Copyright
©The Author(s) 2023.
World J Clin Cases. Aug 6, 2023; 11(22): 5322-5328
Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5322
Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5322
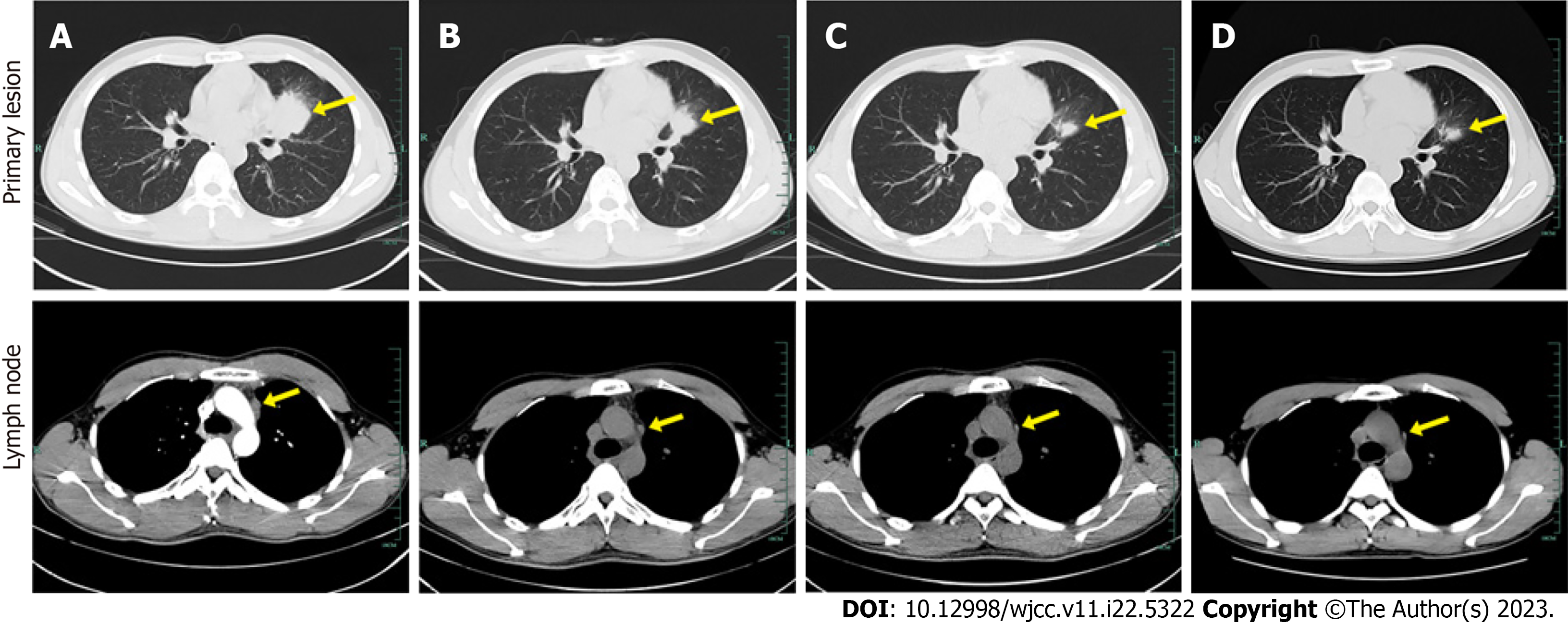
Figure 1 Computed tomography images of lungs in different treatment stages.
A: Baseline computed tomography scan performed in August 2019 shows primary lesion in the upper lobe of left lung near the hilum with mediastinal lymph node metastases; B: 10 wk after initiation of alectinib treatment (November 2019); C: Sustained response to disease is observed in June 2020; D: 14 mo after initiation of alectinib treatment (November 2020). The tumor lesions are indicated by yellow arrow.
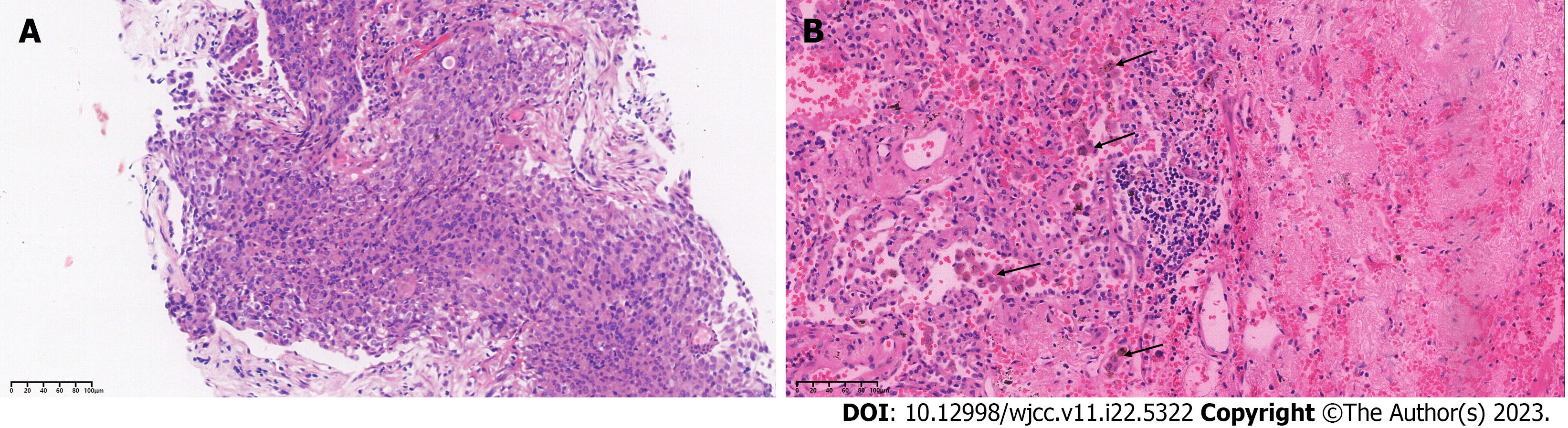
Figure 2 hematoxylin-eosin stained sections of tumor tissue at baseline and 14 mo later.
