Copyright
©The Author(s) 2022.
World J Clin Cases. Oct 6, 2022; 10(28): 9970-9984
Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.9970
Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.9970
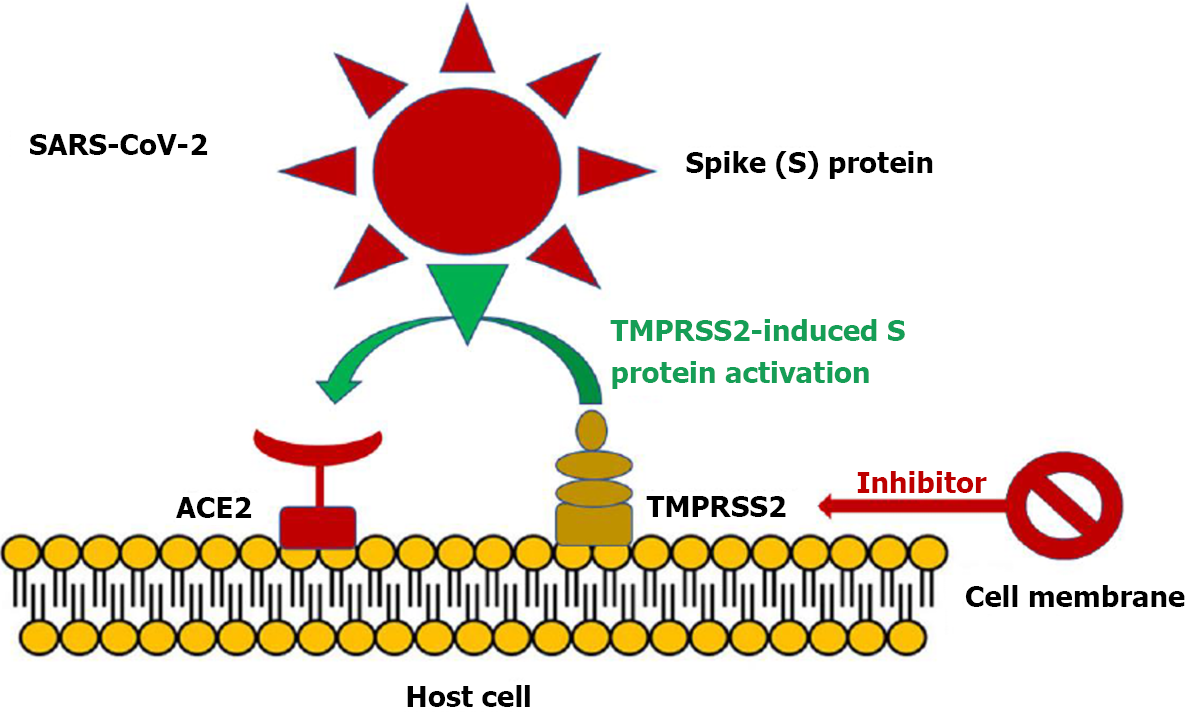
Figure 1 Severe acute respiratory syndrome coronavirus 2 entry into angiotensin converting enzyme 2 is facilitated by transmembrane protease serine 2 induced activation of the S protein via its serine protease activity.
Inhibition of transmembrane protease serine 2 (TMPRSS2) may furnish an alternative strategy to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by blocking viral entry into host cells. ACE2: Angiotensin converting enzyme 2. Citation: Triposkiadis F, Xanthopoulos A, Giamouzis G, Boudoulas KD, Starling RC, Skoularigis J, Boudoulas H, Iliodromitis E. ACE2, the Counter-Regulatory Renin-Angiotensin System Axis and COVID-19 Severity. J Clin Med 2021; 10: 3885. Copyright ©The Author(s) 2021. Published by MDPI.
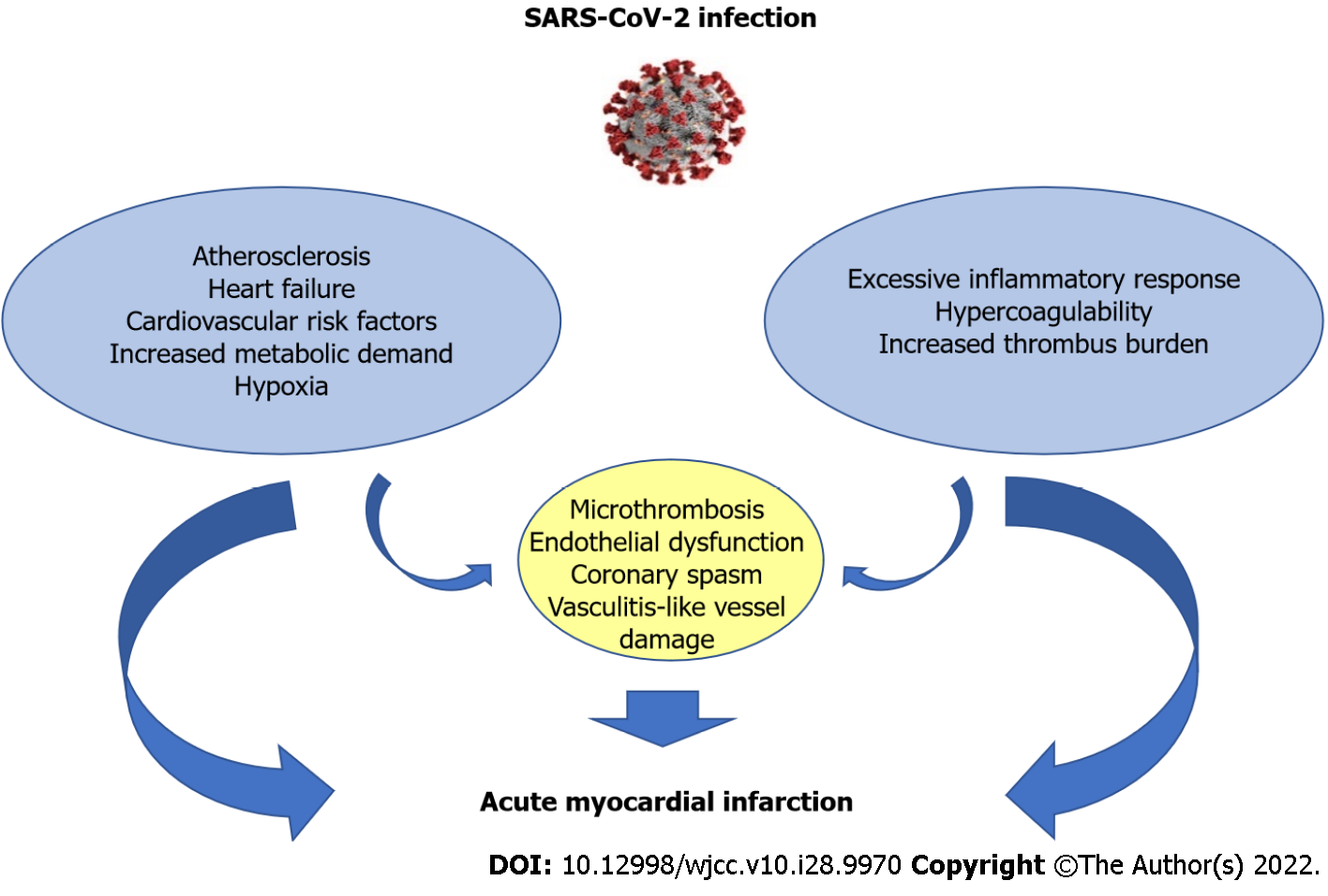
Figure 2 Mechanisms of acute myocardial injury in coronavirus disease 2019 patients.
