Copyright
©The Author(s) 2022.
World J Clin Cases. Jun 26, 2022; 10(18): 6009-6020
Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6009
Published online Jun 26, 2022. doi: 10.12998/wjcc.v10.i18.6009
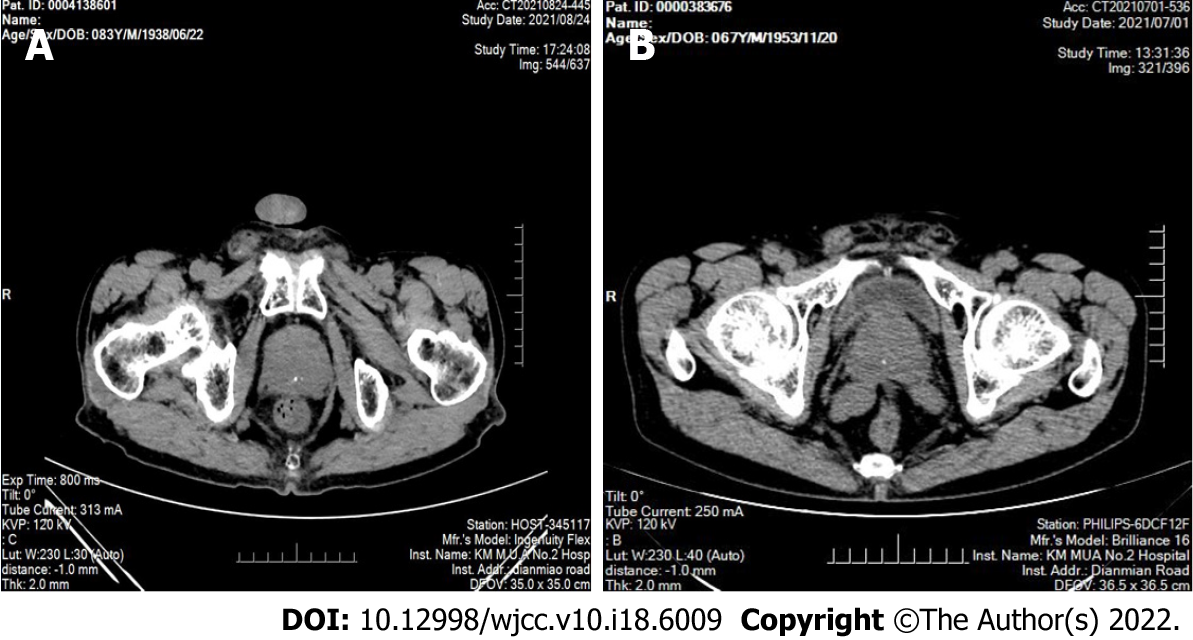
Figure 1 Computed tomography images of sclerosing adenopathy of the prostate.
A: Computed tomography (CT) showing an enlarged prostate with multiple calcifications; B: CT showing that the prostate was enlarged and calcified, partially protruding into the trigone of the bladder, and the enhancement was not uniform.
