Copyright
©The Author(s) 2022.
World J Clin Cases. Oct 6, 2022; 10(28): 10085-10096
Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10085
Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10085
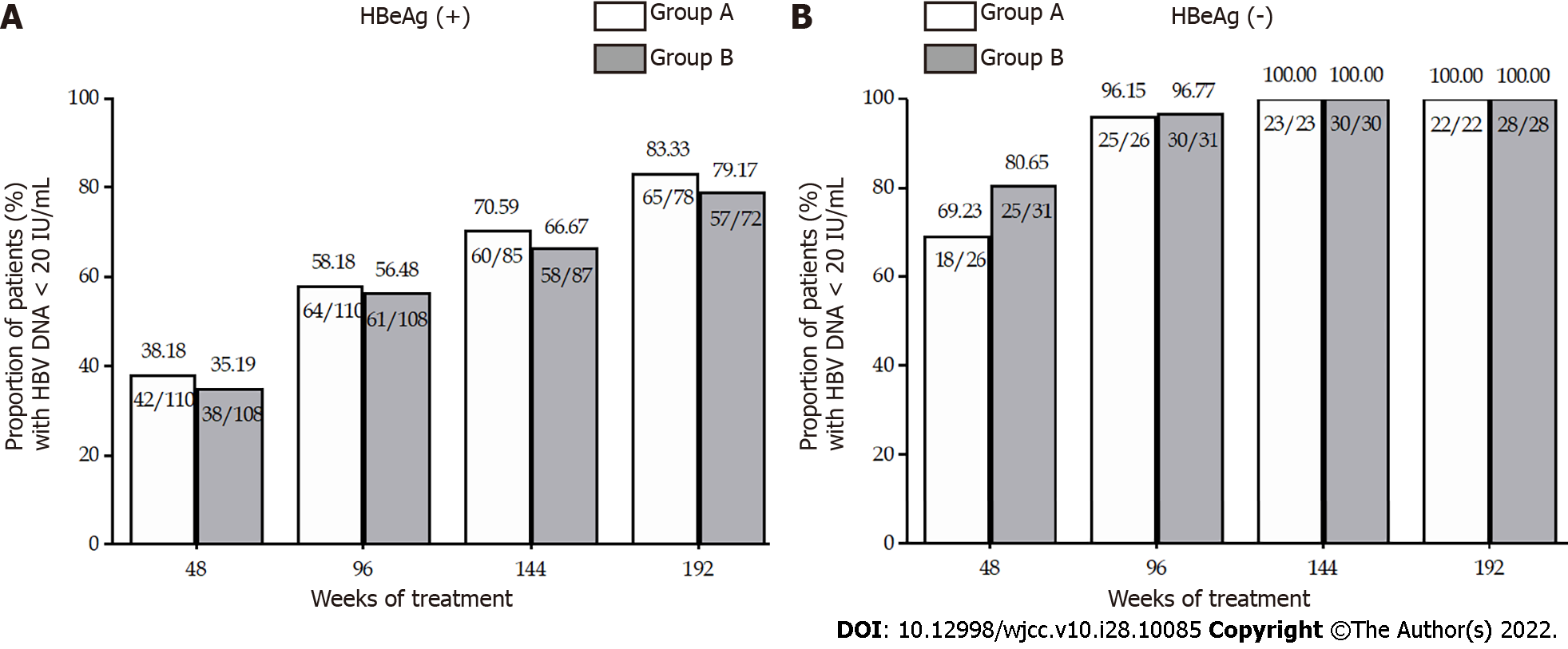
Figure 1 Rates of patients with undetectable hepatitis B virus DNA through week 192.
A: Proportion of patients with HBV DNA < 20 IU/mL in Group A and B in HBeAg positive (+) patients; B: Proportion of patients with HBV DNA < 20 IU/mL in Groups A and B in HBeAg negative (-) patients. HBeAg: Hepatitis B e antigen.
