Copyright
©The Author(s) 2020.
World J Clin Cases. May 26, 2020; 8(10): 1767-1792
Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1767
Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1767
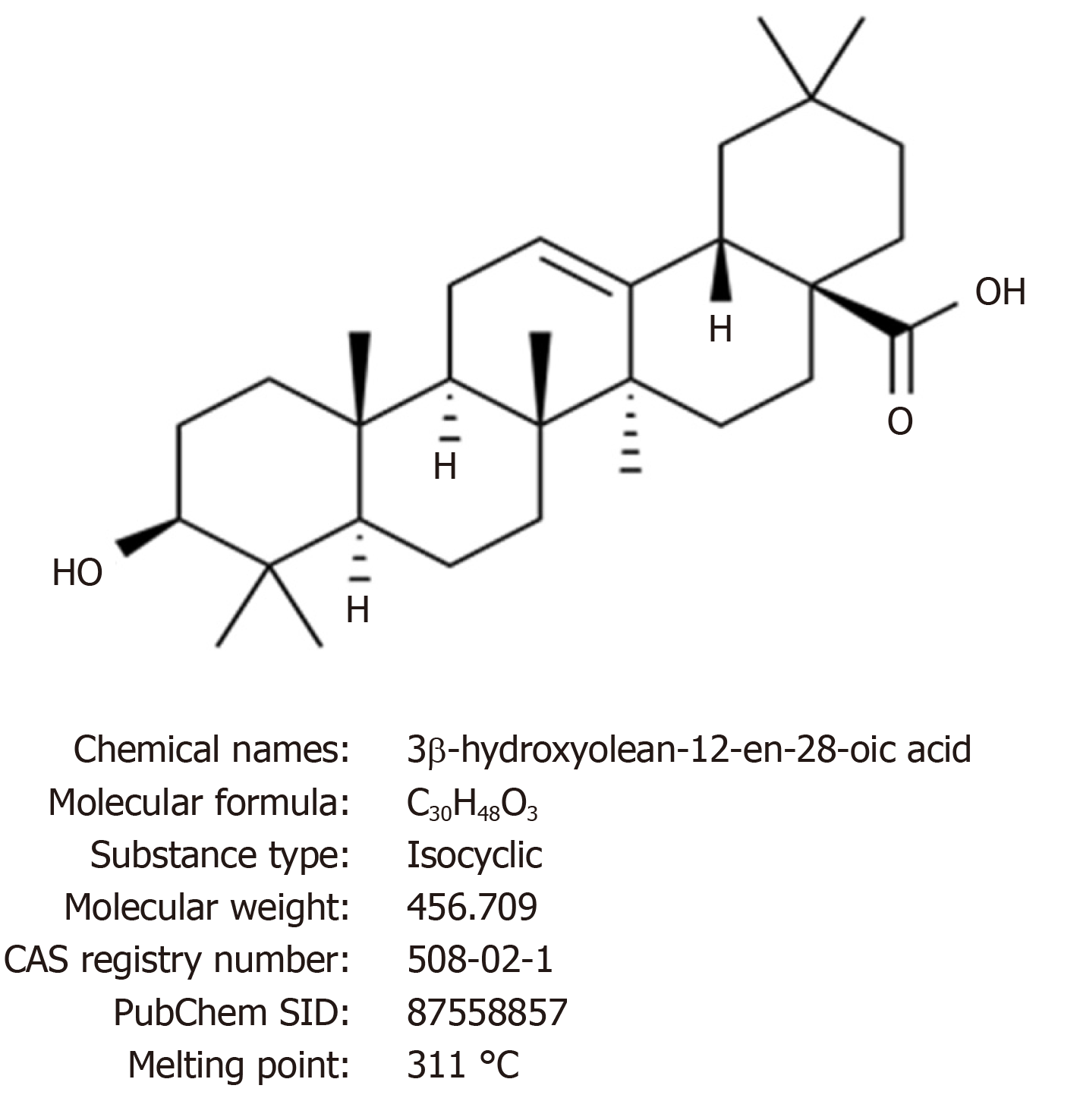
Figure 1 Chemical structure and properties of oleanolic acid.
Figure 2 Structures of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid and its C-28 methyl ester.
CDDO: 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid; CDDO-Me: C-28 methyl ester of CDDO.
