Copyright
©The Author(s) 2020.
World J Clin Cases. Sep 6, 2020; 8(17): 3859-3866
Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3859
Published online Sep 6, 2020. doi: 10.12998/wjcc.v8.i17.3859
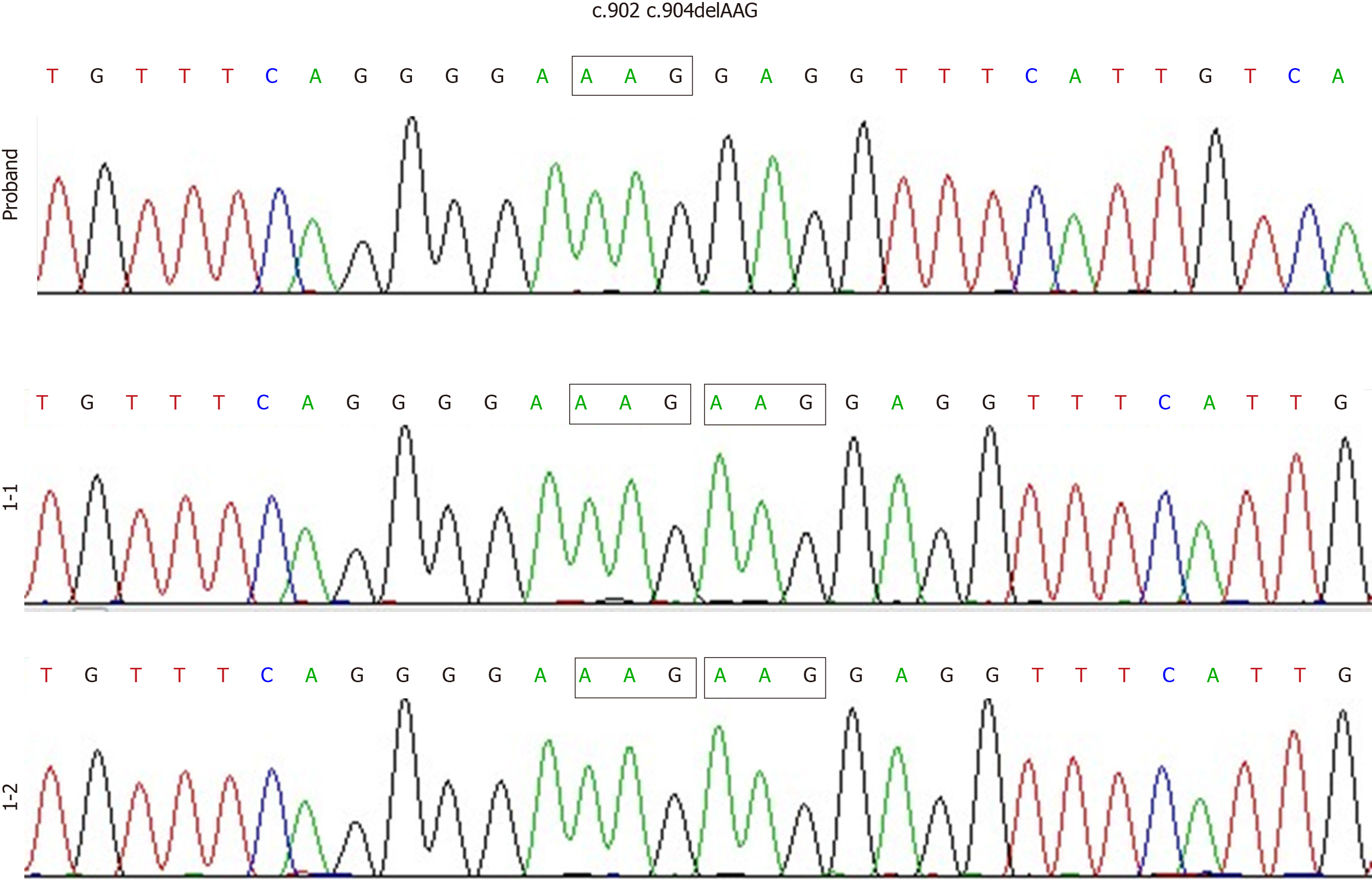
Figure 1 No Bruton’s tyrosine kinase variants were detected in the proband’s parents’ genomes.
