is a promising antineoplastic platinum derivative with a more favorable toxicity profile than cisplatin. Response rates of to the FOLFOX regimen and CapeOx (capecitabine + LOHP) regimen have been reported to be 39%-44%, suggesting be that they might be equivalent to the current standard FP regimen[5-7]. Lorenzen et al[8] conducted a randomized phase II trial that investigated the activity and safety of adding Cet to the standard FP regimen (FP + Cet). The overall response rate and disease control rate were 19% and 75%, respectively, in the FP + Cet group, and superior to the rates in the FP alone group (13% and 57%, respectively). Moreover, there was no evidence that L-OHP or Cet aggravated the known toxicities.
In our patient, objective clinical and pathological response was stable disease, which indicated FOLFOX + Cet was not necessarily effective. However, rapid progression of esophageal cancer could be avoided, although it was untreated for 6 mo between the last administration of the chemotherapy and the esophageal cancer surgery. FOLFOX + Cet might suppress the growth of esophageal cancer.
In conclusion, we have reported the case of synchronous advanced rectal cancer involving the urinary bladder massively associated with multiple liver metastases, and esophageal cancer. FOLFOX + Cet therapy could provide a favorable control of not only rectal origin accompanied by liver metastases but also esophageal cancer, which made possible to undergo two-stage curative resection. FOLFOX + Cet regimen might be a useful option for such refractory rectal and esophageal cancer.
P- Reviewer Hakenberg OW S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1441] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 2. | Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 3. | Morita M, Yoshida R, Ikeda K, Egashira A, Oki E, Sadanaga N, Kakeji Y, Yamanaka T, Maehara Y. Advances in esophageal cancer surgery in Japan: an analysis of 1000 consecutive patients treated at a single institute. Surgery. 2008;143:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1048] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 5. | Mauer AM, Kraut EH, Krauss SA, Ansari RH, Kasza K, Szeto L, Vokes EE. Phase II trial of oxaliplatin, leucovorin and fluorouracil in patients with advanced carcinoma of the esophagus. Ann Oncol. 2005;16:1320-1325. [PubMed] |
| 6. | van Meerten E, Eskens FA, van Gameren EC, Doorn L, van der Gaast A. First-line treatment with oxaliplatin and capecitabine in patients with advanced or metastatic oesophageal cancer: a phase II study. Br J Cancer. 2007;96:1348-1352. [PubMed] |
| 7. | Qin TJ, An GL, Zhao XH, Tian F, Li XH, Lian JW, Pan BR, Gu SZ. Combined treatment of oxaliplatin and capecitabine in patients with metastatic esophageal squamous cell cancer. World J Gastroenterol. 2009;15:871-876. [PubMed] |
| 8. | Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P, Arnold D, Greten T. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20:1667-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Gigot JF, Schulick RD, Choti MA, Aldrighetti L. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755-764, 764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |