Copyright
©The Author(s) 2023.
World J Clin Cases. May 26, 2023; 11(15): 3542-3551
Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3542
Published online May 26, 2023. doi: 10.12998/wjcc.v11.i15.3542
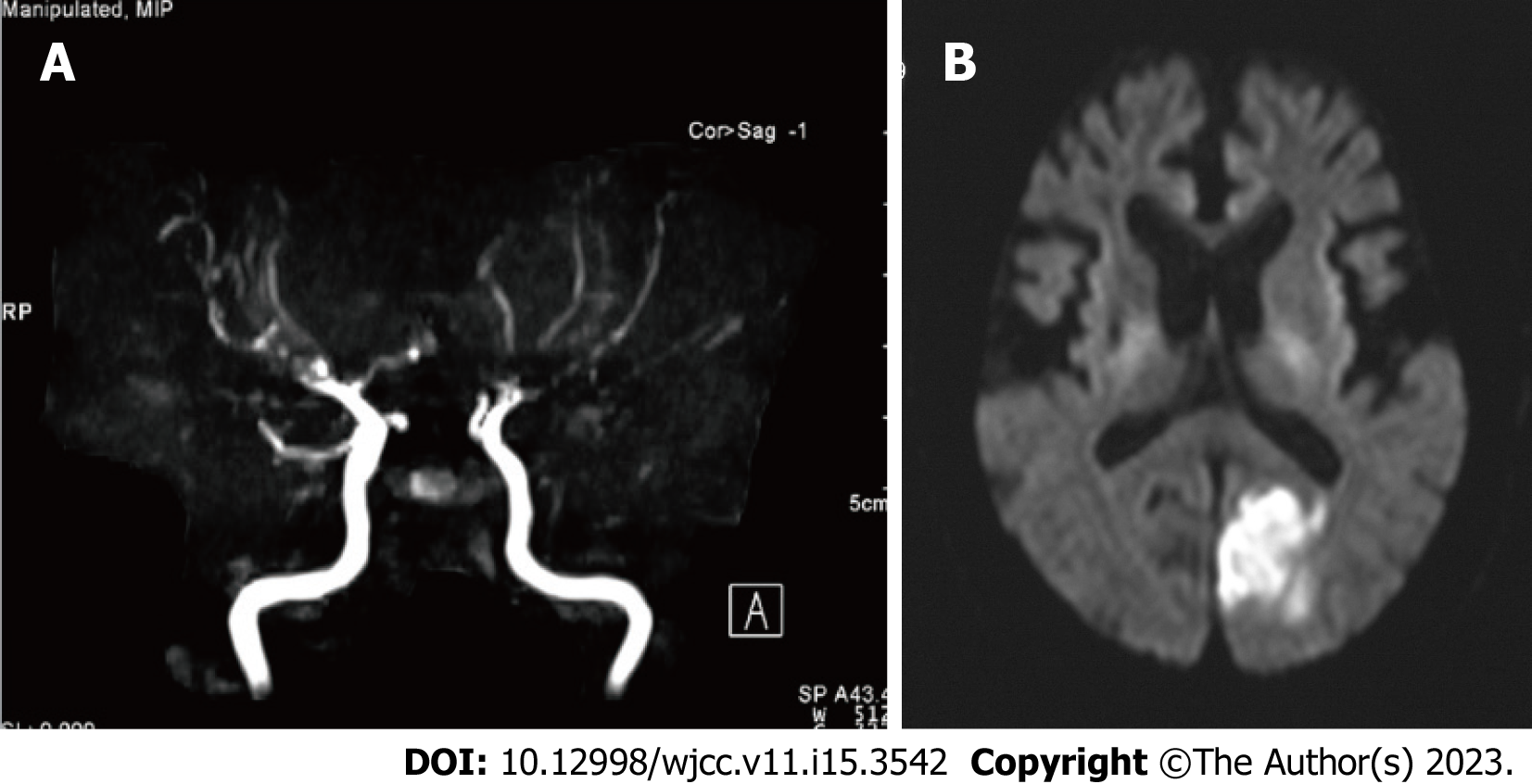
Figure 1 Magnetic resonance angiography and diffusion-weighted magnetic resonance imaging findings of Case 1.
