Copyright
©The Author(s) 2022.
World J Clin Cases. Mar 26, 2022; 10(9): 2773-2782
Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2773
Published online Mar 26, 2022. doi: 10.12998/wjcc.v10.i9.2773
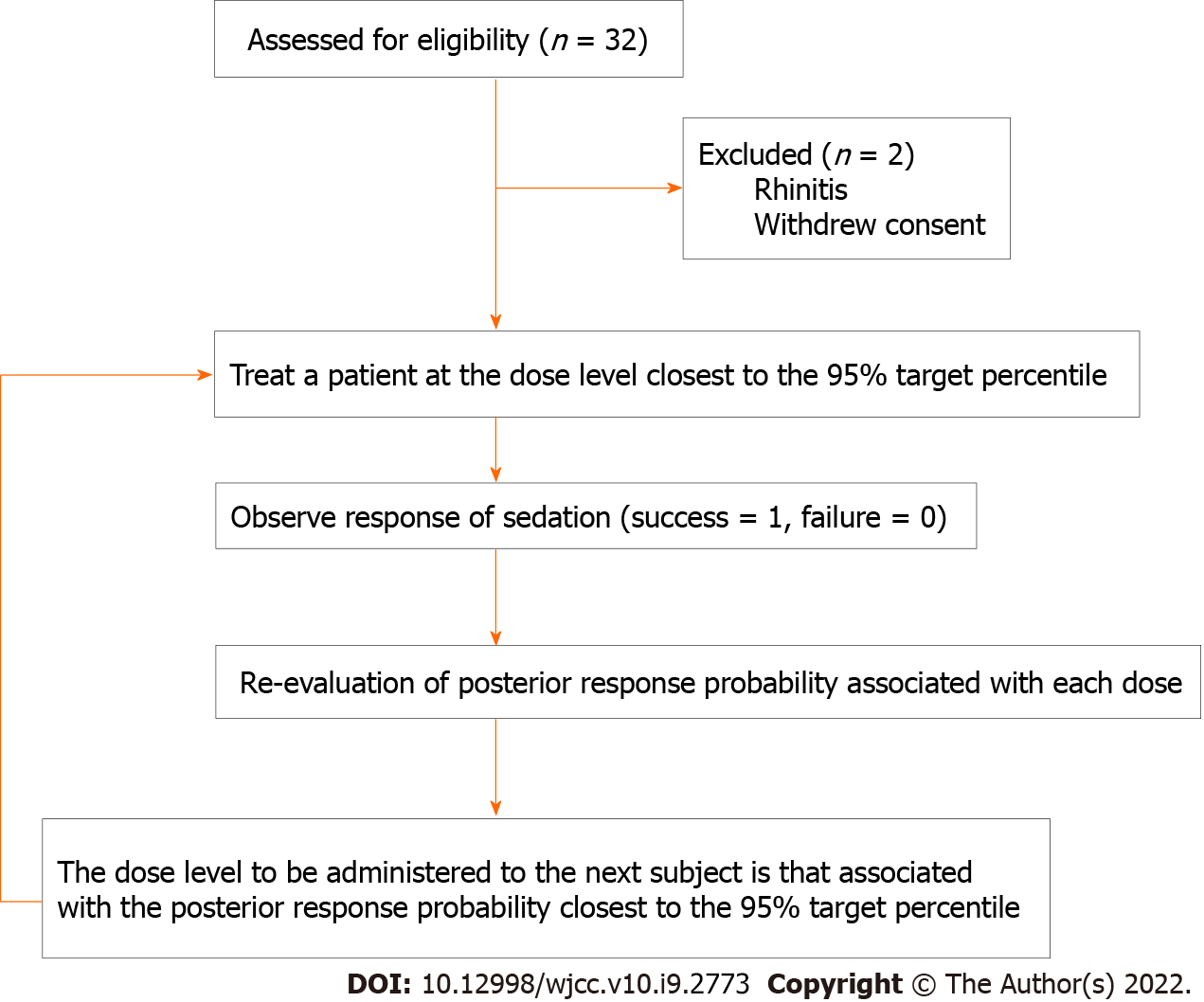
Figure 1 Schematic representation of patients’ flow and the continual reassessment method applied in this applied in this clinical trial.
