Copyright
©The Author(s) 2019.
World J Clin Cases. Dec 6, 2019; 7(23): 4029-4035
Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4029
Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4029
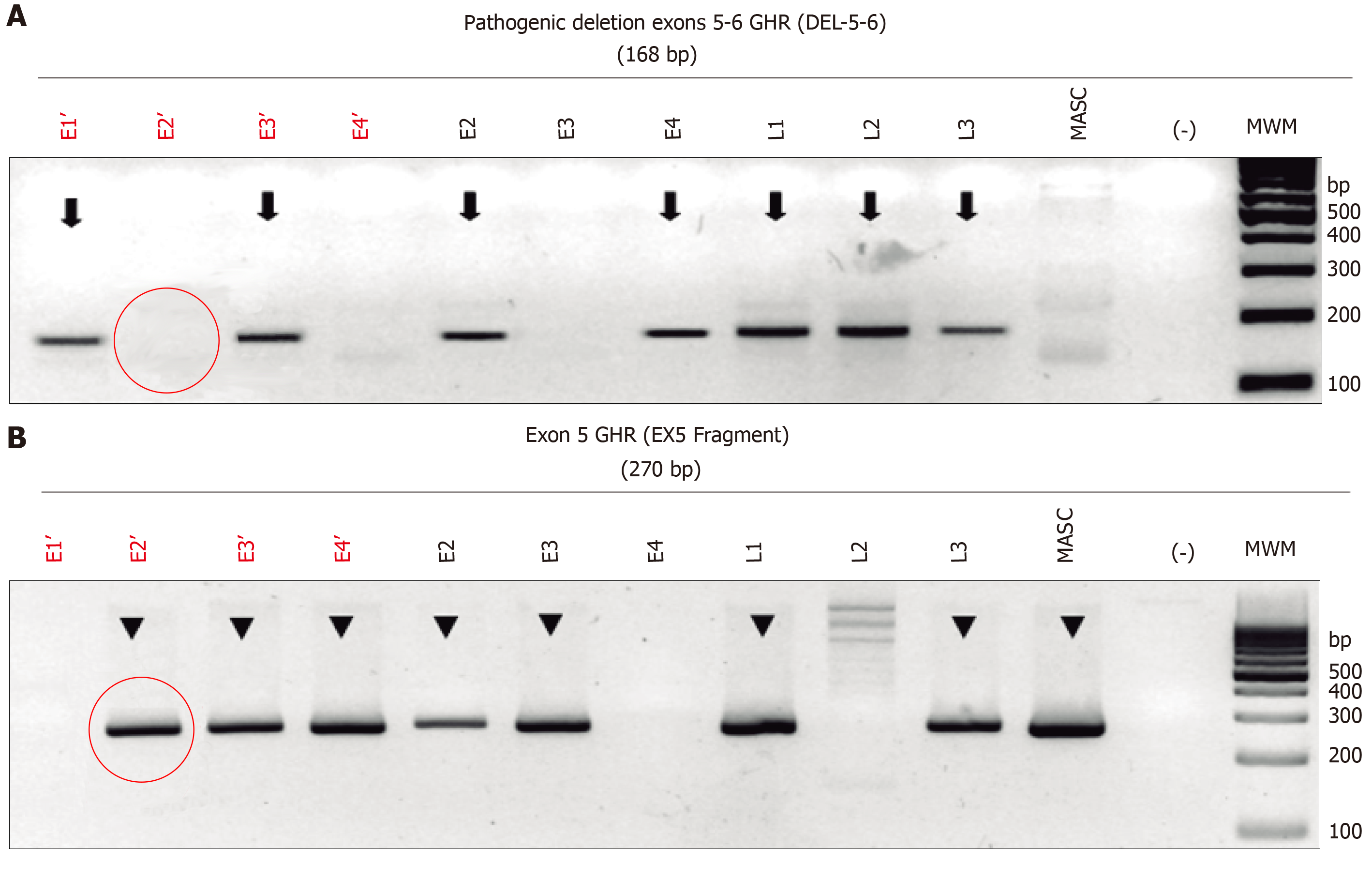
Figure 1 Electrophoresis of the monoplex polymerase chain reaction assay to identify the deletion of exons 5 and 6 (del5-6) of the growth hormone receptor gene for in vitro fertilization embryos.