A: Magnetic resonance angiography indicated the main branches of the internal carotid artery, including the middle cerebral artery, anterior cerebral artery, and vertebral artery, were tapered or disrupted; B: Diffusion-weighted magnetic resonance imaging indicates left occipital cerebral infarction.
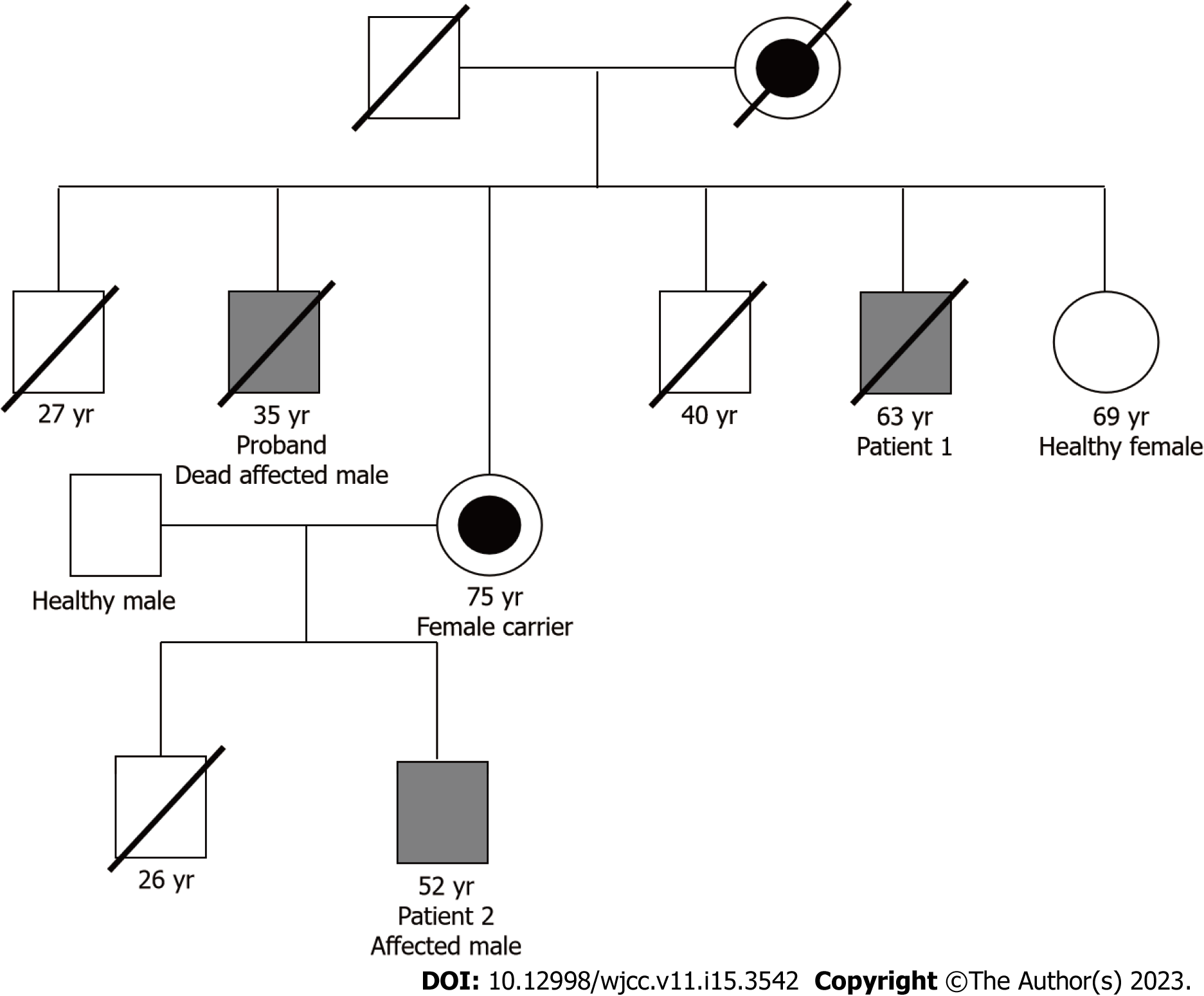
Figure 2 Family tree of both patients.
