Copyright
©The Author(s) 2019.
World J Clin Cases. Dec 6, 2019; 7(23): 3915-3933
Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.3915
Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.3915
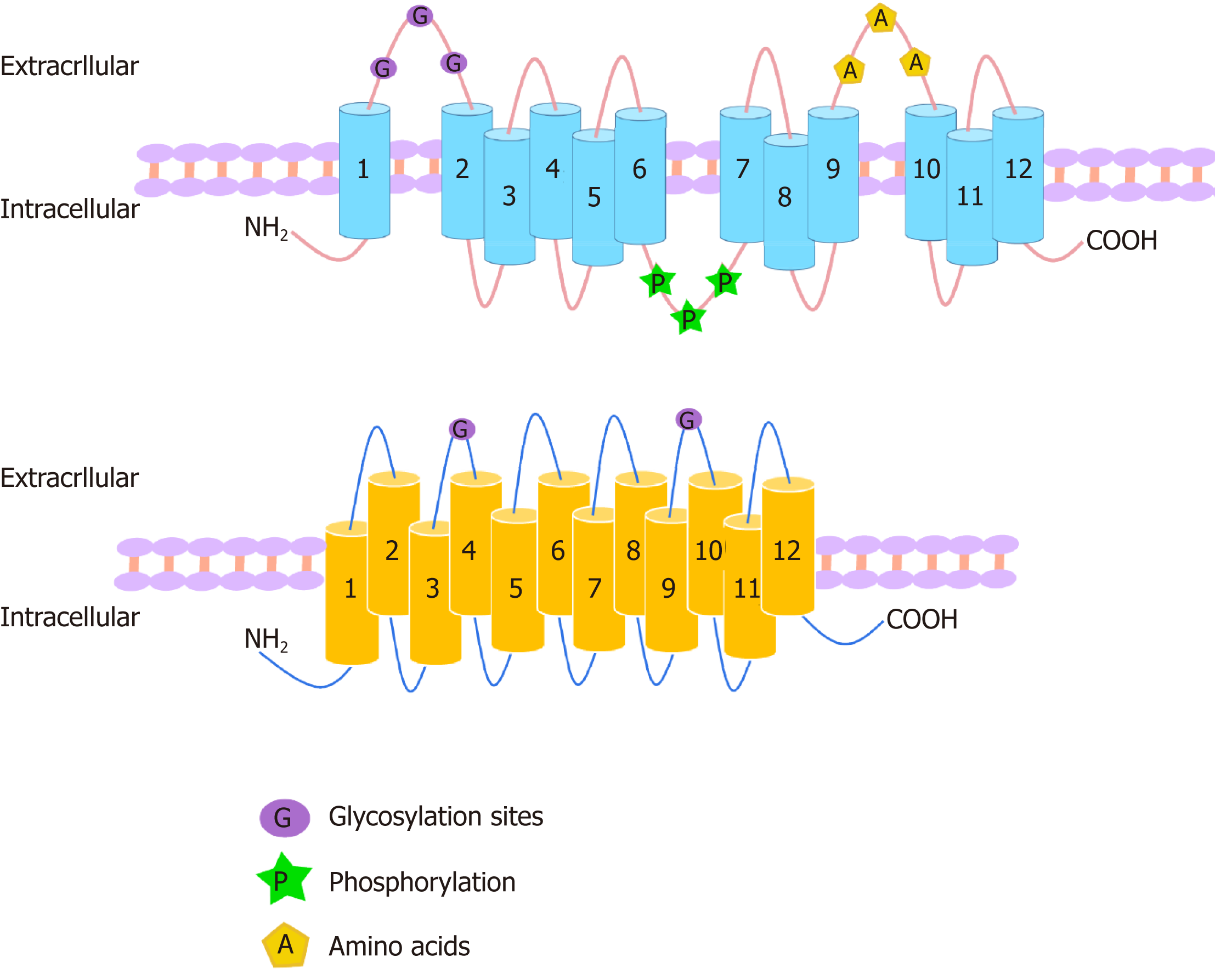
Figure 1 Structures of organic anion transporters and organic anion transporter polypeptides.
The structures of the family members of organic anion transporter (OAT) are similar. They each consist of 12 transmembrane domains (TMD) with three highly conserved regions: (1) The large extracellular loop between TMD1 and TMD2 has many glycosylation sites; and (2) the large intracellular loop between TMD6 and TMD7 has residues that are phosphorylated; and (3) TMD9 and TMD10 contain important amino acids. The C-terminus and N-terminus of OATs are located in the cytoplasm. The organic anion transporter polypeptide (OATP) family members have 12 transmembrane segments, which can form six extracellular loops and five intracellular loops. The C-terminus and N-terminus are located in the cytoplasm. One of the OATP members, OATP1B1, is glycosylated in the 2nd and 5th extracellular loops, and it is predicted that other OATPs may also be glycosylated in these two extracellular loops.
