Published online Sep 26, 2016. doi: 10.5662/wjm.v6.i3.190
Peer-review started: April 15, 2016
First decision: May 19, 2016
Revised: June 19, 2016
Accepted: July 14, 2016
Article in press: July 16, 2016
Published online: September 26, 2016
Processing time: 160 Days and 1.3 Hours
To study immunogenicity of Pseudomonas N terminal flagellin as an adjuvant for Acinetobacter baumannii (A. baumannii) biofilm associated protein (Bap).
The N terminal flagellin gene was amplified. The pET28a (+) and polymerase chain reaction products were digested with HindIII and EcoR I. The ligation of N terminal flagellin into pET28a (+) was performed using T4 DNA ligase and was then transformed into Escherichia coli BL21 (DE3) as a suitable expression host. pET28a (+) vector harboring a conserved region of Bap from our previous work was used. The recombinant proteins were expressed, analyzed by SDS-PAGE method and was purified by affinity chromatography with His-Tag residues followed by confirmation with western blotting. Mice were immunized with recombinant N terminal flagellin and Bap subunits. The immunized animals were intranasally (i.n) challenged with A. baumannii and Pseudomonas aeruginosa (P. aeruginosa).
The flagellin enhanced the immunogenicity of Bap causing an increase in specific IgG titers in serum (P < 0.001). Internal organs, i.e., liver, lung and spleen of the Bap-Flagellin immunized group challenged with A. baumannii showed significantly lower bacterial load compared to the control group. The bacterial loads were studied in internal organs. A. baumannii infected immunized animals with Bap-Flagellin exhibited internal organs with minor bacterial load while P. aeruginosa PAO1 infected group showed heavy bacterial load of (4.3 ± 0.12) × 106, (1.1 ± 0.01) × 106 and (2.2 ± 0.22) × 106 per gram of lungs, liver and spleen respectively. Bacterial loads were detected per gram of lungs, liver and spleen of the mice group immunized with Bap were (1.2 ± 0.06) × 107, (11.1 ± 0.041) × 105 and (3.6 ± 0.42) × 106 respectively. In vivo neutralization assay indicated that all experimental mice groups, except for Flagellin administered group was significantly (P < 0.05) protected against A. baumannii.
These results demonstrate that P. aeruginosa Flagellin as an adjuvant for BapA. baumannii could be a useful model to evaluate new vaccine against A. baumannii.
Core tip: The increasing frequency of Acinetobacter baumannii (A. baumannii) infections and its drug resistance challenge health authorities. Flagellin is an effective immune activator stimulating various biologic functions identified by Toll like receptor 5. Conserved regions of biofilm associated protein (Bap) have already been identified and their immunoprotectivity against A. baumannii have been established. In order to enhance their immunogenic activities, we designed a study on adjuvant role of flagellin from Pseudomonas aeruginosa for Bap.
- Citation: Sefidi MD, Rasooli I, Owlia P, Talei D, Astaneh SDA, Nazarian S. Adjuvant role of Pseudomonas flagellin for Acinetobacter baumannii biofilm associated protein. World J Methodol 2016; 6(3): 190-199
- URL: https://www.wjgnet.com/2222-0682/full/v6/i3/190.htm
- DOI: https://dx.doi.org/10.5662/wjm.v6.i3.190
Acinetobacter baumannii (A. baumannii) an opportunistic pathogen, causes severe infections of major concerns[1,2]. It survives on harsh dreadful environment such as medical devices. This ability has been related to forming multicellular complex named biofilm on abiotic and biotic surfaces[3,4]. The A. baumannii survival has also been contributed with clinical strains resistance to antimicrobial stressors and desiccation[5,6]. Formation by A. baumannii clinical strains of biofilms on abiotic surfaces has been documented[5]. Production of factors like poly-β-1,6-N-acetyl glucosamine (PNAG), in different bacteria that produce PNAG could also act as a major virulence factor for emerging biofilm-dependent pathogens[7]. Biofilms are encapsulated bacterial constructions within exopolysaccharide, a polymeric matrix important in medicine[8]. Identifying a biofilm associated protein (Bap) homologue of staphylococcus[9], in an isolate of A. baumannii from bloodstream showed that Bap is the most important factor in A. baumannii biofilm formation[10]. The role of high molecular