Copyright
©The Author(s) 2024.
World J Clin Cases. Aug 16, 2024; 12(23): 5410-5415
Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5410
Published online Aug 16, 2024. doi: 10.12998/wjcc.v12.i23.5410
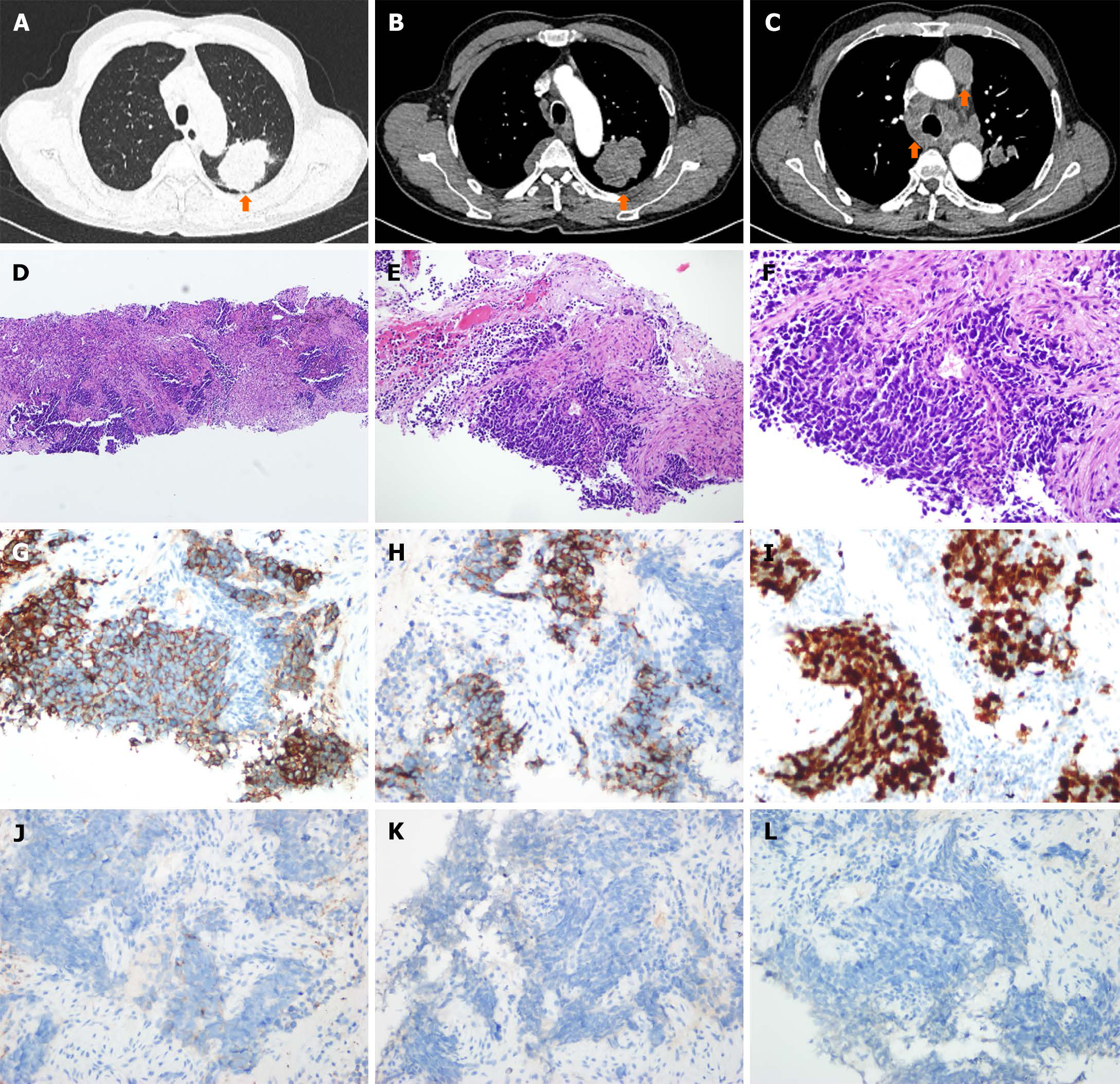
Figure 1 Chest computed tomography and histological evaluation.
A-C: The baseline chest computed tomography assessment in May 2021 included evaluation of lung lesions, lymph node lesions, and pleural lesions; D-F: Hematoxylin and eosin staining with magnifications of 100 x, 200 x, and 400 x revealed extensive-stage disease small cell lung cancer (SCLC) classification; G-L: Immunohistochemical staining displayed cytokeratin CAM5.2, CD56, Ki67, synaptophysin, chromogranin A, and thyroid transcription factor-1 in the SCLC tumor biopsy.