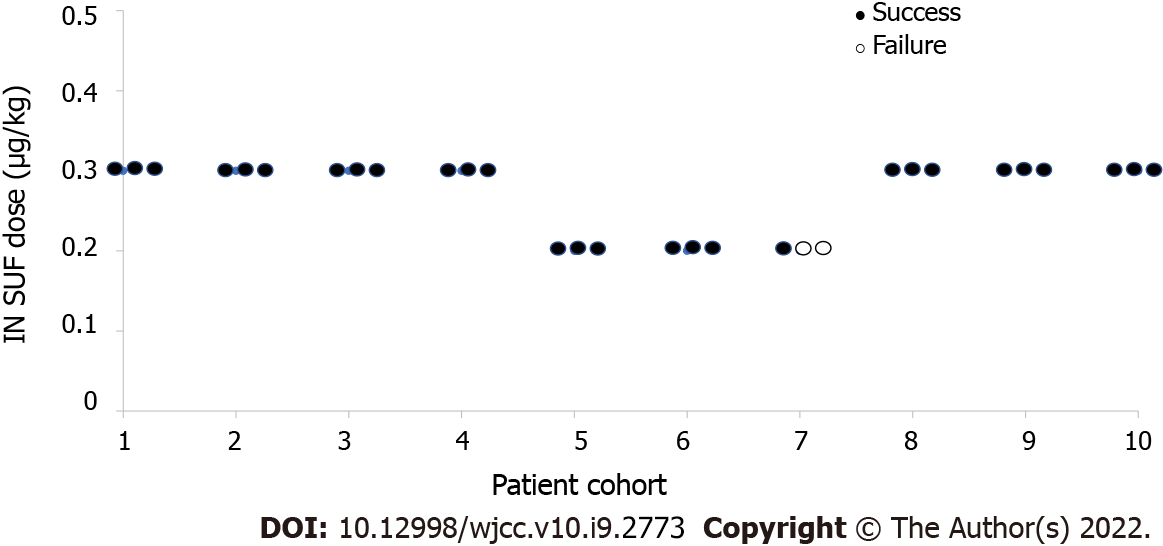
Figure 2 Dose-response of the series of successful and unsuccessful moderate sedation.
In total, 28 out of 30 sedations were successful in ensuring MOAA/S ≤ 3. ● Indicates success. ○ Indicates failure. IN SUF: Intranasal sufentanil.
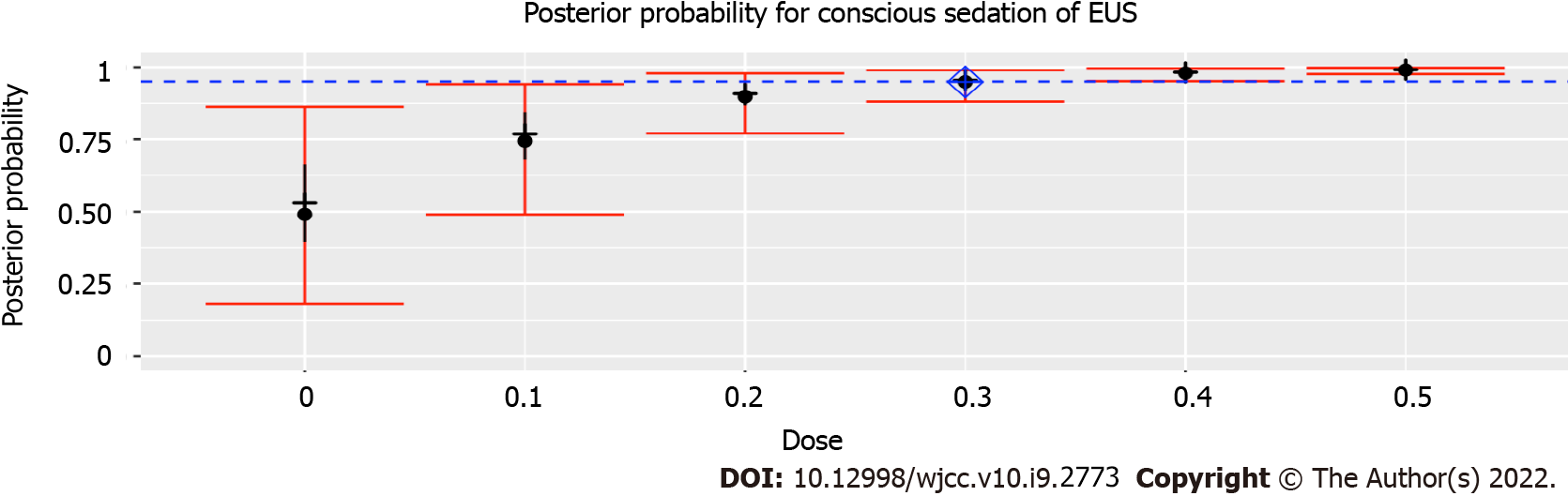
Figure 3 Posterior probability for moderate sedation during endoscopic ultrasonography.
After 30 subjects been performed, the dose closest to ED95 was 0.3 μg/kg, with an estimated success probability of 94.9% (95% credibility interval: 88.1%-98.9%). Posterior probability quantiles: 2.5, 25, 50, 75, and 97.5%. Diamond shows estimated ED95. Of note, because of the high success rate encountered with the 0.3 μg/kg dose, the CRM never recommended higher or lower doses. Hence, the 0, 0.1, 0.4 and 0.5 μg/kg dose were never tested, and the posterior probability estimated for these doses are therefore based on the prior probabilities and an extrapolation of the results from the doses using the dose-response model. EUS: Endoscopic ultrasonography.
- Citation: Zou Y, Li N, Shao LJZ, Liu FK, Xue FS, Tao X. Determination of the ED95 of intranasal sufentanil combined with intranasal dexmedetomidine for moderate sedation during endoscopic ultrasonography. World J Clin Cases 2022; 10(9): 2773-2782
- URL: https://www.wjgnet.com/2307-8960/full/v10/i9/2773.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i9.2773