Copyright
©The Author(s) 2021.
World J Clin Cases. Dec 26, 2021; 9(36): 11122-11147
Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11122
Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11122
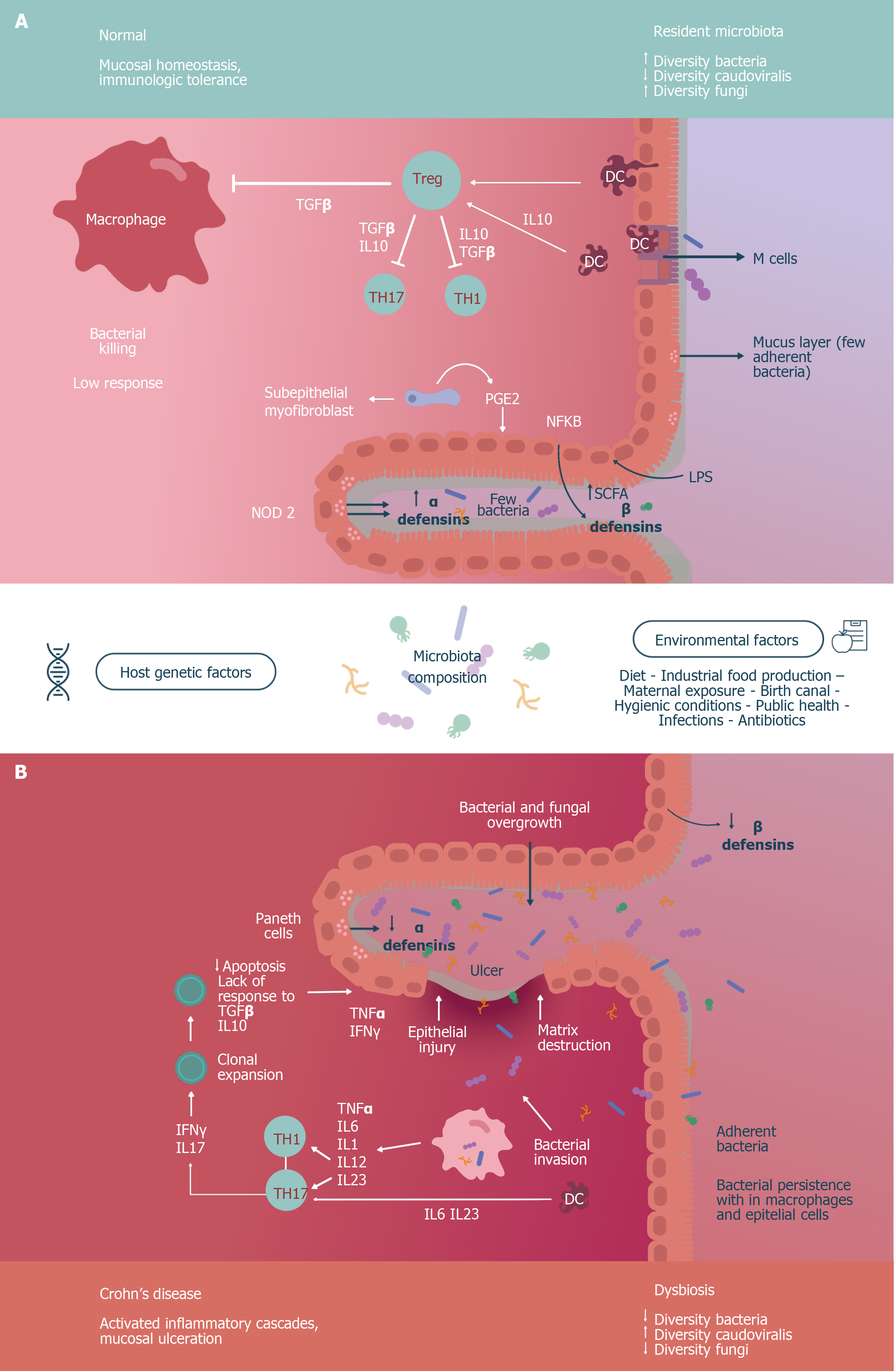
Figure 1 Commensal microbiota modulates the response of the immune system in the intestinal mucosa and participates in the metabolism of the epithelium.
A: In the case of immune regulation, dendritic cells (DCs), activated in M cells, modulate regulatory T lymphocytes (Tregs) through anti-inflammatory signals (such as interleukin [IL]-10), which in turn inhibit other anti-inflammatory signals (such as IL-10 and transforming growth factor-beta [TGFβ]) of T helper cells 1-17 and macrophages. In the case of epithelial metabolism, the synthesis of short chain fatty acids (SCFAs), the secretion of prostaglandins (PGEs, such as PGE2) and the activity of nuclear factor-kappa B (NF-kB) contributes to an adequate secretion of defensins α and β, to the appropriate functioning of the mucus layer and even the microbiome state in homeostasis; B: The persistence of bacteria adhering to a deteriorated mucus film, the decrease in defensins and the invasion of the epithelium produces the activation of DCs and macrophages, that in turn activate the T helper (Th) cells, Th1 and Th17, by proinflammatory signals (IL-1, IL-6, IL-12, and IL-23), thereby promoting a low apoptosis, clonal expansion with loss of response to anti-inflammatory signals, and contributing to tissue damage mediated by interferon gamma (INFγ) and tumor necrosis factor alpha (TNFα). This figure is based upon data published in Reference 11.