A: Hematoxylin-eosin staining (200×) results of lung puncture biopsy confirming lung adenocarcinoma; B: HE staining (200×) results showing interstitial vascular and fibrous tissue hyperplasia, eosinophil infiltration, and chronic inflammation of the lung tissue. The arrows point to activated eosinophils.
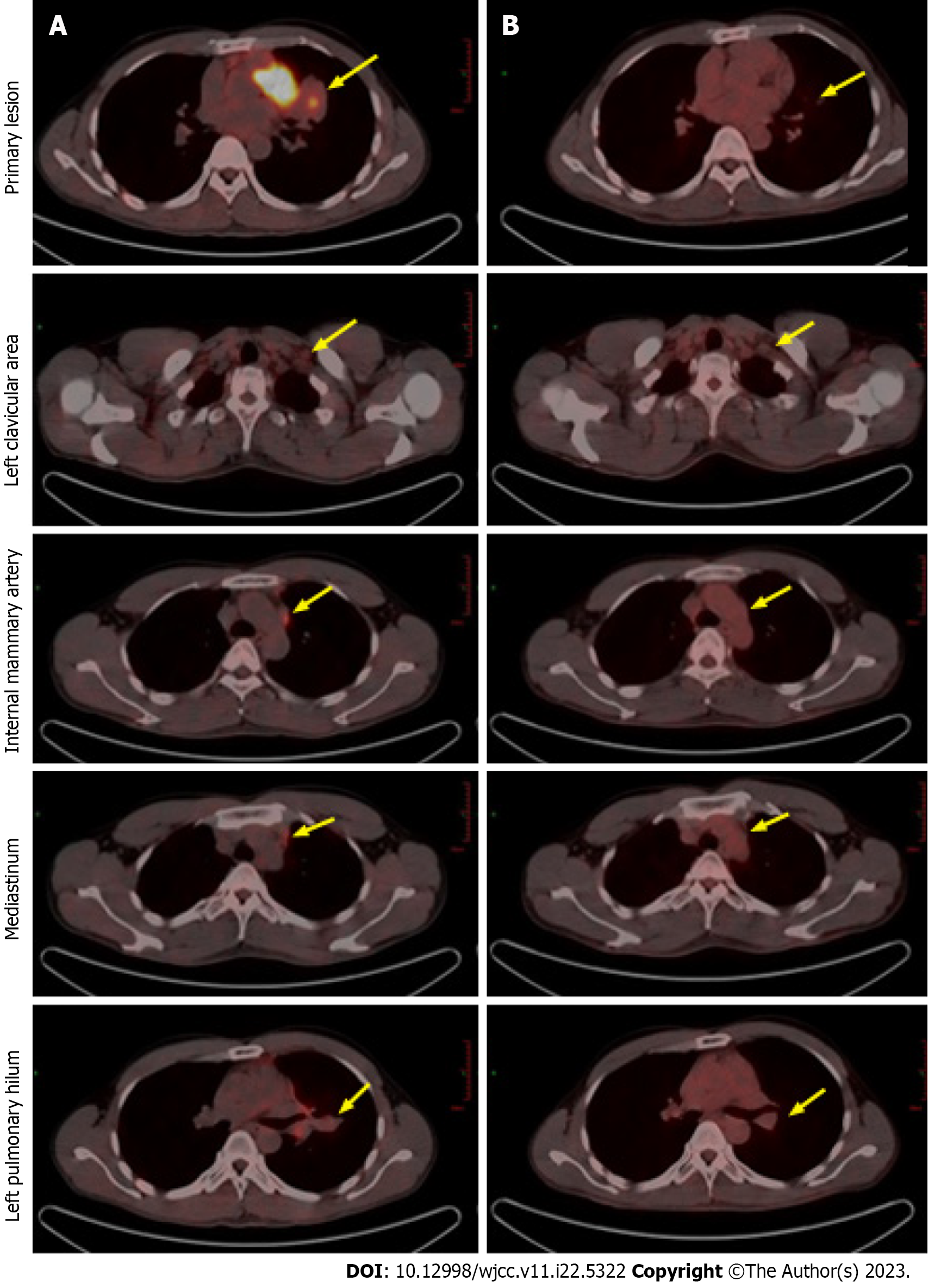
Figure 3 Positron emission tomography computed tomography images after alectinib treatment demonstrating partial remission.
A: Compared to baseline; B: The primary lesion has shrunk and the enlarged lymph nodes have almost disappeared after 14 mo of treatment. Positron emission tomography computed tomography, positron emission tomography-computed tomography.
- Citation: Wang LM, Zhao P, Sun XQ, Yan F, Guo Q. Pathological complete response to neoadjuvant alectinib in unresectable anaplastic lymphoma kinase positive non-small cell lung cancer: A case report. World J Clin Cases 2023; 11(22): 5322-5328
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5322.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5322