Copyright
©The Author(s) 2021.
World J Clin Cases. Apr 6, 2021; 9(10): 2400-2408
Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2400
Published online Apr 6, 2021. doi: 10.12998/wjcc.v9.i10.2400
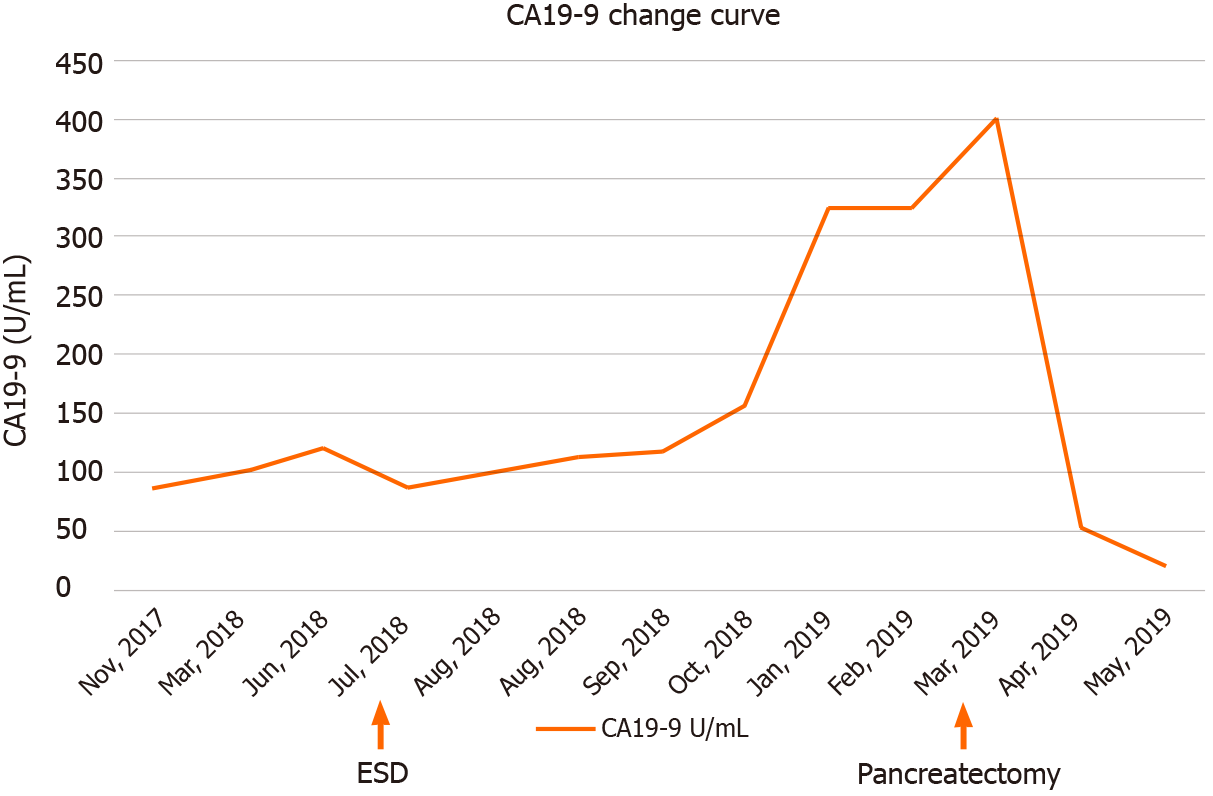
Figure 1 Line chart of blood carbohydrate antigen 19-9 during the diagnosis and operation steps in the case course.
Carbohydrate antigen (CA) 19-9 level of this patient increased continuously, suggesting an invasive cancer. The CA19-9 level increased to 400.1 U/mL before the pancreatic cancer was removed and decreased after the operation. CA19-9: Carbohydrate antigen 19-9; ESD: Endoscopic submucosal dissection.