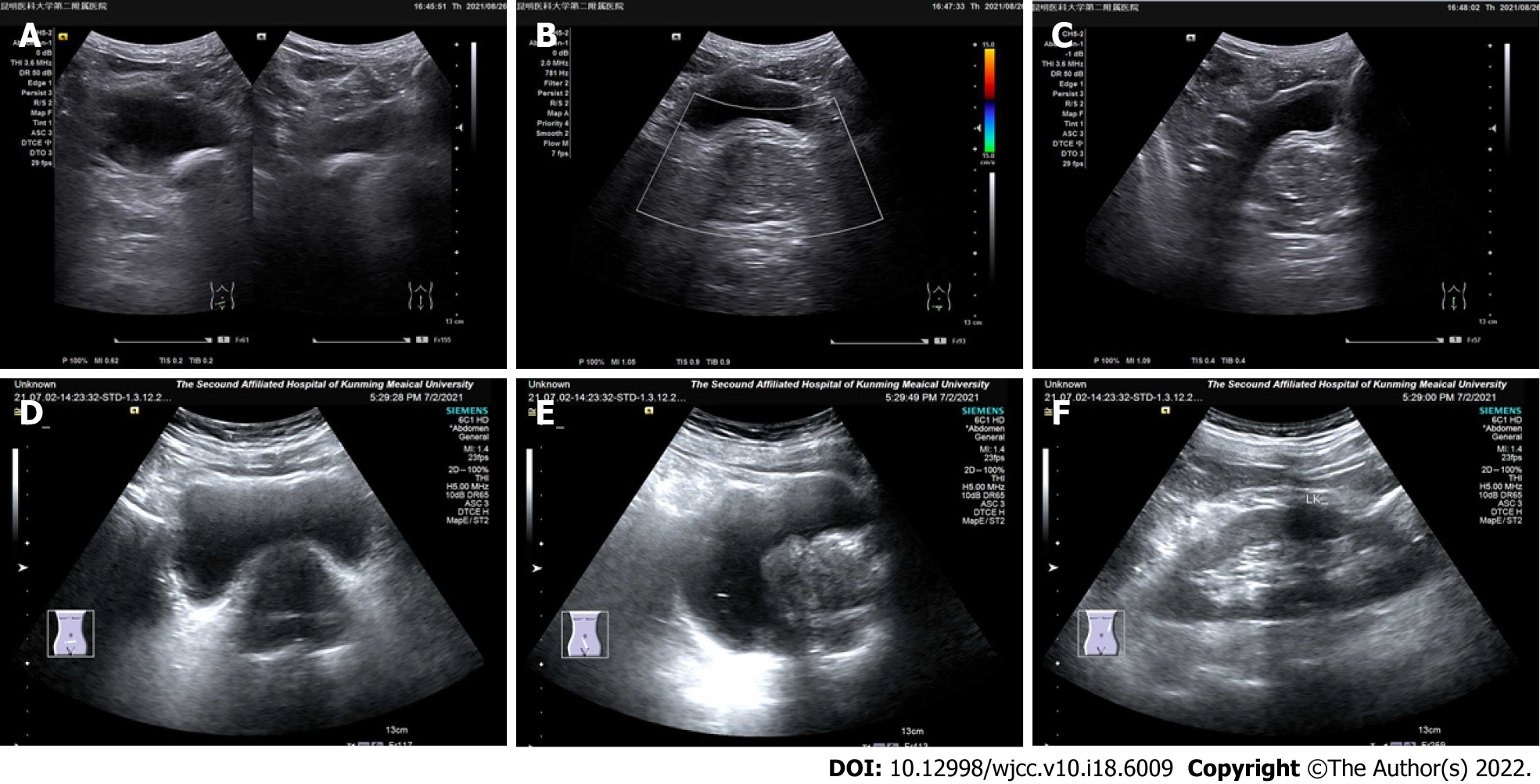
Figure 2 Ultrasound image of sclerosing adenopathy of the prostate.
A-C: The prostate shape was full, showing regular margins, a normal ratio of internal to external glands, an uneven echo, and a sonographic image of benign prostatic hyperplasia; D-F: The prostate gland was enlarged, its shape was plump, the internal gland was enlarged, the external gland was compressed and thinned, the parenchymal echo was not uniform, and the parenchyma was probed with multiple hyperechoic spots.