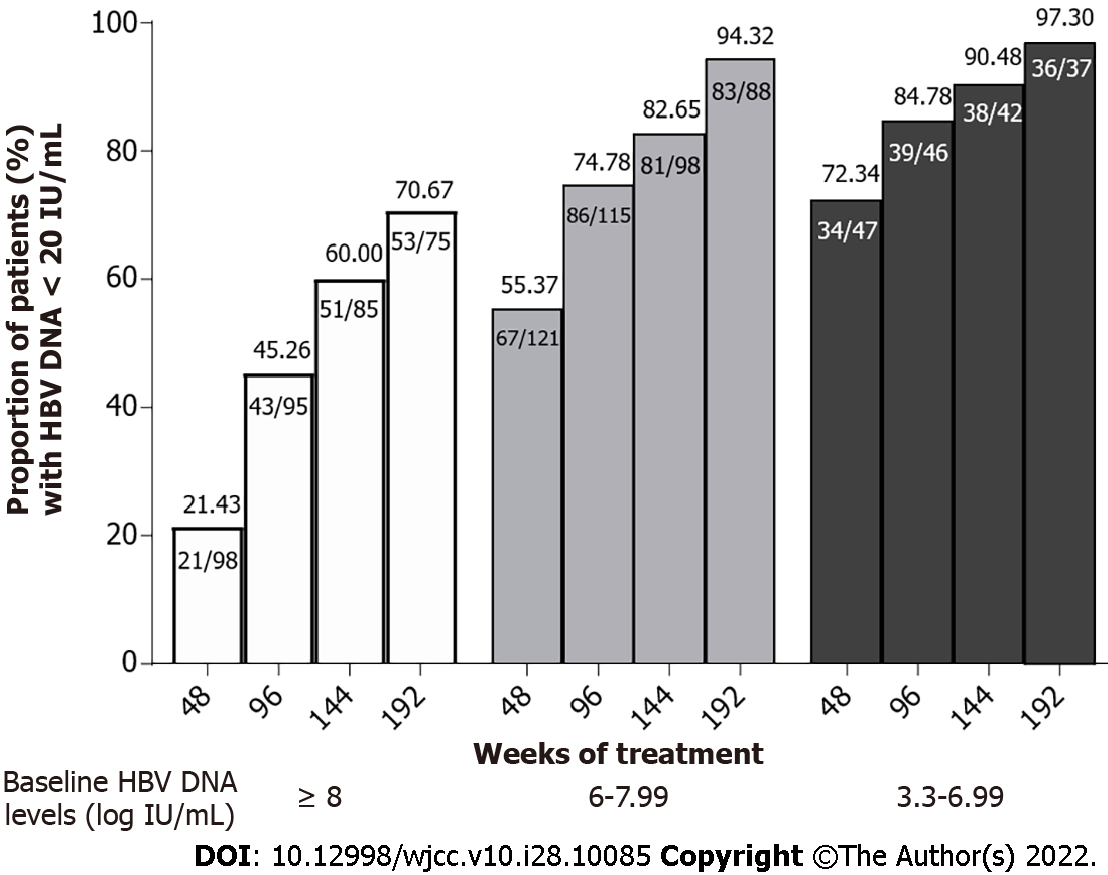
Figure 2 Rates of undetectable hepatitis B virus DNA levels in patients with different baseline hepatitis B virus DNA levels from 48 wk to 192 wk.
HBV: Hepatitis B virus.