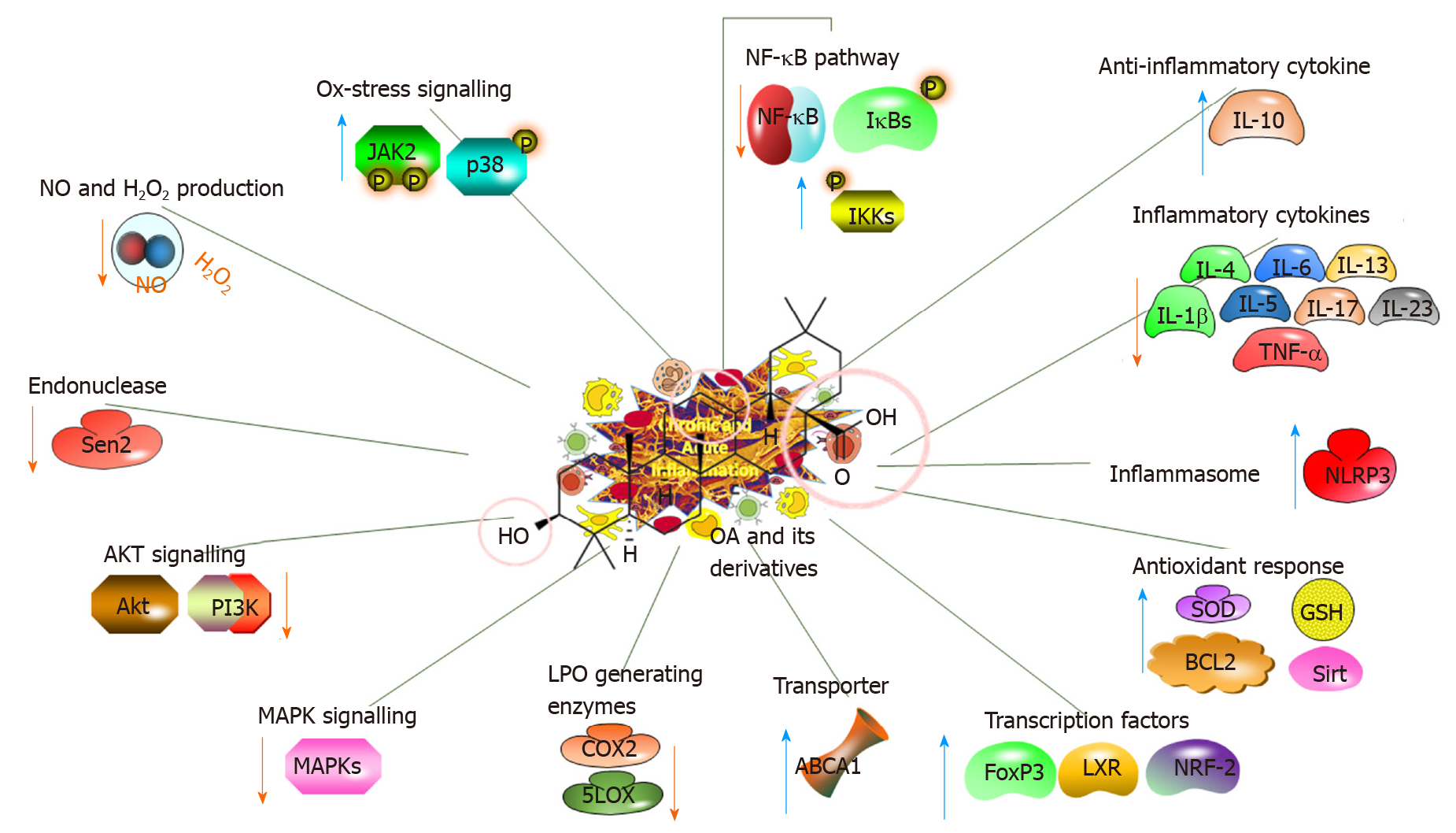
Figure 3 Anti-inflammatory impacts of oleanolic acid and its derivatives, illustrating the molecular mechanisms.
OA: Oleanolic acid; NF-κB: Nuclear factor-κB; IL: Interleukin; TNF-α: Tumour necrosis factor-α; Akt: Serine/threonine kinase; GSH: Glutathione; LXR: Liver X receptor; NRF-2: Nuclear factor erythroid-2-related factor 2.