Lanes E1 to E4 show the whole genome amplification-DNA corresponding to the embryos being analyzed. Lanes L1 to L3 shown the controls previous known to have the deletions. L1 (paternal: heterozygous for del5-6), L2 (affected child: homozygous for del5-6), L3 (maternal: heterozygous for del5-6), masculine (positive control for a homozygous male normal), (-) water control, MWM molecular weight marker (right lane). E1 and E3 show a del5-6, only E1 shows a deletion of ex5, meaning that E1 is homozygous affected, E3 is a heterozygous carrier, and E2 and E4 are wild-types for the deletion causing Laron syndrome. GHR: Growth hormone receptor.
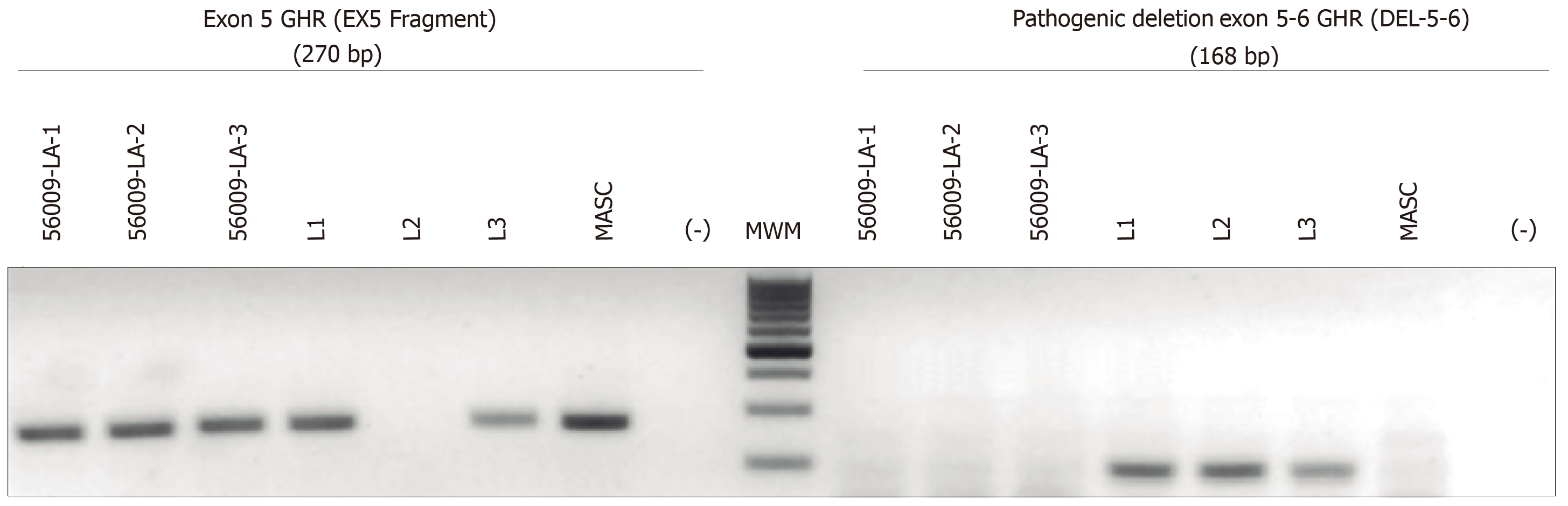
Figure 2 Electrophoresis of the monoplex polymerase chain reaction assay to determine the growth hormone receptor mutational status in the fetus of the pregnant mother.
Amniocytes were extracted and were analyzed in triplicate by generating genomic DNA using the same procedure for embryos. 56009-LA-1 to -3 are the 3 independent analyses of the amniocytes. L1 (paternal: heterozygous for del5-6), L2 (affected child: homozygous for del5-6), L3 (maternal: heterozygous for del5-6), masculine (positive control for a homozygous male normal), (-) is the water control and the molecular weight marker. Results showed the presence of at least one copy of exon 5 of the growth hormone receptor gene; therefore, it can be concluded that the fetus will not present with Laron Syndrome but be a carrier (heterozygote) of Laron syndrome. GHR: Growth hormone receptor.
- Citation: Neumann A, Alcántara-Ortigoza MÁ, González-del Ángel A, Camargo-Diaz F, López-Bayghen E. Diagnosis of Laron syndrome using monoplex-polymerase chain reaction technology with a whole-genome amplification template: A case report. World J Clin Cases 2019; 7(23): 4029-4035
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4029.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4029