Polymerase chain reaction direct sequencing revealed a compound heterozygous Bruton’s tyrosine kinase sequence variant (c.902_c.904delAAG/p.E301del) in the proband. The parents of the proband both had normal Bruton’s tyrosine kinase genes.
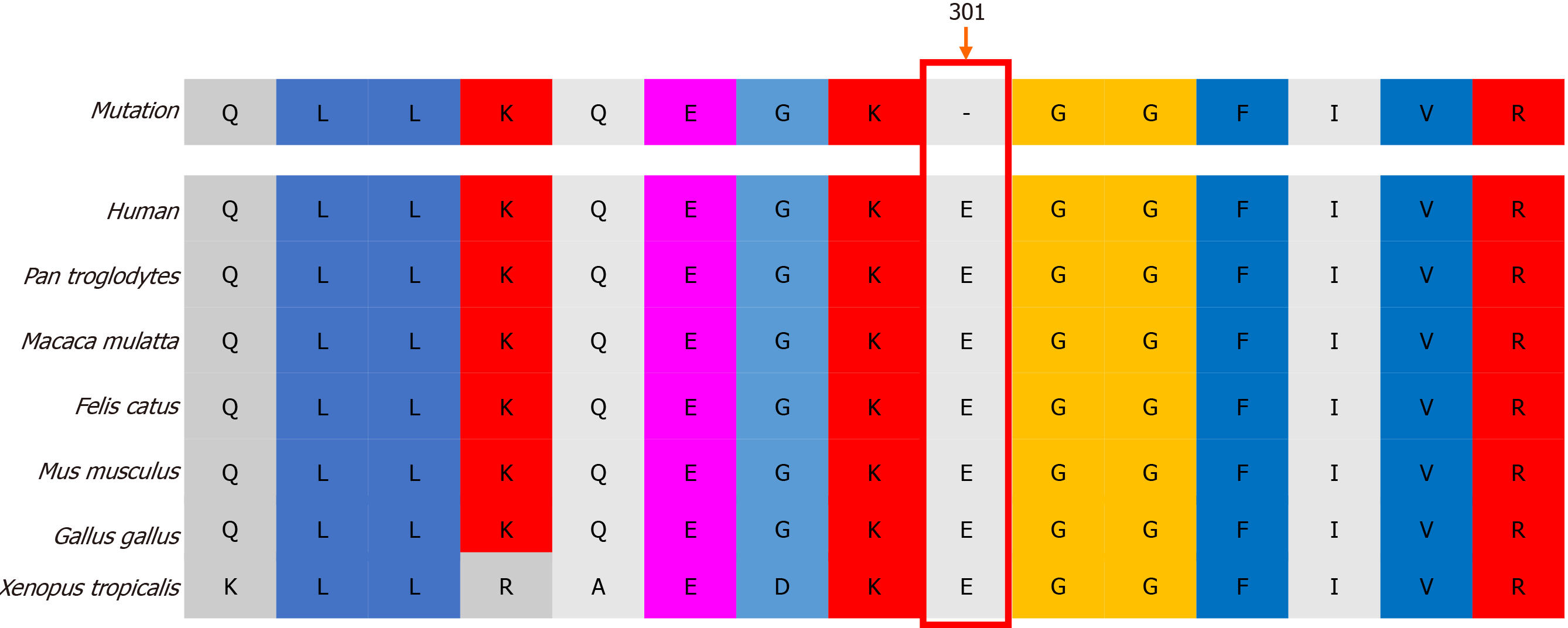
Figure 2 Bruton’s tyrosine kinase sequence conservation analysis.
Amino acid E301 (arrow) was found to be conserved across diverse vertebrates.