The corresponding number indicates either the current age or the age at which a patient died. This is a part of the family tree, and the entire family tree has been previously published[9]. The mother of patient 2 was not revealed to be a carrier of Fabry disease until two years after the diagnosis of patient 2.
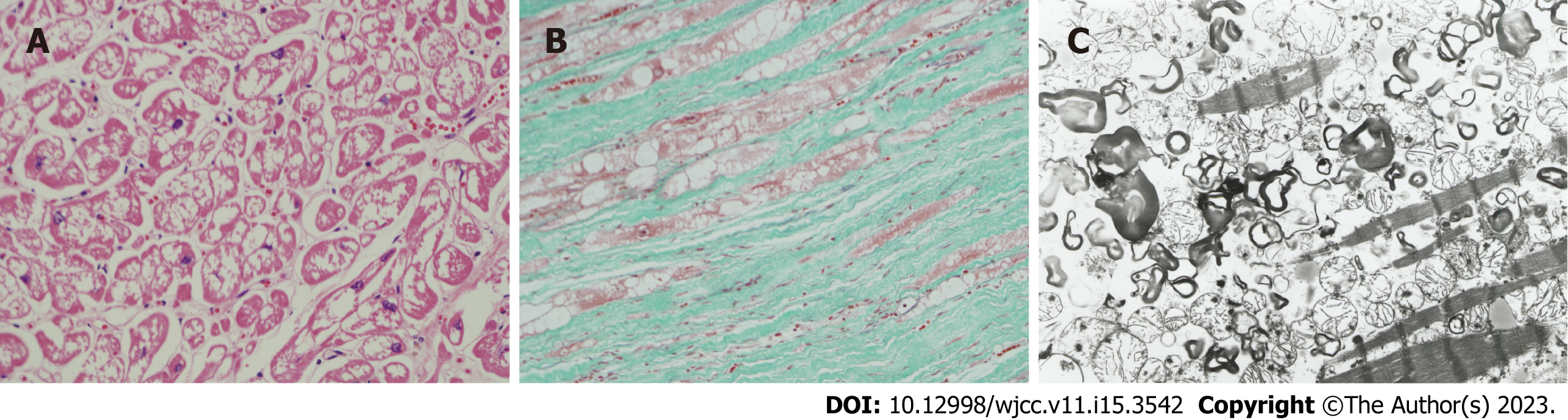
Figure 3 Microscopic examination (A and B) and electron microscopic examination (C) of cardiac muscles on autopsy.
A: Histological findings of the autopsy. Hematoxylin-eosin staining (× 100). Marked vacuolation of the cardiac muscles in the left ventricle wall of the heart. This autopsy finding has been previously published[10]; B: Histological findings in autopsy. Elastica-Masson staining (× 100). Cardiac muscles vacuolation and marked interstitial fibrosis in the left ventricular wall of the heart. This autopsy finding of this case has previously been published[10]; C: Electron microscopic findings in autopsy. Many lamellar structures in the cardiac muscles of the left ventricular wall of the heart (× 6000) observed. This autopsy finding has been previously published[10]. Annotation: These pictures were kindly provided by co-author O. Suzuki, who is the first author of reference[10]. These pictures were not published in their study[10], but were taken for the current study.