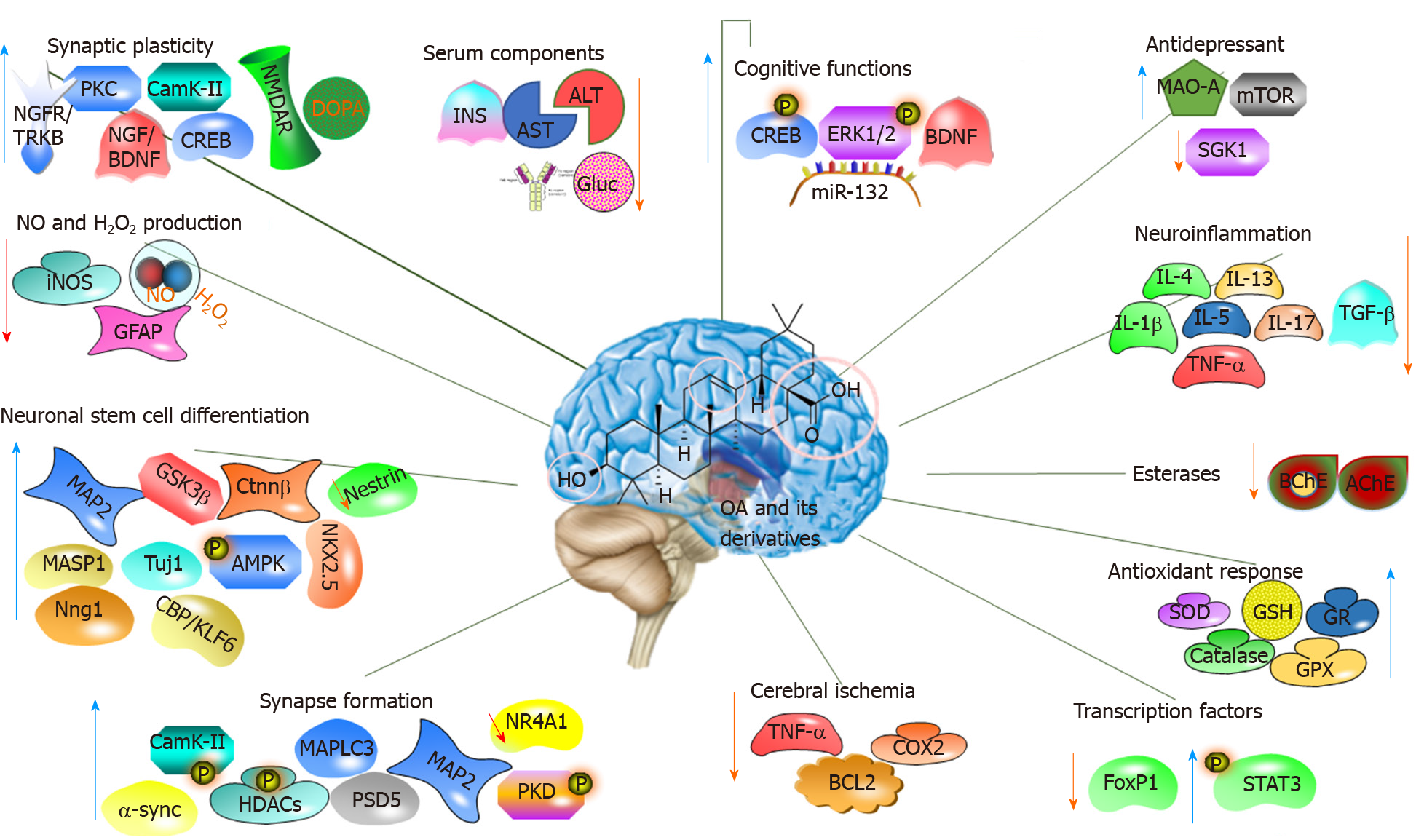
Figure 4 Molecular mechanism of the action of oleanolic acid and its derivatives on the nervous system.
OA: Oleanolic acid; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; IL: Interleukin; TNF-α: Tumour necrosis factor-α; GSH: Glutathione; STAT3: Signal transducer and activator of transcription 3.