weight of superficial Bap conferring biofilm formation capacity upon bacterial attachment and infection has been reported[10]. Bap A. baumannii is one of the biggest and the most acidic bacterial proteins with a predicted pI-3 is composed of about 8620 amino acids(aa). Seven tandem repeats are the major functional and conserved regions components of Bap[11]. Using adjuvants and immune modulators during vaccination helped to increase immune responses[12]. Adjuvant can enhance host response against an immunogen or a vaccine without imparting antigenic effect. Various substances acting as vaccine delivery vehicles such as mineral salts, particulate and surfactants or liposomes and virosomes have been extensively used as adjuvants[13]. Pattern recognition receptors called “Toll like receptors (TLRs)” are present on various types of microbial cells[14]. TLR5 recognizes flagellin, a potent immune activator stimulating diverse biological functions. In a classic study, a recombinant fusion protein strategy was used with TLR5s like Flagellin to show TLR5 role in the development of vaccine[15]. N-terminal region amino acids of Pseudomonas aeruginosa (P. aeruginosa) PAO1 flagellin was administrated to play significant binding role to TLR5. Specific involvement of different amino acids in TLR5-flagellin interactions was particularly predicted[16], so researches show significant role of N-flagellin terminal region in immunization and can play as well as whole flagellin. The present study was designed to examine the role of Pseudomonas flagellin as an adjuvant to Bap against infections caused by A. baumannii.
The kits for extraction of plasmids for purification of gels were purchased from a local dealer. Synthesis of the designed primers was done by Gene Fanavaran (Tehran, Iran). Standard quality restriction enzymes, Nickel Nitrilotriacetic Acid (Ni-NTA), T4 DNA ligase, nitrocellulose membrane, anti-polyhistidine antibodies, anti-mouse HRP conjugated IgG, microtiter plates, and other standard chemicals and reagents were procurred from local market.
A. baumannii (ATCC 19606), P. aeruginosa (PAO1) and Escherichia coli (E. coli) BL21 (DE3) grown in Luria Bertani (LB) medium on a shaker at 37 °C /220 rpm were used.
The N terminal Flagellin gene (Gen Bank accession No.: AGY69124.1) with 1-180aa of the mature Flagellin protein weight of 53.79 kDa from P. aeruginosa was amplified by polymerase chain reaction (PCR) using its genomic DNA. N terminal F (5’-ATATGAATTCATGGCCCTTACAGT-3’) and N terminal R (5’-TATAAAGCTTTTAACCGCTGATCT-3’) primers. The F primer contained EcoRI site and R primers had HindIII site. PCR conditions started at 95 °C/ 5 min followed by 35 cycles at 94 °C/30 s, 58 °C/1 min, 72 °C/90 s, and a 72 °C for 5 min as the final extension. One percent agarose gel was used to analyze the amplified fragments. Double Digestion of PET28a (+) vector and PCR products was carried out and T4 DNA ligase was used for ligation of N terminal Flagellin into pET28a (+). Transformation of the recombinant DNA plasmids, N Flagellin, pET28a into E. coli BL21 was then carried out.
Vector harbouring pET28a-n Flagellin construct was incubated for 3 h at 37 °C in LB agar incorporated with 70 μg/mL kanamycin to an OD620 of 0.6. Induction was brought about with 1 mmol IPTG for 3 h at 37 °C. The cell pellet was collected after 10 min centrifugation at 5000 rpm and re-suspended in denaturation lysis/binding buffer (buffer B). Lysate sonication at 200 w with a 10 s cooling time between each burst was done for 6 times. The lysate was then centrifuged at 14000 rpm at 4 °C for 20 min to pellet the cell debris. The affinity Ni-NTA agarose column loaded with supernatant was washed with denaturing buffer (buffer C, pH = 6.3), and denaturing elution buffer D (pH = 5.9). The recombinant Bap subunit was then eluted using denaturing elution buffer E (ph = 4.5). Eight mol/L urea was present in all buffer solutions. Protein analysis was carried out by 10% SDS-PAGE. Dialysis was performed in descending order against PBS (pH = 7.4) containing 6 to 0 mol/L urea. Huleatt et al[15] method was employed to determine the purified protein concentration. Bovine serum albumin (BSA) was used as a standard protein. Production of the recombinant Bap and its and purification was according to Fattahian et al[17].