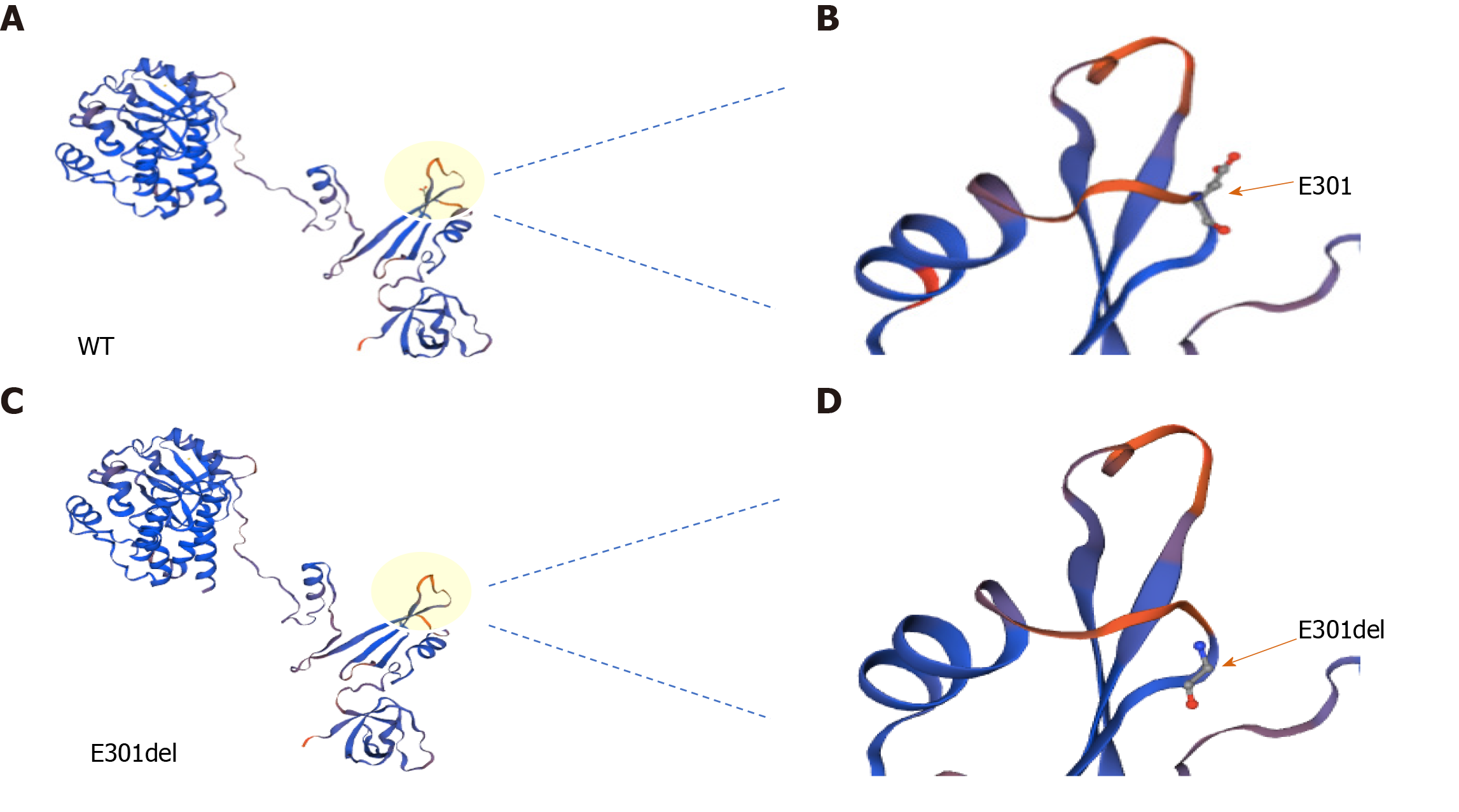
Figure 3 Three-dimensional structural model of Bruton’s tyrosine kinase.
A and C: Total views of wild-type (A) and E401del (C) Bruton’s tyrosine kinase structures. Local views of amino acid 301 in wild-type (B) and E301del (D) Bruton’s tyrosine kinase; arrows point to position 301. WT: Wild-type.