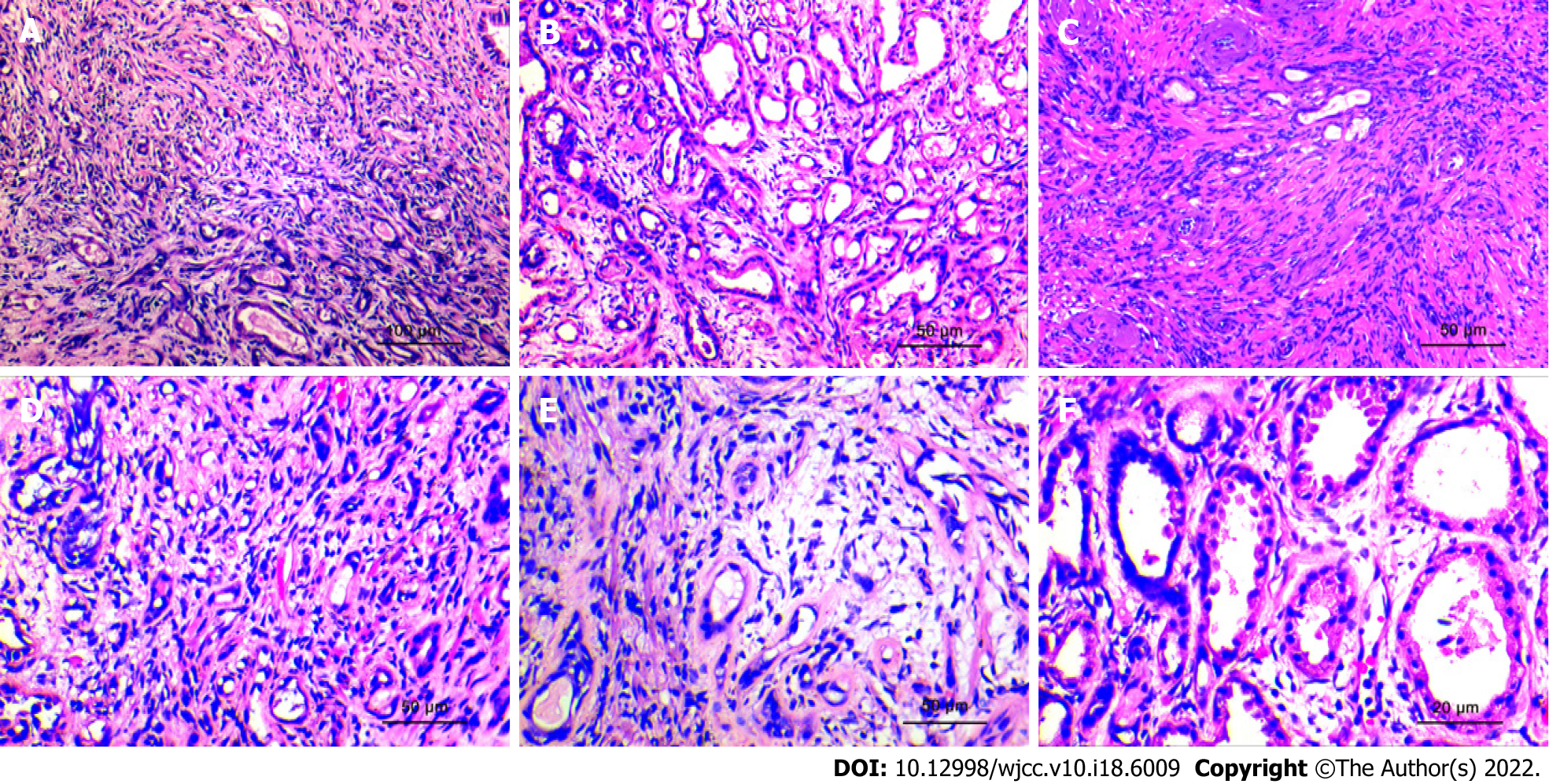
Figure 3 Histopathological morphological characteristics.
A: The lesions are distributed uniformly, have a nodular shape, without obvious capsules, and the simulated prostate adenocarcinoma presents an "invasive growth" pattern. (HE × 100; scale bar, 100 μm); B: The hyperplastic stroma squeezes the glands to form glandular tubes of varying sizes. Eosinophilic shoed spike-like protrusions can be seen in part of the lumen (HE × 200; scale bar, 50 μm); C: The glands of sclerosing adenopathy are squeezed into a cord-like, thread-like, or even single-cell-like arrangement, and the cells have a shuttle-like shape (HE × 200; scale bar, 50 μm); D: When the glandular transition is squeezed, it can form a vacuole-like structure, and the increase in the number of vacuole-like cells leads to a signet ring-like morphology (HE × 200; scale bar, 50 μm); E: The interstitium of the disease was composed of mildly morphological spindle cells, often with collagenous or mucinous changes (HE × 200; scale bar, 50 μm); F: At high power, the glandular epithelium and the compressed myoepithelial components can be seen, the cell cytoplasm is eosinophilic, and the nucleus is located at the base (HE × 400; scale bar, 20 μm).