Western blotting was performed to confirm the expression of N-flagellin and Bap. Anti-His. 0.5 mg from each of purified recombinant Bap subunit was adsorbed on a nitrocellulose strip and dried. The transformed lysates from uninduced cell were used as control. The nitrocellulose strip was incubated for 1 h at room temperature with gentle shaking in the blocking buffer containing 5% skim milk. The strip was then subjected to three washes with phosphate buffered saline containing Tween 20 (PBST). Diluted anti-His conjugated was incubated with 1:8000 dilution of horseradish peroxidase for one hour. The strip was then subjected to three washes of 5 min each with PBST. Membrane visualization with the substrate diaminobenzidine was complete by observing brownish dots. Color development was terminated by washing with PBST.
Sixty BALB/c mice were divided equally in six groups: (1) the first group was administered with four 20 μg doses of the recombinant Bap; (2) the second group was administered with four 20 μg doses of the recombinant N Flagellin; (3) a single 20 μg dose of recombinant Bap followed by administration of N-flagellin in subsequent vaccinations was administration plan for the third group; (4) the fourth group received a single dose of 20 μg of recombinant N-flagellin followed by administration of Bap in subsequent vaccinations; (5) the fifth group received combination of Bap and N-flagellin simultaneously in all the vaccinations; and (6) the control group received combination of PBS and Freund’s adjuvant.
All groups except group (5), first received complete Freund’s adjuvant only The subsequent vaccinations were with the incomplete Freund’s adjuvant emulsified recombinant proteins. Ten days after the second dose the blood samples were collected through infra-orbital plexus.
The coating solution was used to dilute the recombinant Bap and N-flagellin subunit to an optimal concentration of 20 μg/mL to coat a 96-well plate. Each well was added with 100 μL of the resulting solution followed by incubation at 4 °C for 12-18 h. The wells were washed once with 0.05% Tween 20 (PBST) incorporated PBS to block the unoccupied sites. The plate was then incubated for 1 h at 37 °C with 100 μL of PBST + 5% skimmed milk. The plates were washed 3 times with PBST. Serial dilutions of 1:100 to 1:1600 from each serum were added to the wells. The plates were incubated at 37 °C for 1 h followed by 3 washes as described above. One hundred microlitre of 1:1000 horseradish peroxidase - conjugated secondary antibody diluted in PBST was added to each well and the plates were incubated at 37 °C for one hour followed by three washes with PBST and incubation with 100 μL per well of TMB (3,3’,5,5’-tetramethylbenzidine solution) substrate until a desired absorbance was reached. Addition of 2 mol/L H2SO4 stopped the reaction. Sample absorbance at 450 nm was read on an ELISA plate reader.
The clinical A. baumannii isolates incubated overnight in LB broth were resuspended in PBS to an OD620 of 0.3[17]. Fifty microlitres of the bacterial suspension was added to each well in 96-well plates and dried at 37 °C. The plates were washed five times with after blocking with 200 μL of PBS with 5% skimmed milk for 1 h. This was followed by incubation with different dilutions of immunized mice sera. The plates were then incubated with HRP conjugated secondary antibody for one hour. PBS was used to wash the plates for five times. One hundred microlitres of TMB substrate was added per well and incubated until desired absorbance. The reactions were stopped with 2 mol/L H2SO4 and OD450 was read.
Cyclophosphamide (Cy) regimen (150 mg/kg of body weight) was used to bring about neutropenia of short duration but able to facilitate the onset of the infectious process. LD50 was determined with intranasal (i.n) administration of A. baumannii at 109 CFU concentration 4 d after treatment of five BALB/c mice with Cyclophosphamide. PBS was administered as a control instead of bacteria. General anesthesia was brought about by intraperitoneal (i.p) injection of 0.2 mL of 1.3 mg/mL xylazine and 6.7 mg/mL of ketamine in 0.9% saline. This was 100-200 mg Ketamine and 5-16 mg Xylazine per kilogram body weight.