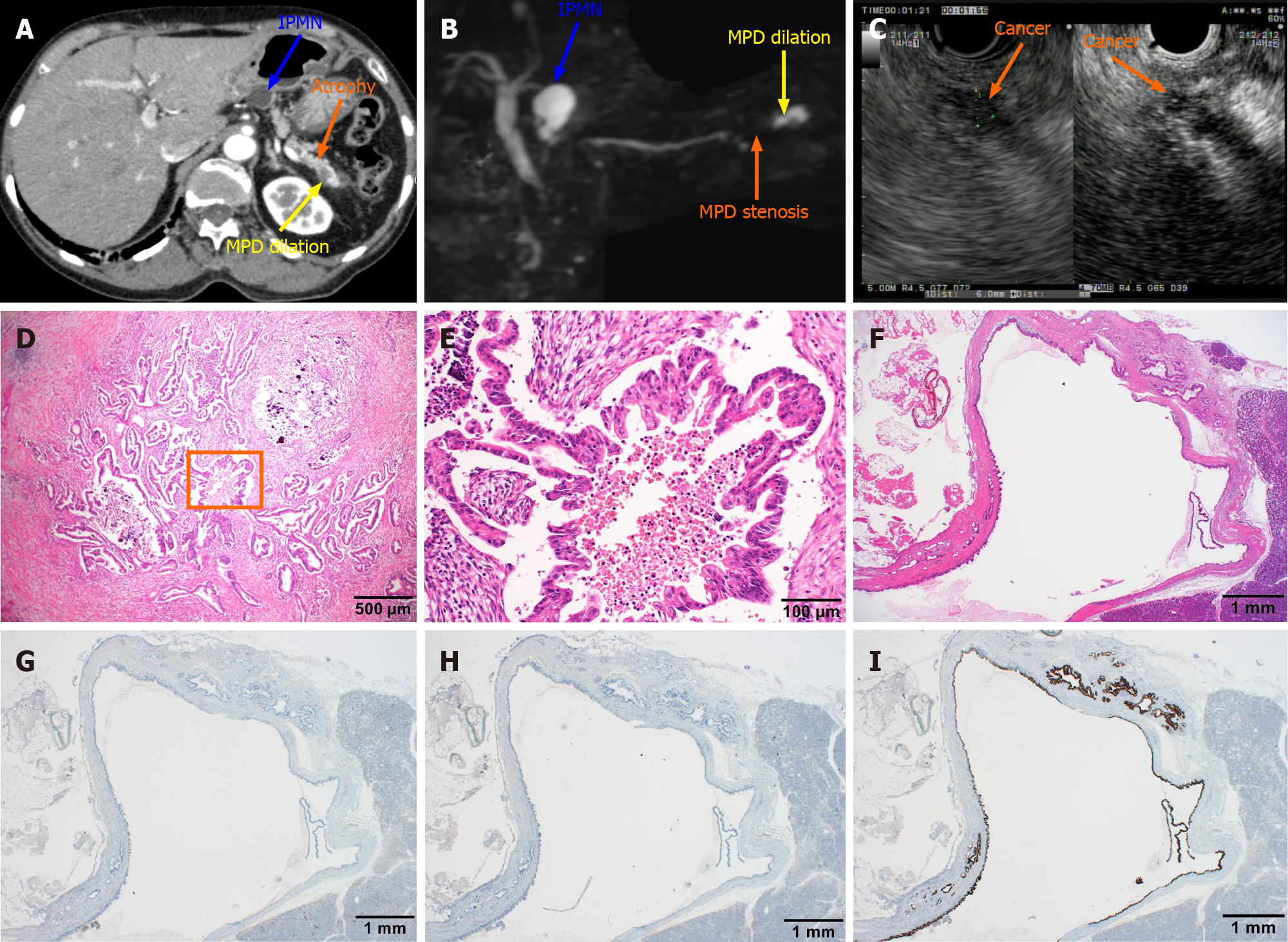
Figure 2 Computed tomography, magnetic resonance cholangiopancreatography, and endoscopic ultrasound images obtained after admission and postoperative pathological diagnosis.
A: The blue arrow indicated a lesion of intraductal papillary mucinous neoplasm (IPMN) with a circular low-density shadow of the pancreatic body with clear boundaries. The orange arrow indicates atrophy in the tail of the pancreas with decreased density. The yellow arrow indicates local dilation of the pancreatic duct in the tail of the pancreas; B: The blue arrow indicates a lesion of IPMN with a high signal of the pancreatic body, which communicated with the pancreatic duct. The orange arrow indicates pancreatic duct stenosis in the tail of the pancreas. The yellow arrow points to local dilation of the pancreatic duct in the tail of the pancreas; C: The endoscopic ultrasound dynamic scan shows a dilated caudal pancreatic duct with a hypoechoic mass at the root of the dilatation, with uneven internal echogenicity and unclear borders. The orange arrow points to the hypoechoic mass on contrast-enhanced endoscopic ultrasound angiography, suggesting a high probability of pancreatic cancer; D: Hematoxylin and eosin staining of the pancreatic tissue section shows dendritic and papillary growth of pancreatic cancer associated with localized calcification; E: At high magnification, cancer cells show the papillary proliferation with necrosis at the center marked inside panel D; F: The histological features of IPMN are cystic lesion composed of dilated ducts and with the cystic surface lined by a layer of columnar epithelial cells, with basal nuclei showing minimal atypia; G-I: Serial sections were also immunohistochemically stained with antibodies against mucin (MUC) 1 (G) , MUC2 (H) and MUC5AC (I), but only MIC5AC was positively stained. IPMN: Intraductal papillary mucinous neoplasm; MPD: Methylpentedrone.