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
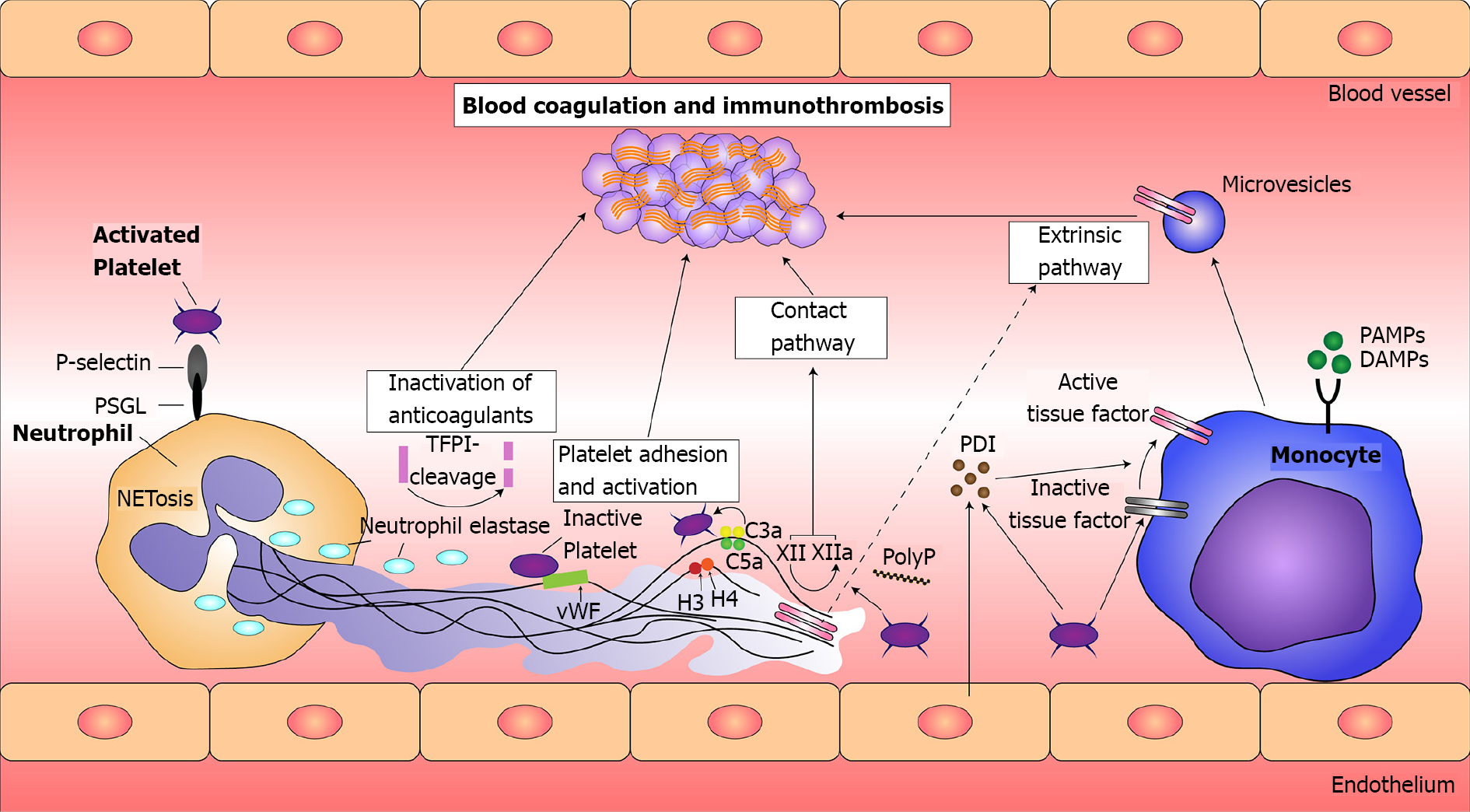
Figure 3 Principles of immunothrombosis.