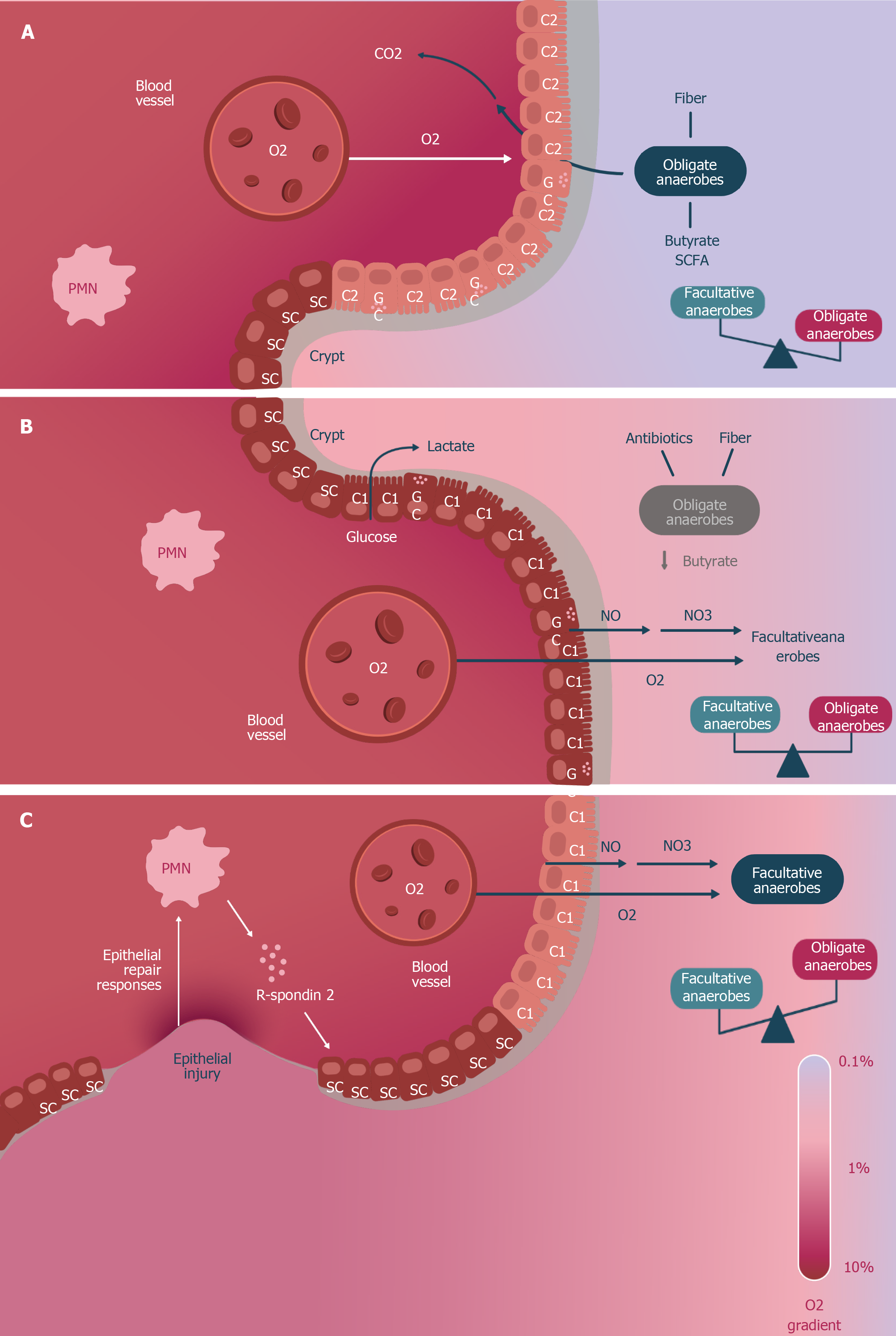
Figure 2 Metabolic epithelial changes in dysbiosis due to loss of obligate anaerobes.
A: The eubiosis microbiome contains a significant number of obligate anaerobes that convert dietary fiber into short chain fatty acids (SCFAs), contributing to a C2-type epithelial trophism with high oxygen consumption (O2), which limits its diffusion to the intestinal lumen and maintains the epithelium in relative hypoxia; B: The deterioration of the microbiome with loss of obligate anaerobes and SCFAs orients the metabolism of the epithelium to a C1-type, with greater glucose fermentation, low O2 consumption, and generation of lactic acid and nitric oxide (NO). The higher partial pressure of the O2 not consumed by the C1 colonocytes and the conversion of NO to nitrate (NO3-) causes an overgrowth of facultative anaerobes; C: The persistence of the loss of strict anaerobes and their fermentation products ends up causing epithelial damage (via polymorphonuclear neutrophils R-spondin 2), the response of which is crypt hyperplasia and the multiplication of stem cells (SCs). The sustained increase in NO3- and O2 contributes to the persistence of facultative anaerobes and dysbiosis. This figure is based upon data published in reference 39.
- Citation: Ceballos D, Hernández-Camba A, Ramos L. Diet and microbiome in the beginning of the sequence of gut inflammation. World J Clin Cases 2021; 9(36): 11122-11147
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11122