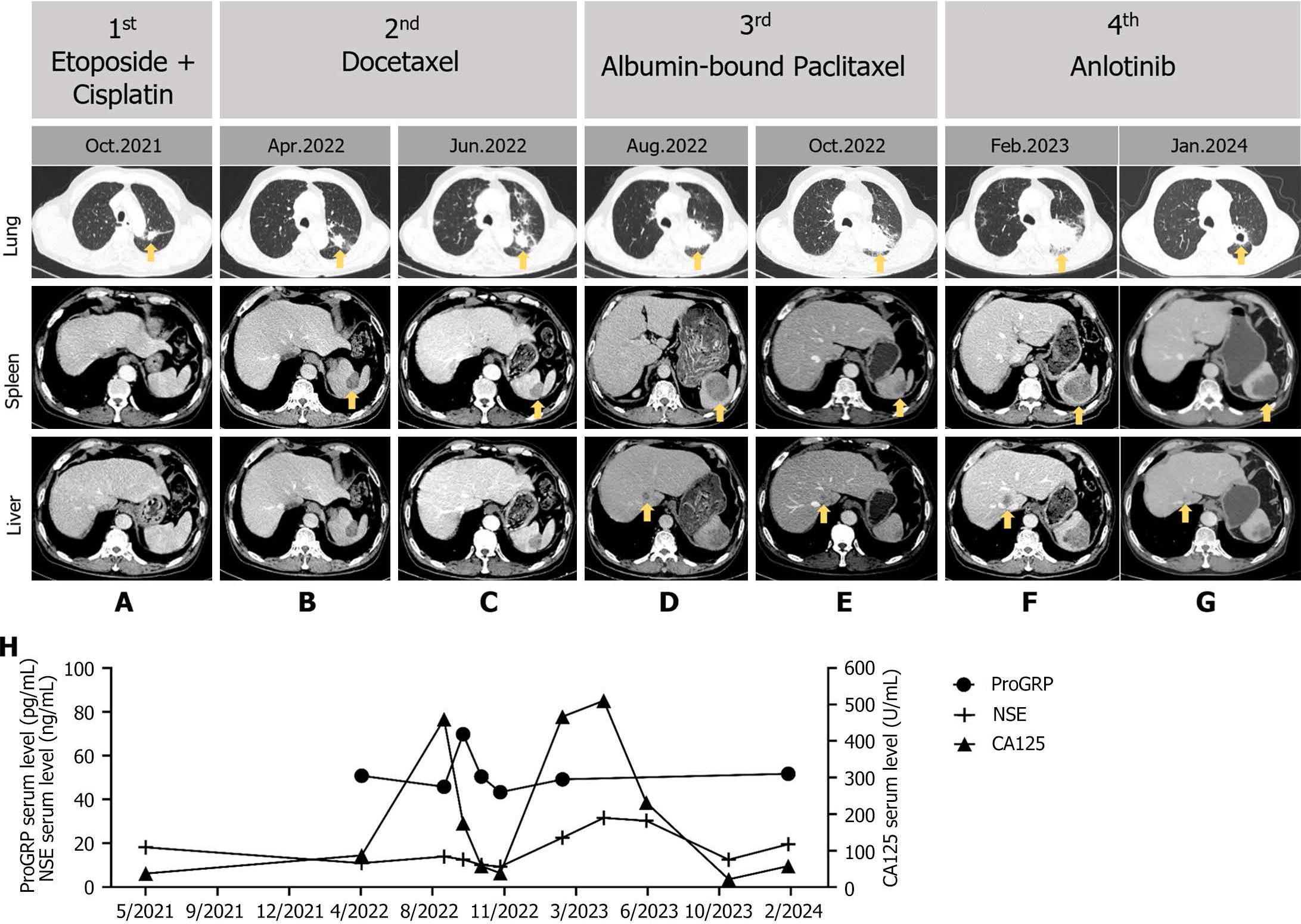
Figure 2 Imaging evaluations and serum tumor biomarker testing during treatment.
A-G: Imaging assessments of the tumor were conducted during the 1st to 4th line chemotherapy, yellow arrows were used to indicate primary tumors and metastatic lesions; H: Serum levels of progastrin-releasing peptide, neuron-specific enolase, and carbohydrate antigen 125 were measured to monitor clinical responses. NSE: Neuron-specific enolase; CA125: Carbohydrate antigen 125; ProGRP: Progastrin-releasing peptide.
- Citation: Zhang R, He YT, Liu YS, Li H, Zhao F. Small cell lung carcinoma with KIF5B-RET fusion partially responded to the 4th-line therapy with anlotinib: A case report. World J Clin Cases 2024; 12(23): 5410-5415
- URL: https://www.wjgnet.com/2307-8960/full/v12/i23/5410.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i23.5410