Monocytes and their microvesicles release activated intravascular tissue factor (TF) in response to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), which initiates the extrinsic pathway of coagulation. Activated platelets and endothelium secrete protein disulfide isomerase (PDI), which activates microvesicle-derived TF. Interaction between P-selectin on activated platelets and P-selectin glycoprotein ligand (PSGL) on leukocytes enhance recruitment of leukocytes and increase TF production. Neutrophil extracellular traps (NETs) provide a scaffold consisting of DNA, histones, and neutrophil serine proteases, and they support immunothrombosis through several pathways. (1) NETs bind to von Willebrand factor (vWF) and support the recruitment of platelets; (2) Histones H3 and H4 on NETs can trigger the activation of platelets; (3) NETs bind to TF leading to TF-mediated thrombingeneration through the extrinsic pathway (dotted arrow); (4) Neutrophil elastase and other neutrophil serine proteases cleave and inactivate anticoagulants, including TF pathway inhibitor and thrombomodulin; and (5) NETs can directly activate factor XII (the contact pathway of coagulation) mediated by platelet-derived polyphosphates (PolyP). The complement system (specifically the activated complement components C3a and C5a) also enhances platelet activation. TFPI: Tissue factor pathway inhibitor. Citation: Jayarangaiah A, Kariyanna PT, Chen X, Jayarangaiah A, Kumar A. COVID-19-Associated Coagulopathy: An Exacerbated Immunothrombosis Response. Clin Appl Thromb Hemost 2020; 26: 1076029620943293. Copyright ©The Author(s) 2020. Published by SAGE.
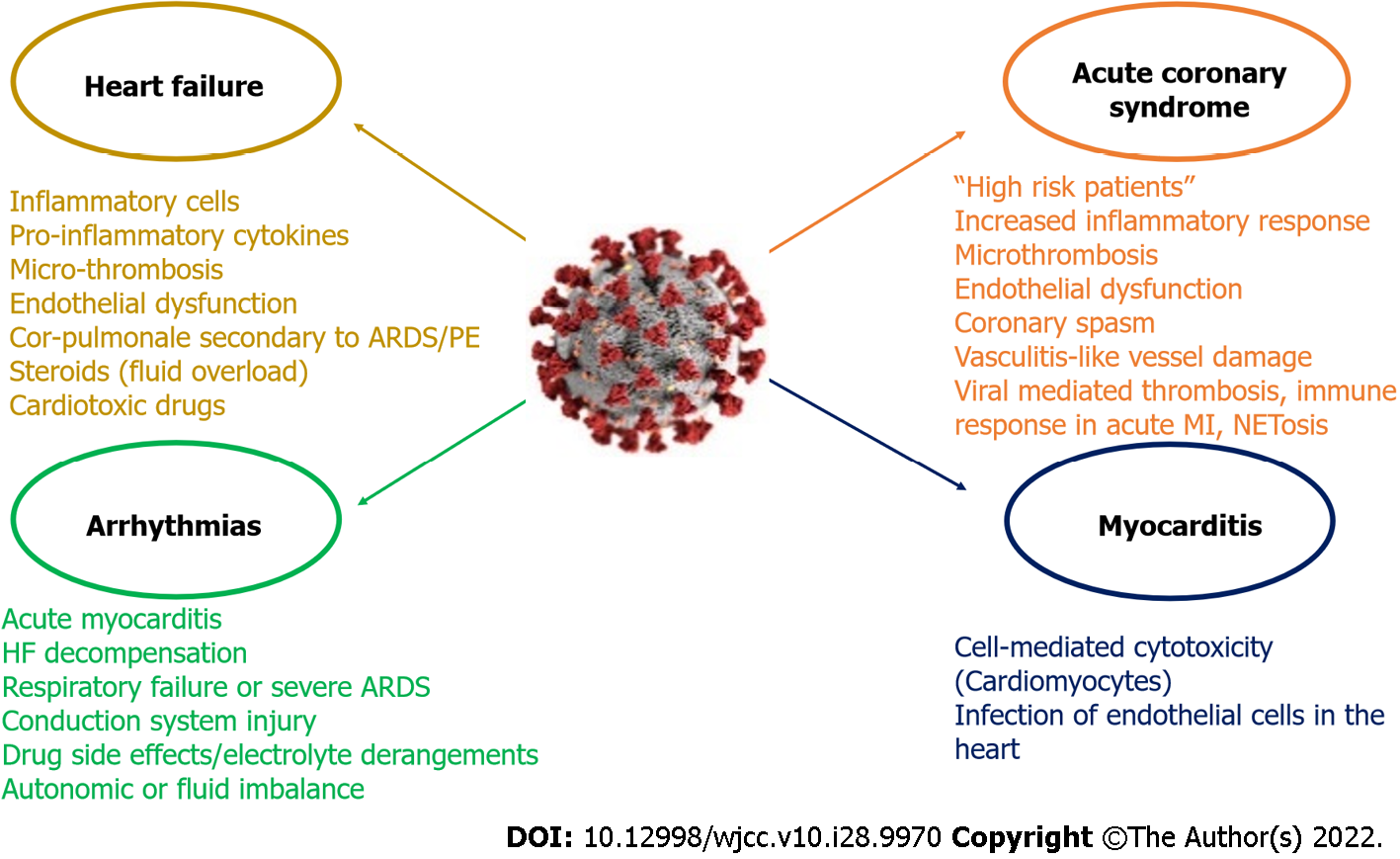
Figure 4 Coronavirus disease 2019 related cardiovascular complications and its main mechanisms.
It is proposed that two different groups of coronavirus disease 2019 (COVID-19) patients with acute coronary syndrome (ACS) exist. The first group includes “high risk” patients (i.e. pre-existing atherosclerosis, a history of heart failure (HF) or multiple cardiovascular risk factors), who are prone to develop acute myocardial injury. In the second group patients may lack previous cardiovascular history or classic