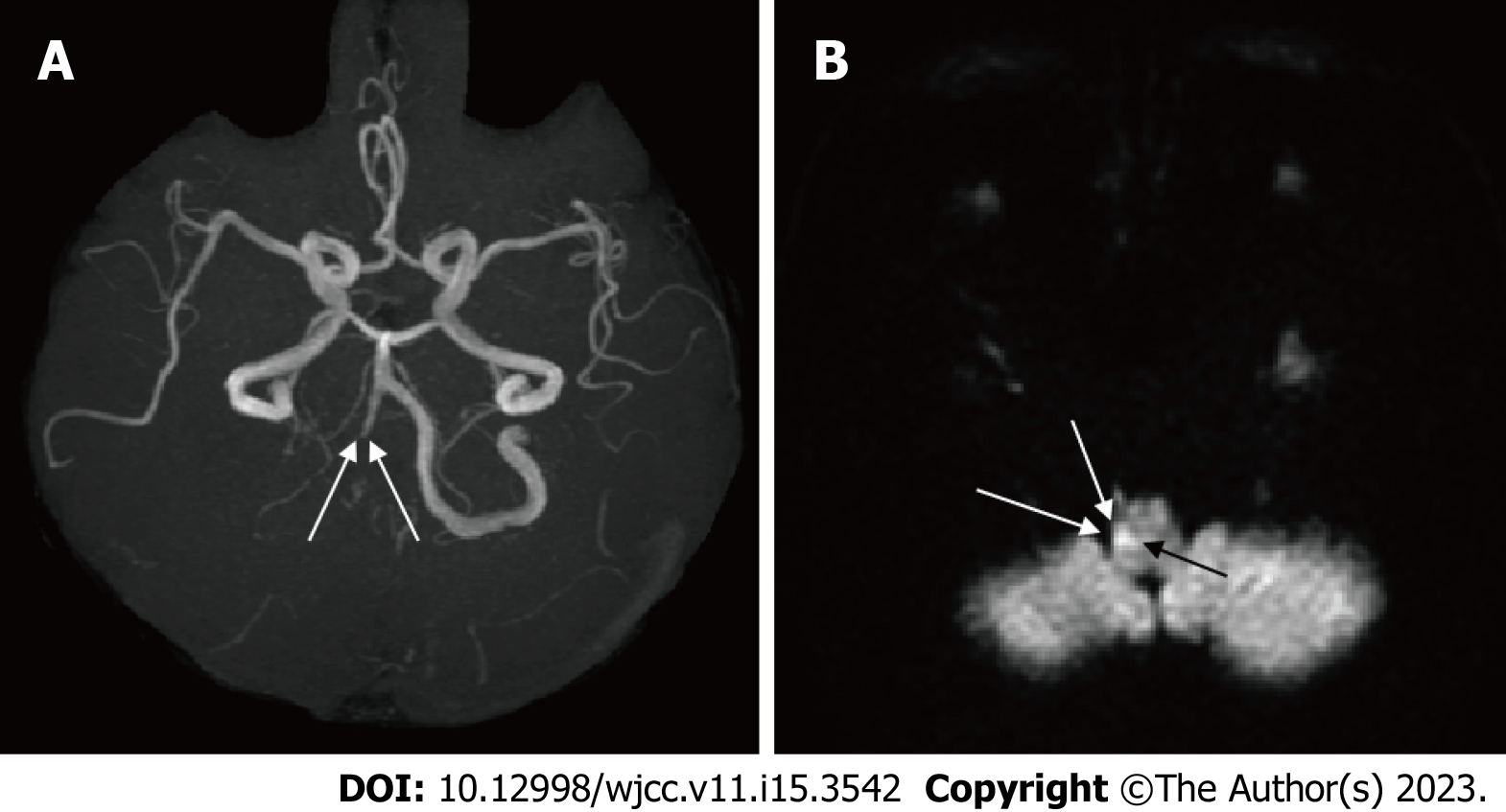
Figure 4 Findings of magnetic resonance angiography (A) and diffusion-weighted magnetic resonance imaging (B) in Case 2.
A: The right vertebral artery is tapered and obstructed (arrow); B: A high-intensity signal is observed in the right medulla oblongata (arrow).