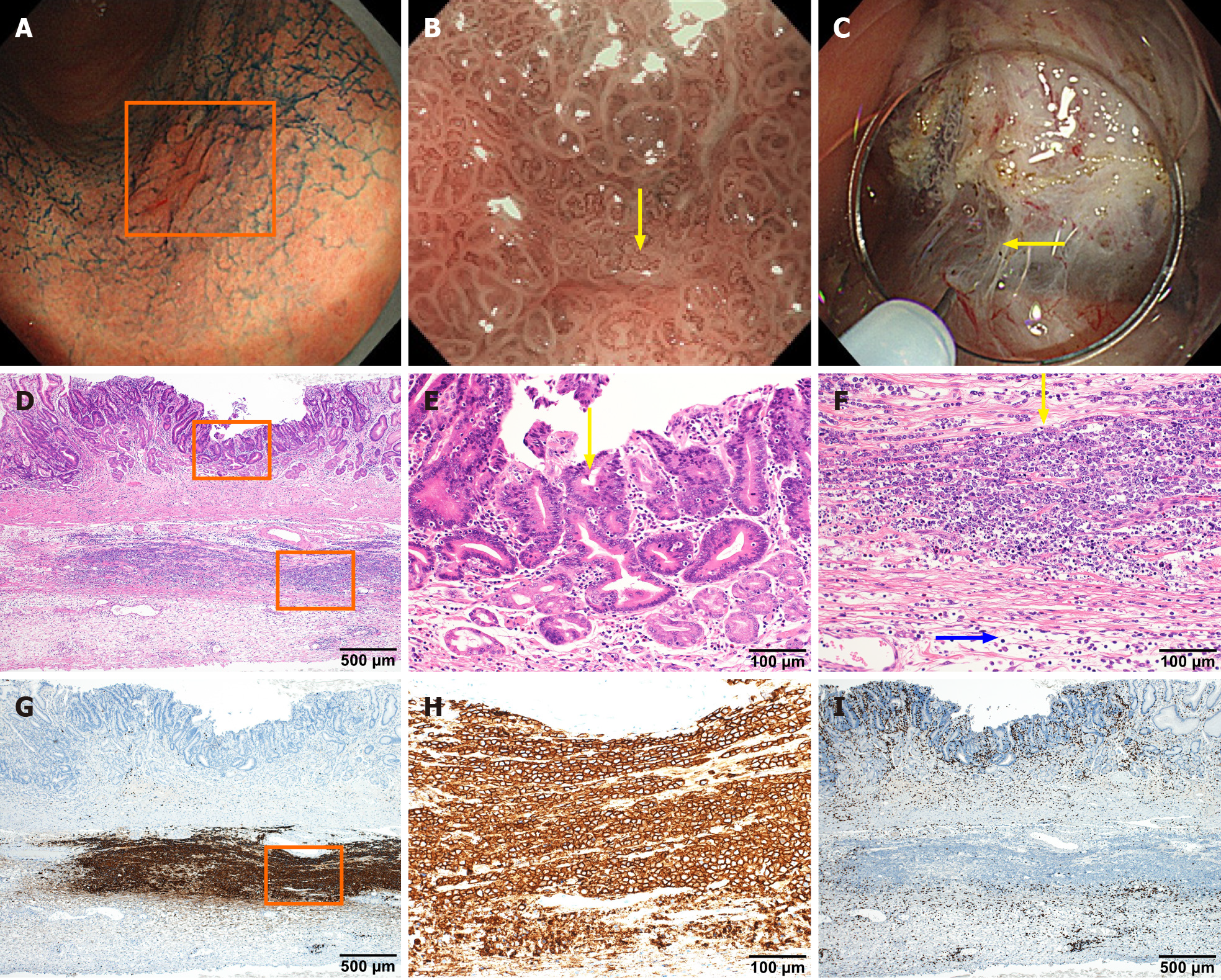
Figure 3 In situ collision between gastric cancer and diffuse large B-cell lymphoma.
A-C: Endoscopic images of lesions were obtained by electronic staining magnifying gastroscopy (GIF-H290Z). Indigo carmine local spray (0.2%) staining of mucous membrane shows uneven staining in the lesion area indicated by the box, with local redness, rough surface, but clear lesion border (A). Narrow band imaging at maximum 85 × magnification under GIF-H290Z gastroscopy clearly shows irregular villi-like structures on the surface of the lesion and vessels of different calibers as indicated by yellow arrows (B). The lesion fibrosis is obvious during endoscopic submucosal dissection operation, as indicated by the arrow, suggesting that the lesion is not confined to the mucosal layer (C); D: Histological examinations revealed that the resected gastric specimen contained a small ulcerated lesion (approximately 5 mm in length) composed of atypical epithelial cells with large nuclei; E: Tubular or papillary proliferation is present, but without submucosal invasion; F: Beneath the gastric carcinoma, there was diffuse infiltration of atypical lymphoid cells in the muscular propria. The yellow arrow indicates atypical lymphoid cells that are large in size, compared with the blue arrow that indicates normal lymphocytes; G-I: Immunohistochemical staining analysis, with brown staining showing abnormal lymphoid cells are positively stained with CD20 (G and H) but negatively stained with CD3 (I).
- Citation: Ma YH, Yamaguchi T, Yasumura T, Kuno T, Kobayashi S, Yoshida T, Ishida T, Ishida Y, Takaoka S, Fan JL, Enomoto N. Pancreatic cancer secondary to intraductal papillary mucinous neoplasm with collision between gastric cancer and B-cell lymphoma: A case report. World J Clin Cases 2021; 9(10): 2400-2408
- URL: https://www.wjgnet.com/2307-8960/full/v9/i10/2400.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i10.2400