risk factors for coronary disease and the excessive inflammatory response to the primary infection is the main mechanism. Nonetheless the two groups may share common pathophysiological mechanisms (microthrombosis, endothelial dysfunction, coronary spasm, vasculitis-like vessel damage). The main mechanisms associated with thrombosis and immune response presented in COVID-19 patients with ACS include viral mediated thrombosis, immune response in acute myocardial infarction (MI) and NETosis. Acute myocarditis has been reported as a possible complication in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive patients. The entrance of SARS-CoV-2 in cardiac myocyte finally leads to the production of primed CD8+ T lymphocytes, which migrate to the cardiomyocytes and may cause myocardial inflammation through cell-mediated cytotoxicity. SARS-CoV-2 can also cause myocardial damage via the infection of endothelial cells in the heart. COVID-19 patients may develop HF due to infiltration of heart by inflammatory cells, destructive action of pro-inflammatory cytokines, micro-thrombosis and new onset or aggravated endothelial dysfunction and respiratory failure. Other possible mechanisms include cor-pulmonale secondary to acute respiratory distress syndrome (ARDS) or pulmonary embolism (PE), the use of steroids which can cause fluid overload, and the administration of cardiotoxic drugs (i.e. hydroxychloroquine). SARS-CoV-2 can engender arrhythmias through direct myocardial damage causing acute myocarditis or through HF decompensation or secondary, through respiratory failure or severe ARDS. Direct viral myocardial injury to the conduction system can also predispose to arrhythmogenesis. Other possible mechanisms include drug side effects, electrolyte derangements, and autonomic or fluid imbalance. NET: Neutrophil extracellular traps.
- Citation: Xanthopoulos A, Bourazana A, Giamouzis G, Skoularigki E, Dimos A, Zagouras A, Papamichalis M, Leventis I, Magouliotis DE, Triposkiadis F, Skoularigis J. COVID-19 and the heart. World J Clin Cases 2022; 10(28): 9970-9984
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/9970.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.9970