Antisera to the Bap and Bap-Flagellin proteins were raised by injecting 20 μg of Bap and Bap-Flagellin per animal in BALB/c mice. Lethal dose (106 CFU) of A. baumannii diluted to 1:400 with PBS was maintained at 37 °C for 30 min. Neutralization test was carried out by peritoneal injection of lethal dose of A. baumannii to the mice groups of five animals per group. In order to rule out interfering role of natural antibodies in mice sera in conferring any resistance to mice against A. baumannii, a lethal dose of A. baumannii and PBS was administered to mice groups as control[18]. Mortality rate was monitored for 48 h.
Mice immunized with Bap and Bap-flagellin were divided into two groups. The groups were challenged with A. baumannii and P. aeruginosa PAO1. The mice were observed for mortality for two days. The animals were sacrificed after 48 h unless they died earlier. The microbial challenge or passive immunization were performed in mice groups treated with Cy. For bacterial challenge, i.n. administration with 20 μL of P. aeruginosa PAO1 or 20 μL of A. baumannii (10 μL/nostril) was carried out[19]. Morbidity and mortality were watched over 48 h.
The experimental data in triplicates were expressed as mean ± SD. In order to calculate P values and to determine the significance of differences in the experimental groups, Student’s t test was used. P < 0.05 was considered as significant for the combined injection of Bap and Flagellin.
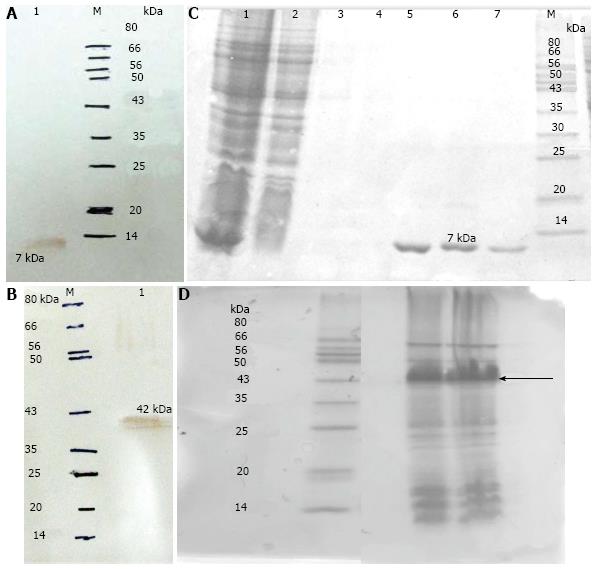
N terminal flagellin gene of P. aeruginosa (PAO1) was successfully amplified. The amplified gene was revealed on agarose gel (1%). N-flagellin gene was cloned into pET28a (+). The N-flagellin and Bap were expressed in E. coli BL21 (DE3). The recombinant proteins were confirmed by SDS-PAGE. The presence of 42 kDa (Bap) and 7 kDa (Flagellin) proteins in the eluted fractions was revealed by SDS-PAGE analysis. Western Blotting was used to confirm the expression of recombinant proteins using anti-His-tag antibodies (Figure 1).