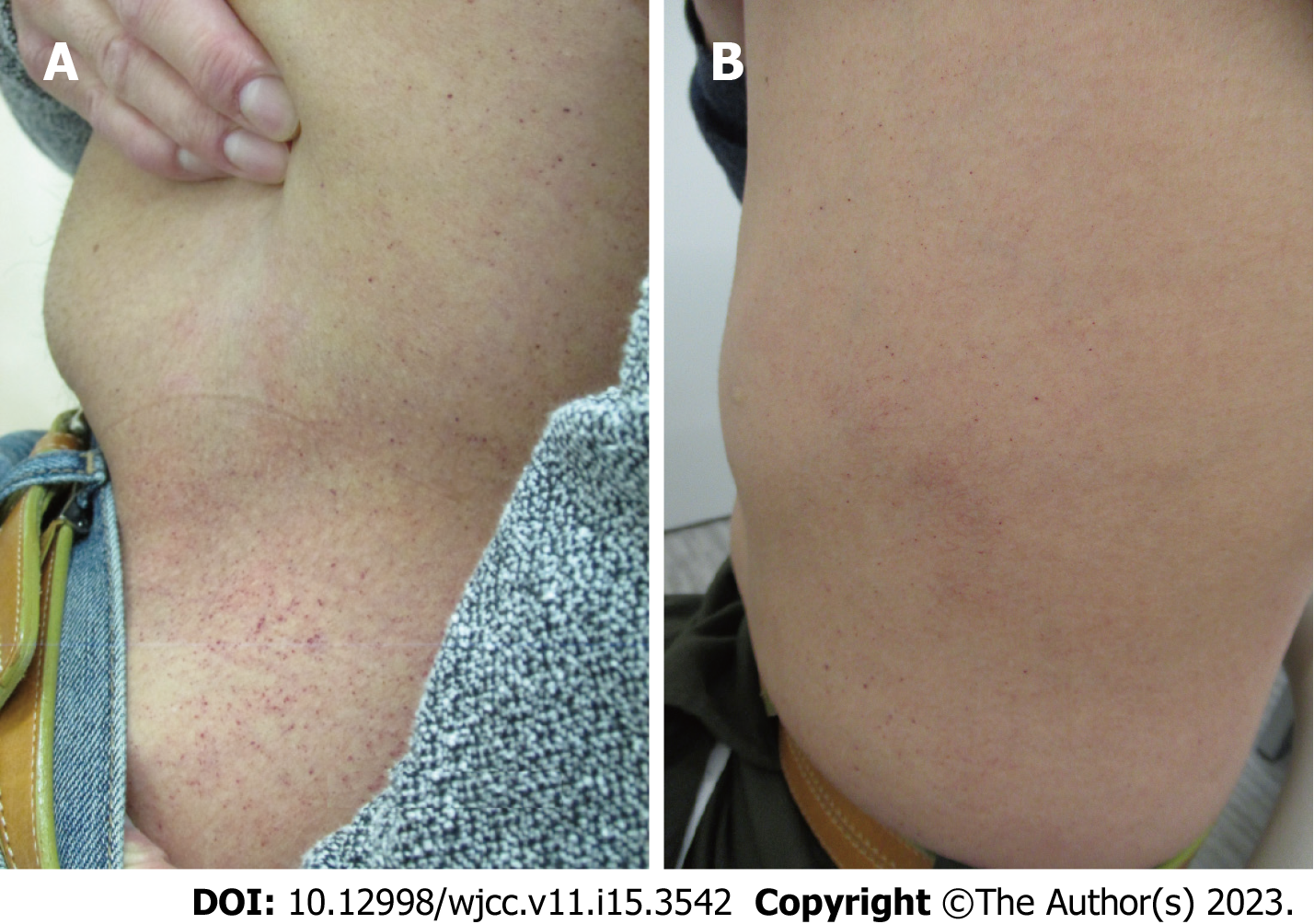
Figure 5 Abdominal angiokeratoma (A) before enzyme replacement therapy and (B) after 16 years of enzyme replacement therapy.
A and B: The angiokeratoma observed 16 years following therapy was less visible in (B) than that in (A).