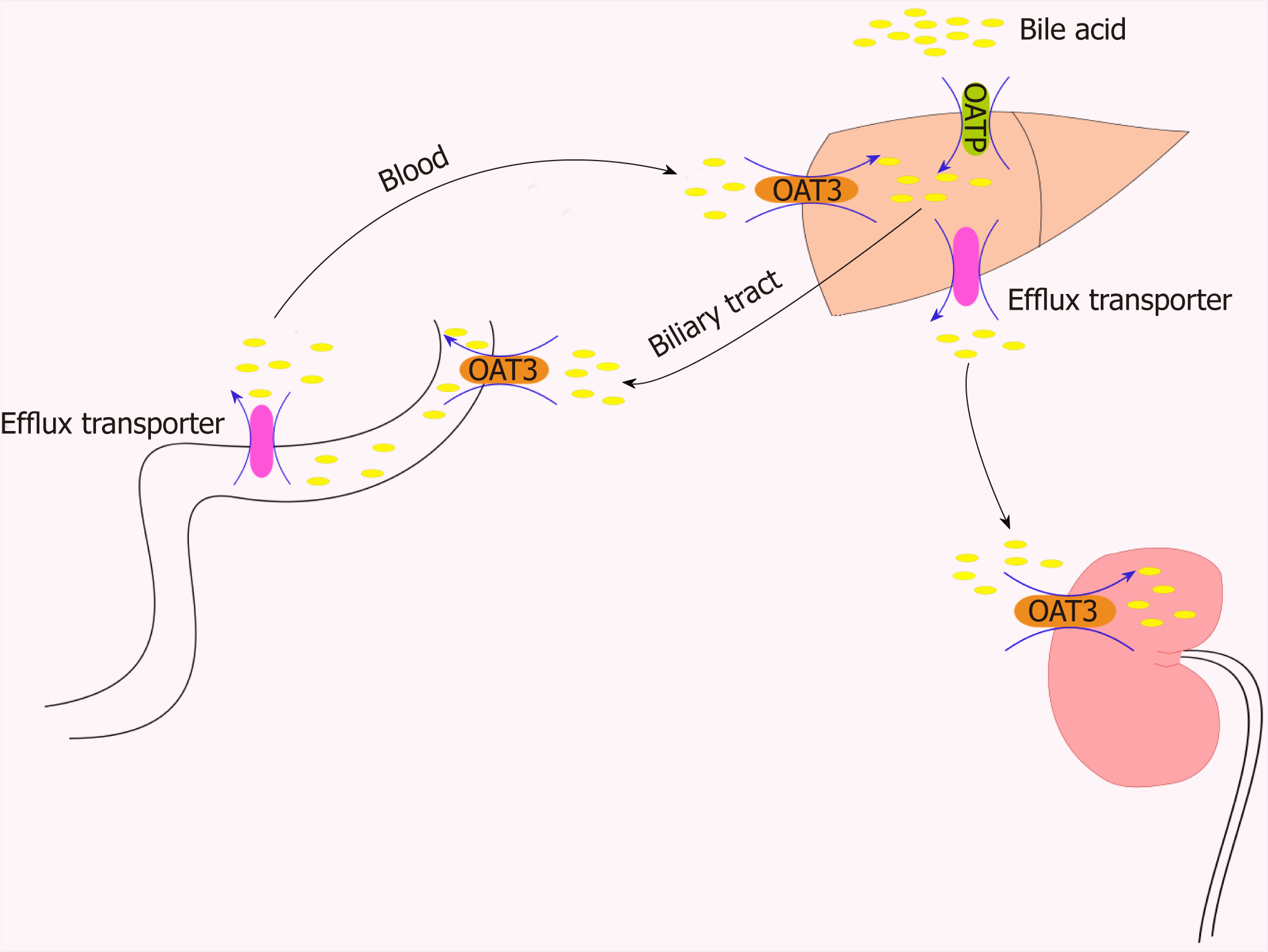
Figure 2 Role of organic anion transporters/organic anion transporter polypeptides in the transport of bile acids through the “intestinal-liver-kidney” axis.
In order to maintain the important physiological process of enterohepatic circulation of bile, hepatocytes recover bile acids from portal vein blood through certain members of the organic anion transporter polypeptide family (e.g., OATP1B1, OATP1B3, and OATP2B1). OAT3 plays a central role in the movement of bile acid through the “intestinal-liver-kidney” axis and is involved in the absorption, metabolism, and excretion of bile acids. OAT: Organic anion transporters.