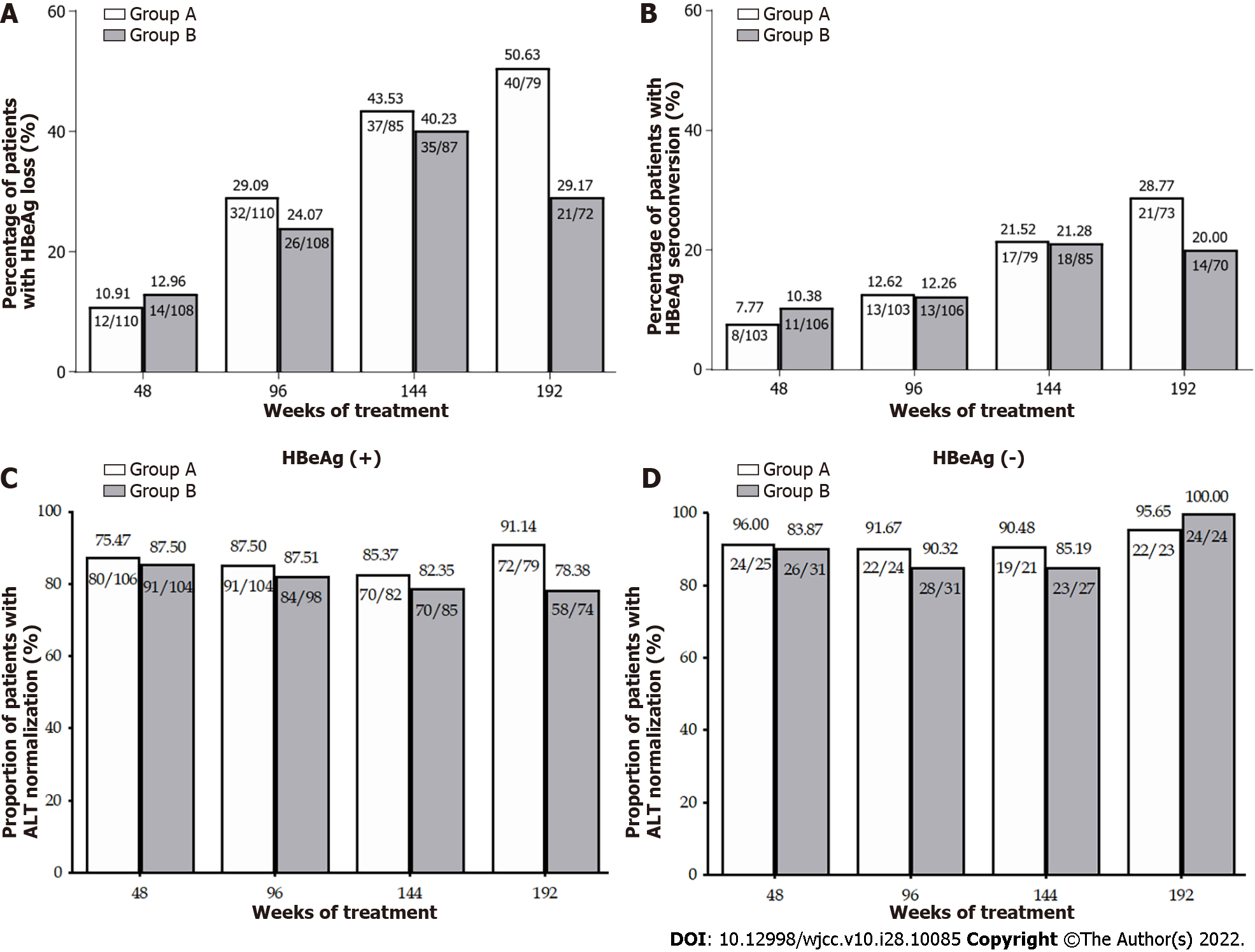
Figure 3 Rates of patients with hepatitis B e antigen loss and hepatitis B e antigen seroconversion, and alanine aminotransferase normalization through week 192.
A: Percentage of patients with hepatitis B e antigen (HBeAg) loss in Groups A and B; B: Percentage of patients with HBeAg seroconversion in Groups A and B; C: Percentage of patients with alanine aminotransferase (ALT) normalization in Groups A and B in hepatitis B e antigen positive (+) patients; D: Percentage of patients with ALT normalization in Groups A and B in in HBeAg positive (+) patients. HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; ALT: Alanine transaminase.
- Citation: Xu JH, Wang S, Zhang DZ, Yu YY, Si CW, Zeng Z, Xu ZN, Li J, Mao Q, Tang H, Sheng JF, Chen XY, Ning Q, Shi GF, Xie Q, Zhang XQ, Dai J. One hundred and ninety-two weeks treatment of entecavir maleate for Chinese chronic hepatitis B predominantly genotyped B or C. World J Clin Cases 2022; 10(28): 10085-10096
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10085.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10085