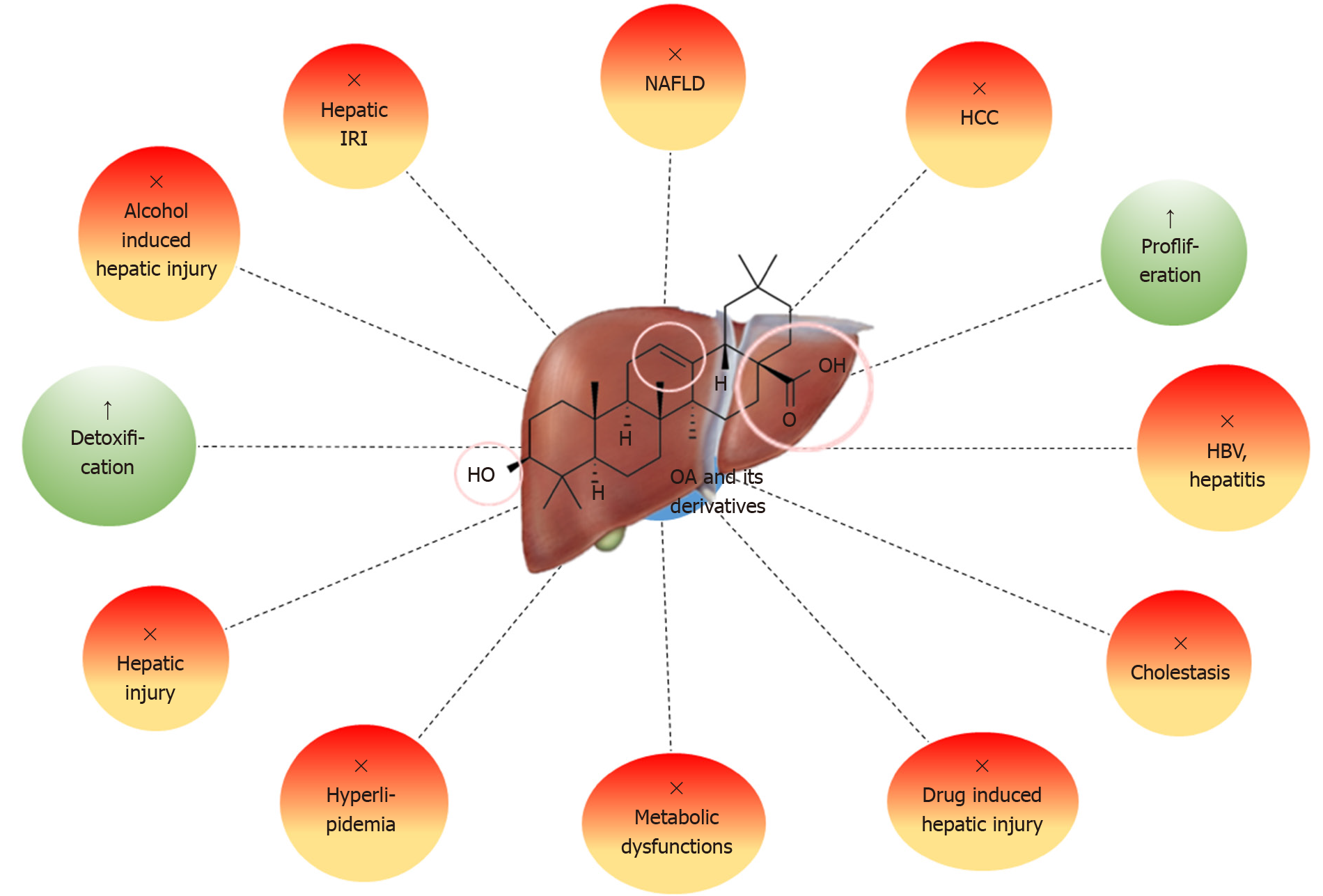
Figure 5 Hepatoprotective effects of oleanolic acid and its natural and synthetic derivatives.
OA: Oleanolic acid; IRI: Ischemia-reperfusion injury; NAFLD: Non-alcoholic fatty liver disease; HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus.