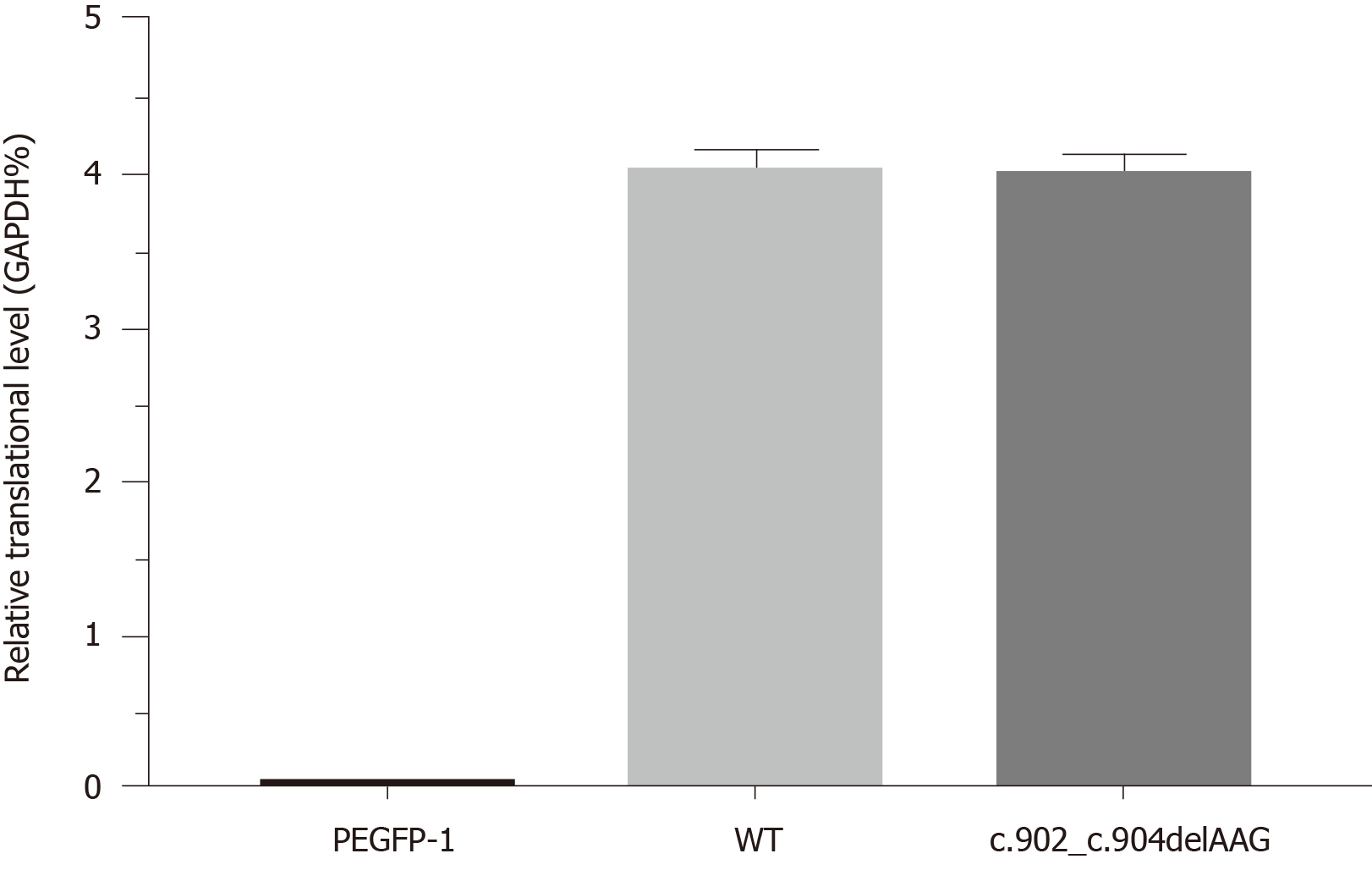
Figure 4 Quantitative polymerase chain reaction results.
Transcript levels were similar between wild-type and E301del Bruton’s tyrosine kinase. Reduced glyceraldehyde-phosphate dehydrogenase was the loading control. WT: Wild-type.