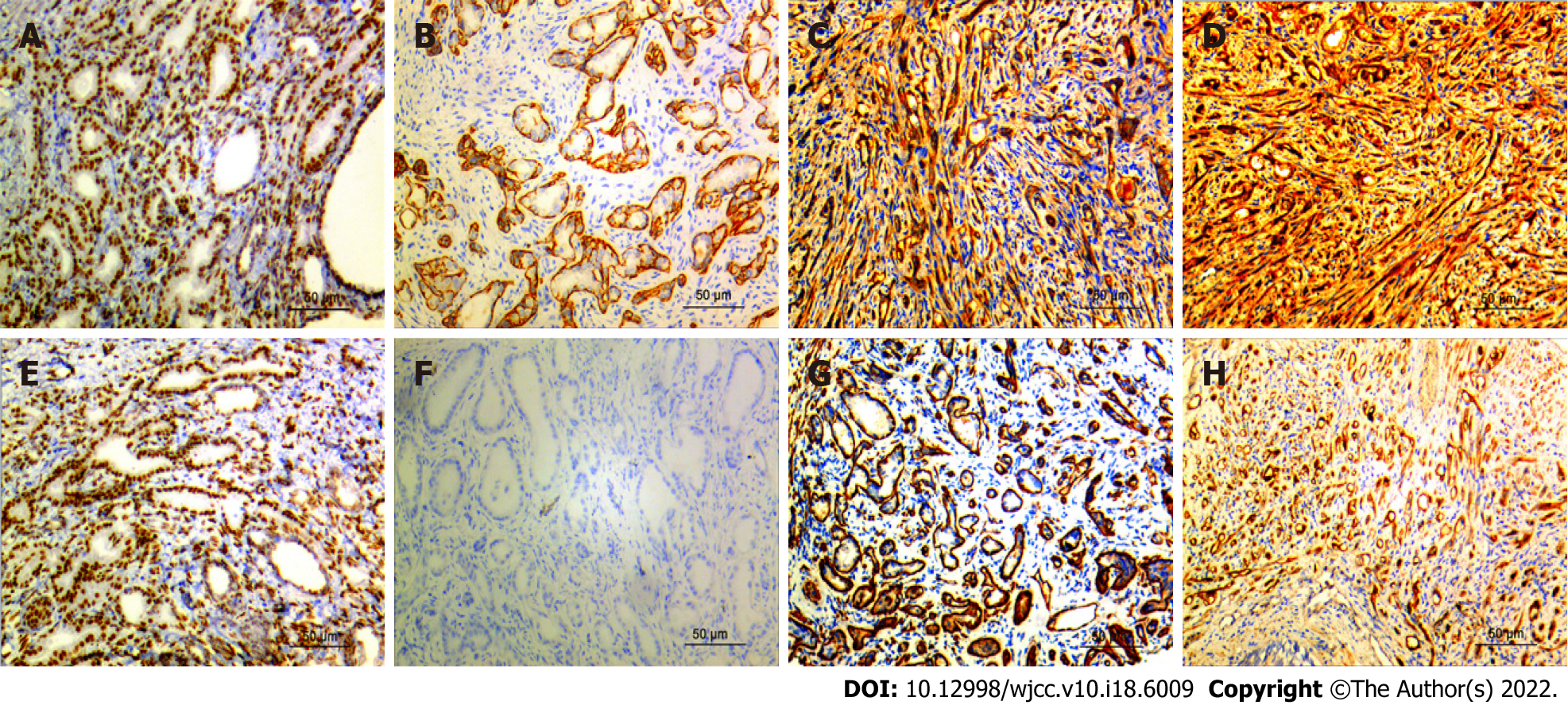
Figure 4 Immunohistochemistry results.
A: AR was strongly expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); B: Calponin is positively expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); C: CK5/6 is positively expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); D: CKH was strongly expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); E: P63 was moderately expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); F: P504S is not expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); G: SMA is moderately expressed in prostatic sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm); H: S100 is strongly expressed in prostate sclerosing adenopathy (Immunohistochemistry; magnification, × 200; scale bar, 50 μm).
- Citation: Feng RL, Tao YP, Tan ZY, Fu S, Wang HF. Prostate sclerosing adenopathy: A clinicopathological and immunohistochemical study of twelve patients. World J Clin Cases 2022; 10(18): 6009-6020
- URL: https://www.wjgnet.com/2307-8960/full/v10/i18/6009.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i18.6009