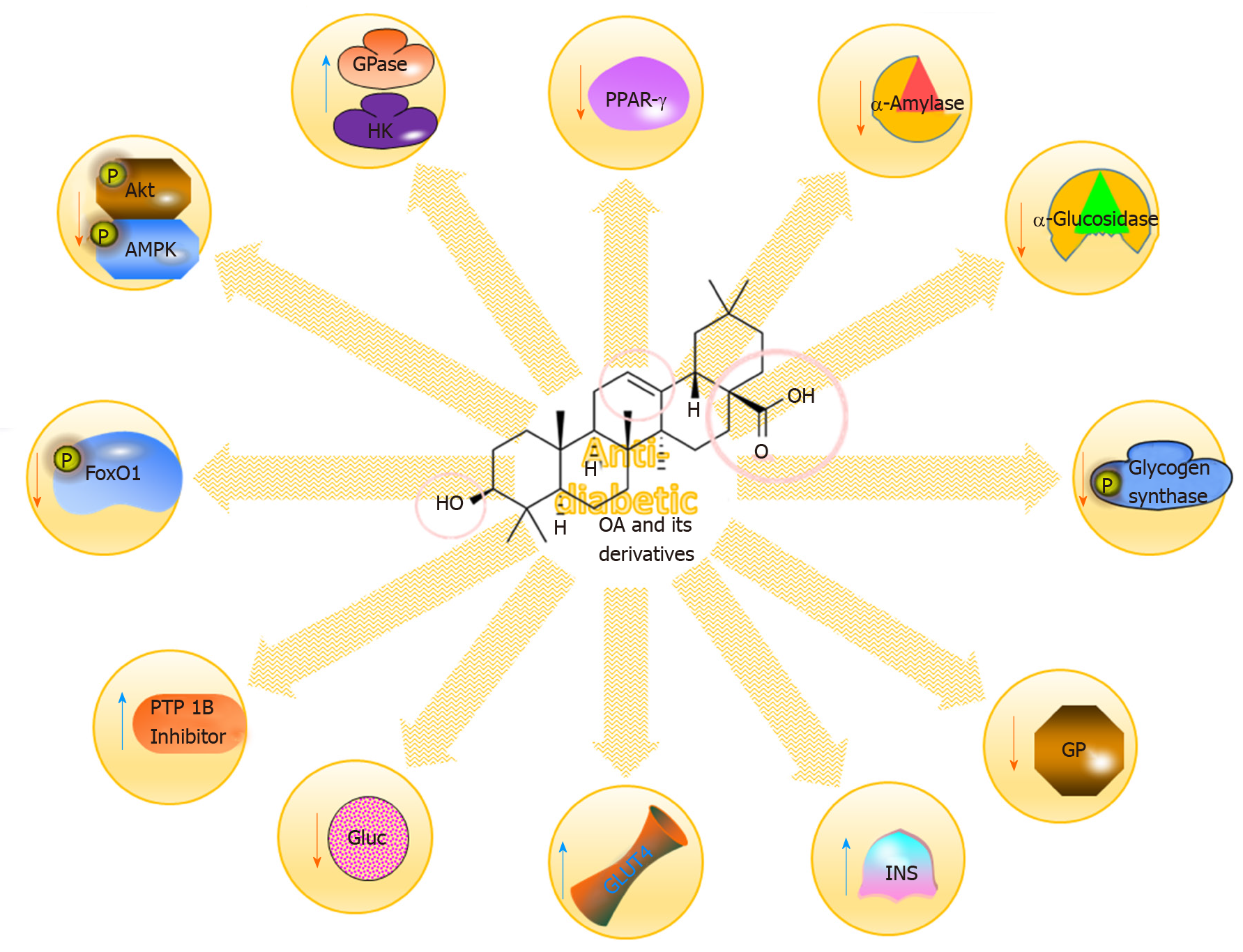
Figure 6 Some of the molecular mechanisms for the anti-diabetic impacts of oleanolic acid and its derivatives.
OA: Oleanolic acid; PPAR: Peroxisome proliferator-activated receptor; Akt: Serine/threonine kinase; AMPK: AMP-activated protein kinase.