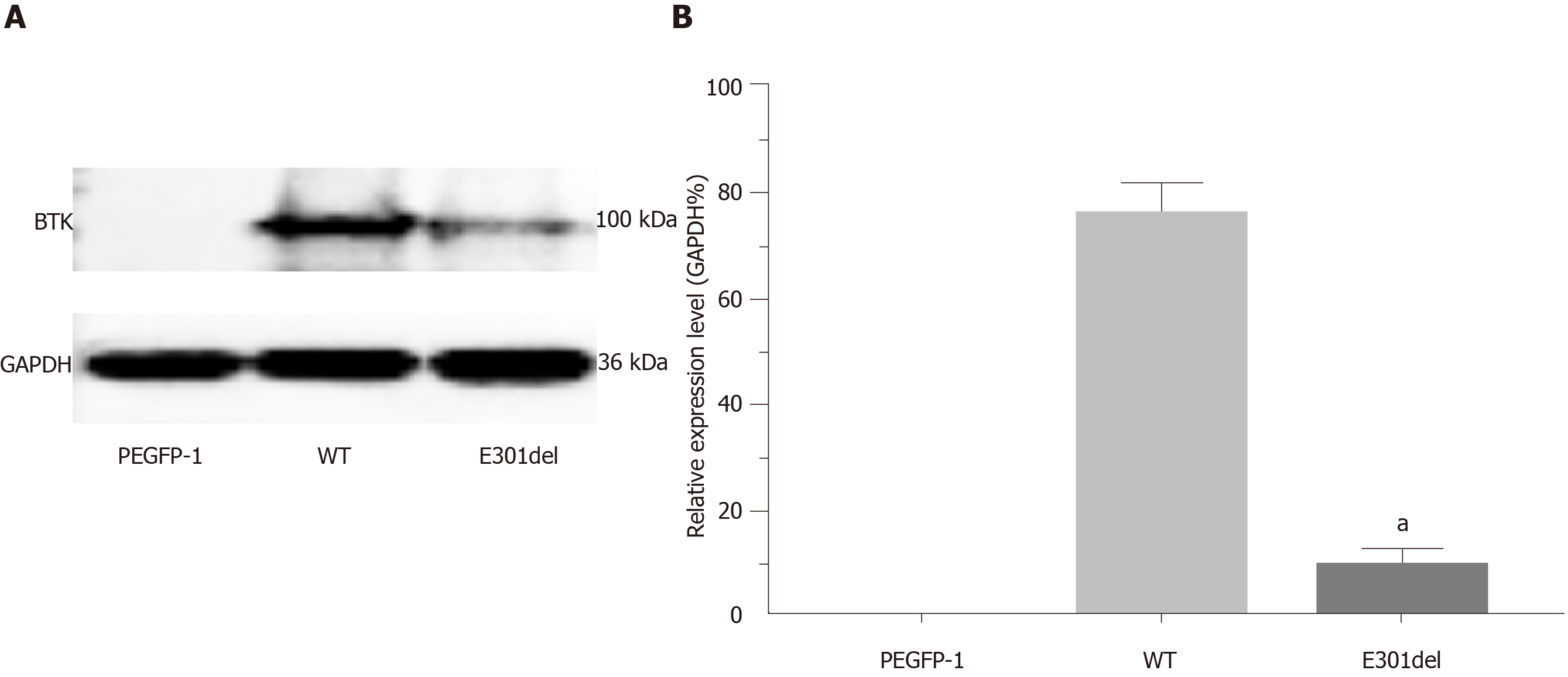
Figure 5 Western blot and relative translation analysis of E301 deletion mutant Bruton’s tyrosine kinase relative to wild-type Bruton’s tyrosine kinase.
Markedly reduced protein band (A) and relative translation (B) were observed with the E301 deletion mutant relative to the wild-type gene. PEGFP-1 is the empty vector control group. Reduced glyceraldehyde-phosphate dehydrogenase was the loading control. Mean ± standard deviation levels are shown; aP < 0.001. BTK: Bruton’s tyrosine kinase; WT: Wild-type.
- Citation: Hu XM, Yuan K, Chen H, Chen C, Fang YL, Zhu JF, Liang L, Wang CL. Novel deletion mutation in Bruton’s tyrosine kinase results in X-linked agammaglobulinemia: A case report. World J Clin Cases 2020; 8(17): 3859-3866
- URL: https://www.wjgnet.com/2307-8960/full/v8/i17/3859.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i17.3859