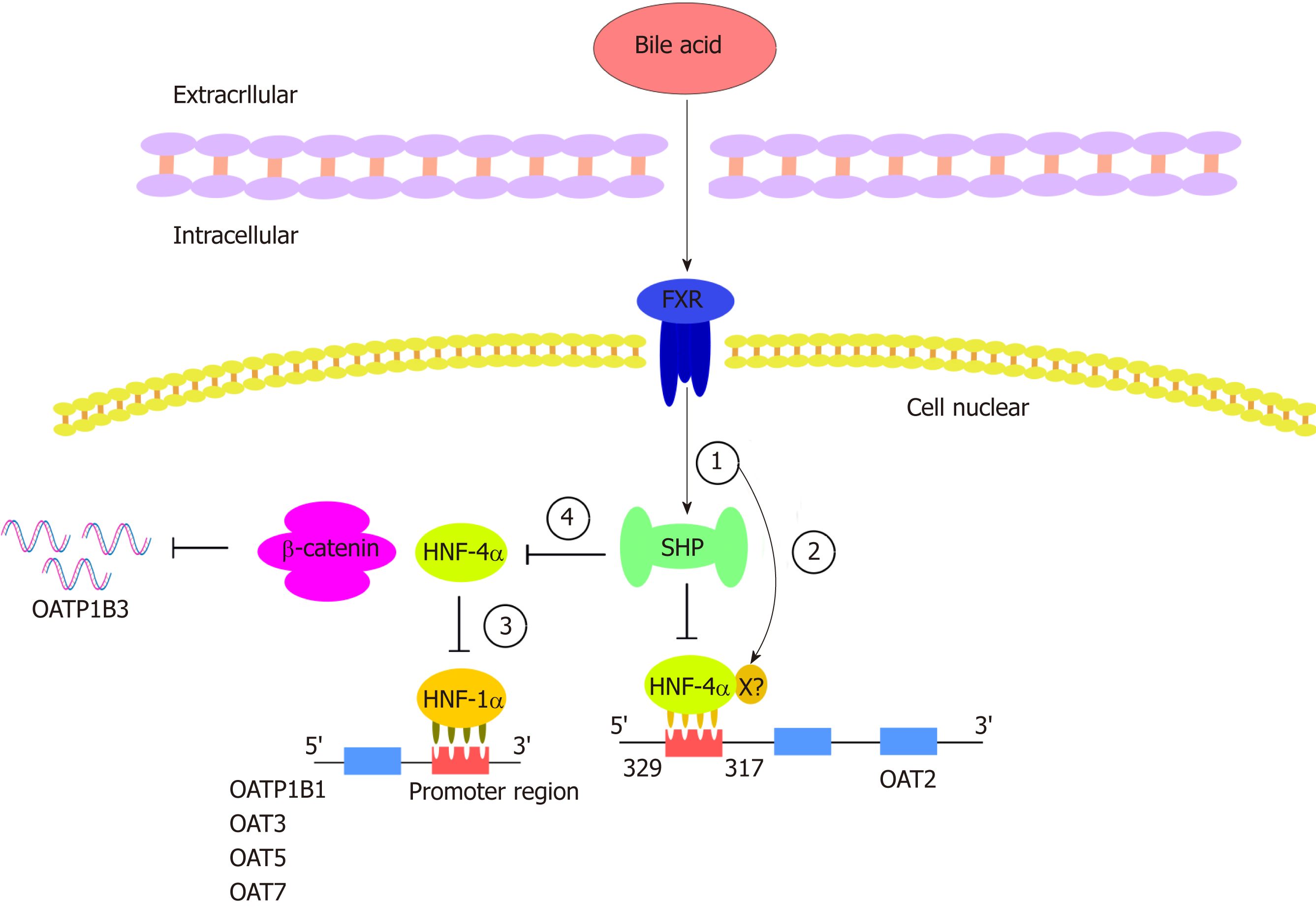
Figure 3 Hepatocyte nuclear factor regulates organic anion transporters and organic anion transporter polypeptides through a mechanism composed of a series of cascade reactions.
In this cascade, various bile acids can enhance the transactivation ability of farnesoid X receptor (FXR). (1) FXR can activate the target gene SHP. SHP negatively regulates HNF-4a, a common target of SHP; (2) In addition to its activation through the SHP-dependent mechanism, FXR may directly bind to negative reaction elements (X) located in the promoter of HNF-4a. The binding of HNF-4a and OAT2 is inhibited by the processes of 1 and 2 (the common binding sites of HNF-4a were identified between 329 and 317 upstream of the transcriptional initiation site of OAT2); (3) HNF-1a is regulated by HNF-4a such that the downregulation of HNF-4a can downregulate OATP1B1, OAT3, OAT5, and OAT7; and (4) the decreased activity of HNF4-alpha inhibits the interaction between HNF4α and β-catenin, thus downregulating the expression of OATP1B3. HNF: Hepatocyte nuclear factor; FXR: Farnesoid X receptor; SHP: A transcriptional repressor belonging to the nuclear receptor family; X: Unknown negative reaction elements in the HNF-4a promoter.
- Citation: Li TT, An JX, Xu JY, Tuo BG. Overview of organic anion transporters and organic anion transporter polypeptides and their roles in the liver. World J Clin Cases 2019; 7(23): 3915-3933
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/3915.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.3915