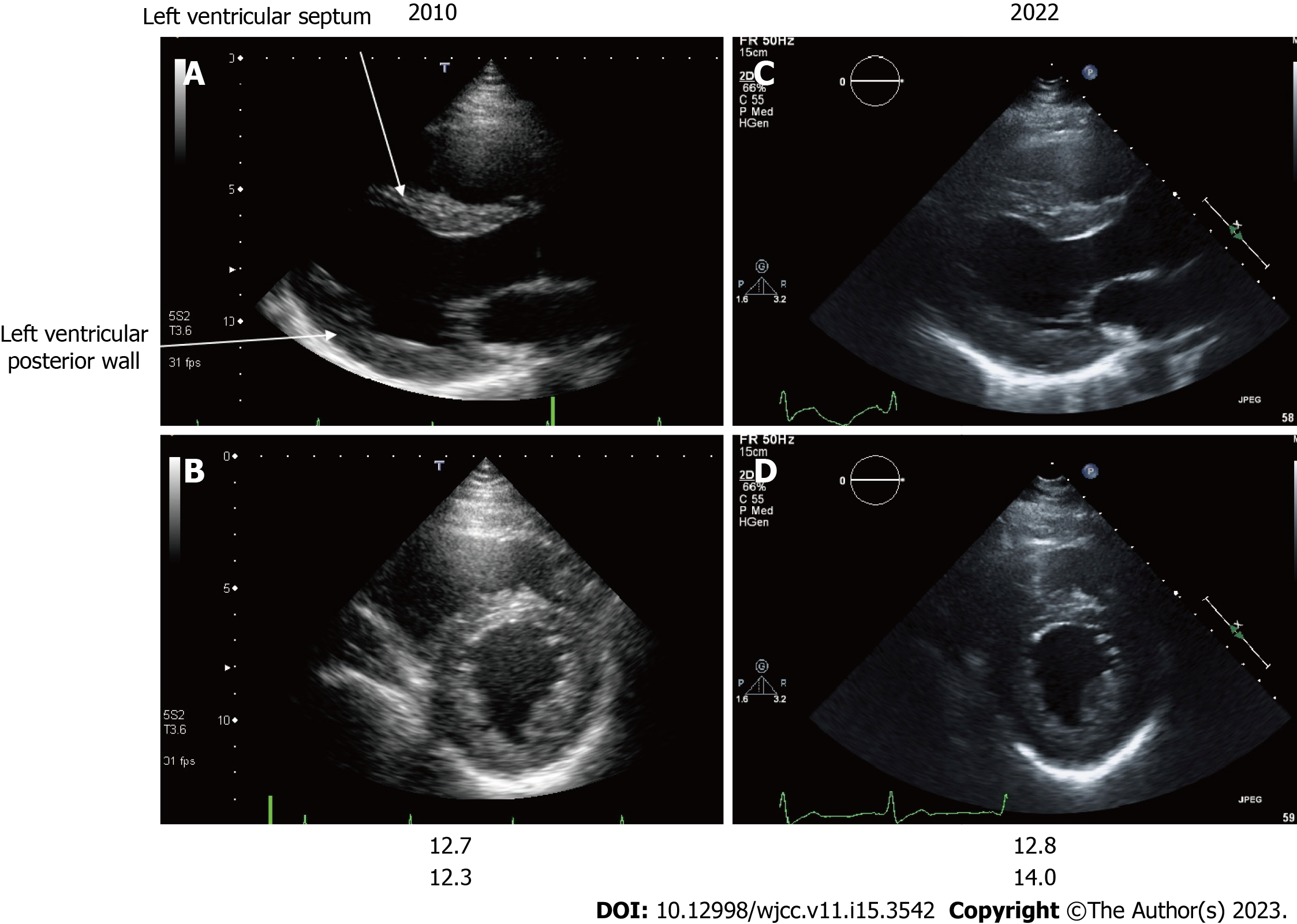
Figure 6 Echocardiography findings of Case 2.
A and C: Long-axis image; B and D: Short-axis image; A and B: Images of patient 2 at 39 years of age; C and D Images of patient 2 at 52 years of age. The echocardiographic findings of the left ventricular septum and left ventricular posterior wall thickness of patient 2 are shown at 39 (A and B) and 52 (C and D) years of age. A change suggestive of asymmetric ventricular septal hypertrophy was observed at 39 years of age. There was a minor progression in ventricular hypertrophy in the left ventricular septal thickness and left ventricular posterior wall thickness 13 years following enzyme replacement therapy initiation.