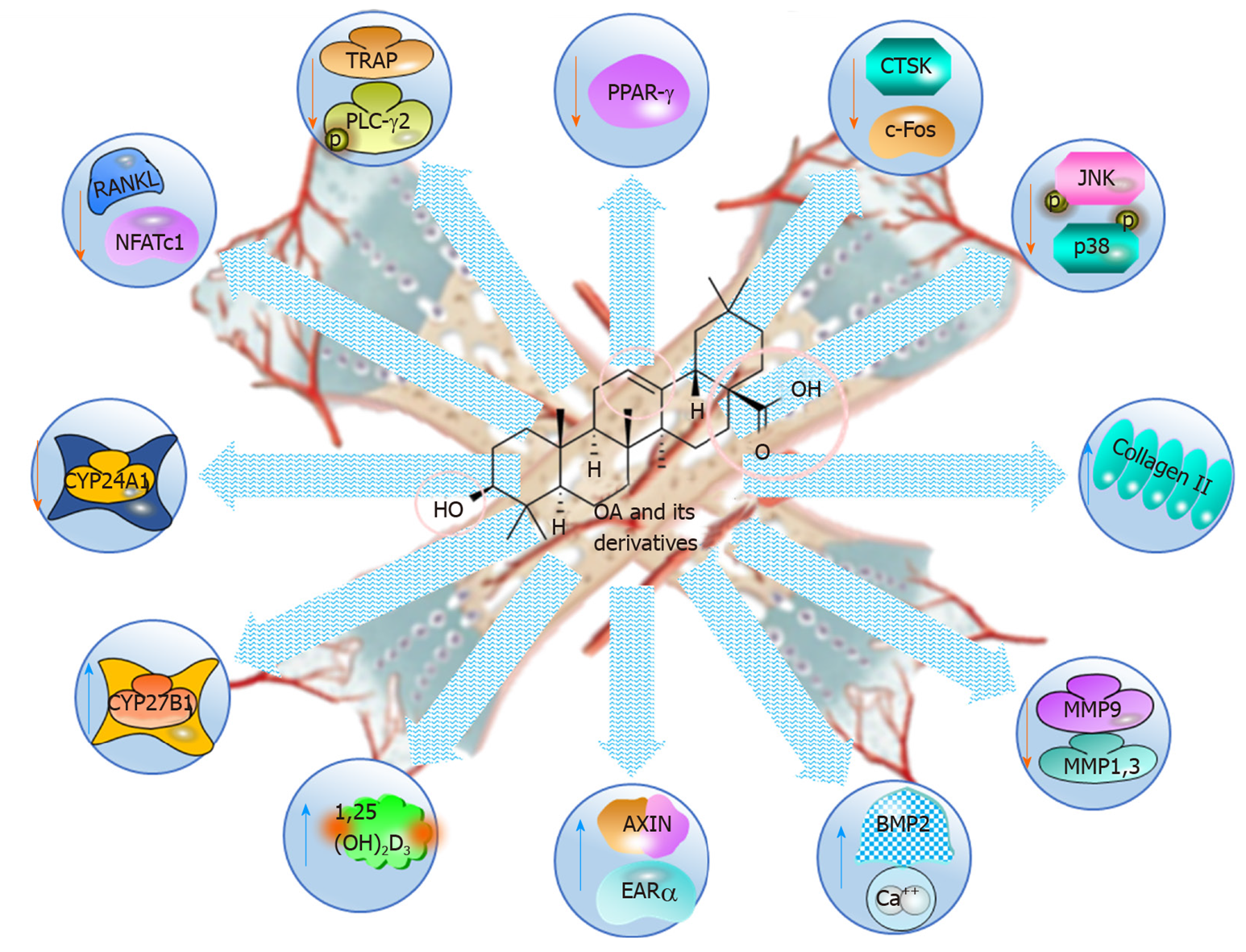
Figure 7 Anti-osteoporotic and bone protective effects of oleanolic acid and its derivatives, illustrating the molecular mechanisms.
OA: Oleanolic acid; PPAR: Peroxisome proliferator-activated receptor; CTSK: Cathepsin K; JNK: cJUN NH2-terminal kinase; MMP: Matrix metalloproteinase; NFAT-c1: Nuclear factor of activated T-cells c1; TRAP: Tartrate-resistant acid phosphatase.
- Citation: Sen A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J Clin Cases 2020; 8(10): 1767-1792
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1767.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1767