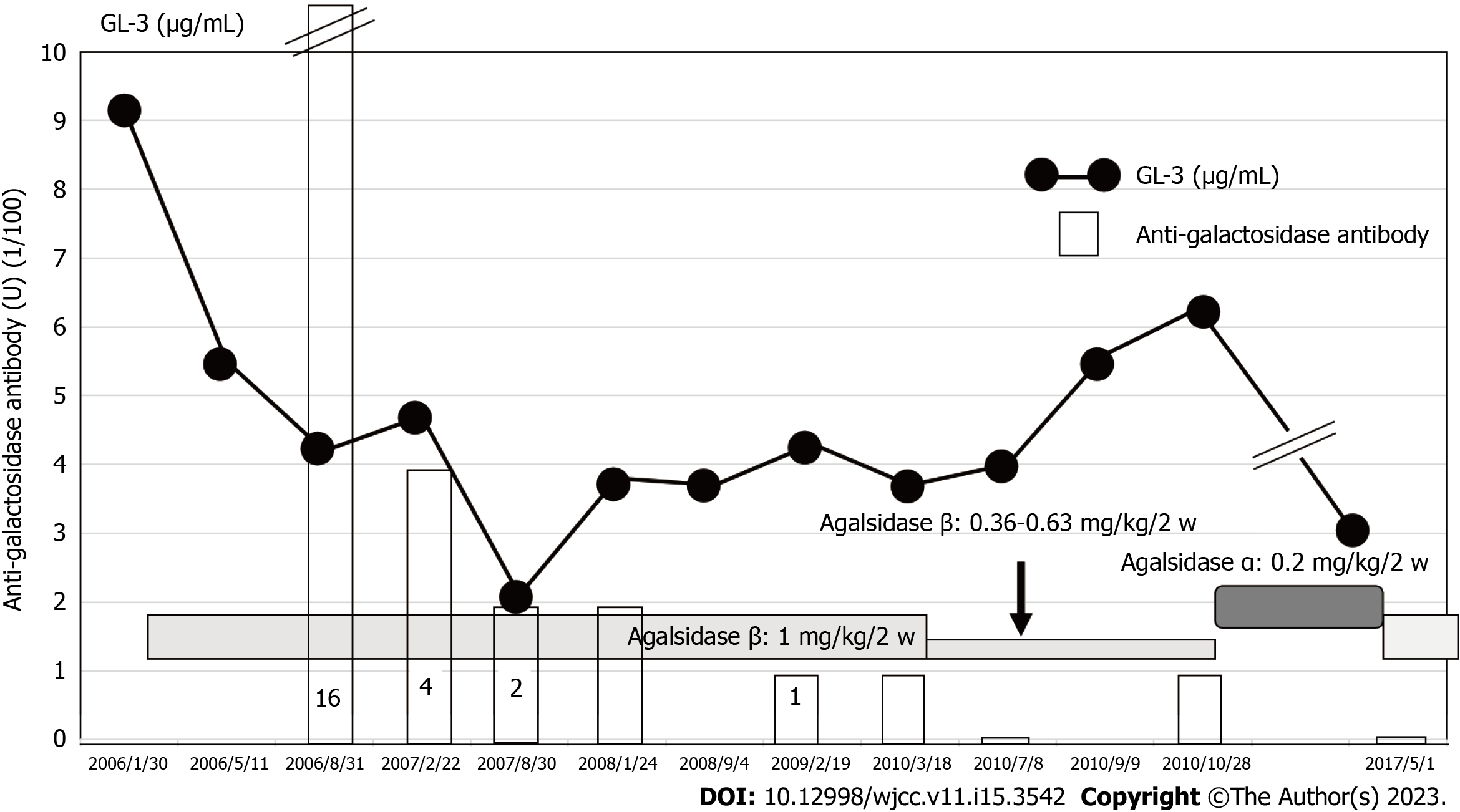
Figure 7 Effects of agalsidase β administration on globotriaosylceramide and anti-galactosidase antibody levels in Case 2.
Globotriaosylceramide (GL-3) was sufficiently reduced when 1 mg/kg of agalsidase β was administered; GL-3 increased as agalsidase β dose decreased. α-galactosidase at a dose of 0.2 mg/kg, initiated from 2010 and replaced by agalsidase β in 2017, suppressed GL-3 level within normal limits. GL-3: Globotriaosylceramide.
- Citation: Harigane Y, Morimoto I, Suzuki O, Temmoku J, Sakamoto T, Nakamura K, Machii K, Miyata M. Enzyme replacement therapy in two patients with classic Fabry disease from the same family tree: Two case reports. World J Clin Cases 2023; 11(15): 3542-3551
- URL: https://www.wjgnet.com/2307-8960/full/v11/i15/3542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i15.3542