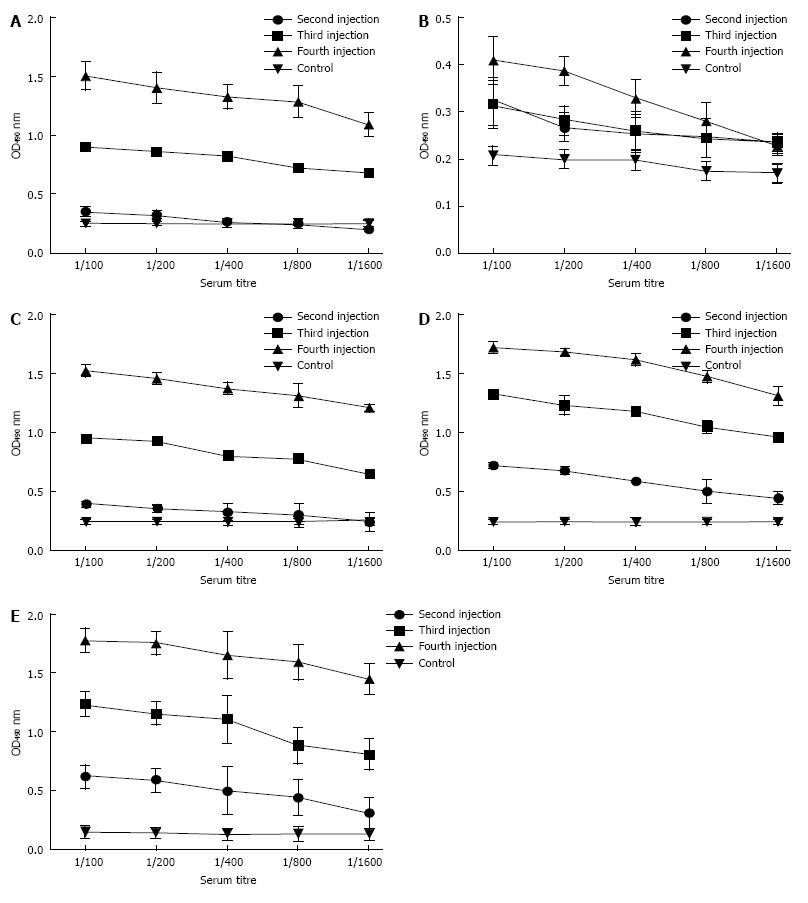
Animals were observed healthy with no post immunization signs of abnormalities. Significant (P < 0.05) levels of IgG were noted in immunized sera compared to control mice. Increased antibody titer was noted after the third booster dose. No specific antibody was detected in sera samples from mice that received adjuvant and PBS. No significant difference was observed in the sera from combined administration of both proteins as compared to that of the single Bap injections (Figure 2A and E). Significant (P < 0.001) increase of antibody titer took place after the third booster, whereas animals administered with adjuvant and PBS or just Flagellin serving as control exhibited no Bap-specific antibodies in their sera.
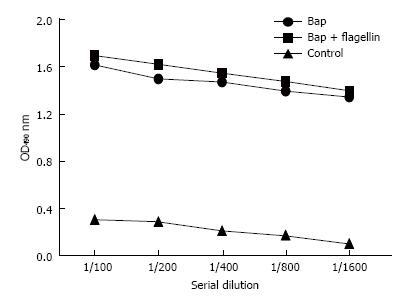
The antibody raised against Bap subunit reacted with A. baumannii. OD values detected were significant compared to control (Figure 3).
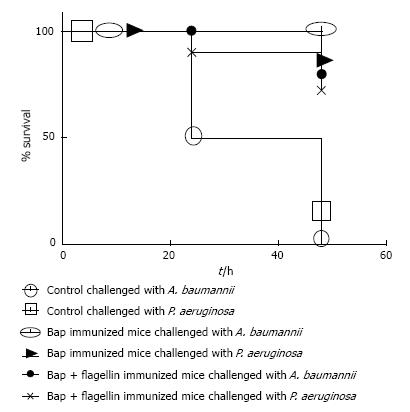
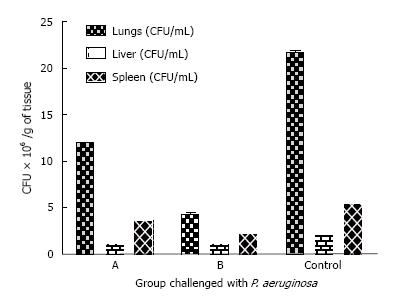
2.5 × 109 CFU/mL was determined as LD50 per mouse via intranasal administration. Rendering mice immunocompromised by prior treatment with Cy makes them susceptible to pneumonia. This susceptibility is accompanied by a drop in the LD50 after a challenge with 1000 CFU of a P. aeruginosa PAO1 or A. baumannii causing lethality. The control group died within the first 24 h of challenge. The Bap immunized groups resisted A. baumannii challenge with no mortality. Twenty percent of the mice population immunized with Bap died upon challenge with P. aeruginosa within 48 h. Eighty percent of the group immunized with Bap-Flagellin survived challenges with A. baumannii or P. aeruginosa (Figure 4).
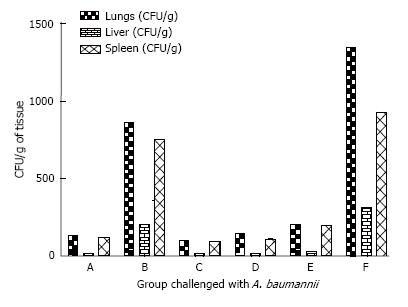
The internal organs were studied for bacterial load. A. baumannii infected immunized animals with Bap-Flagellin exhibited internal organs with minor bacterial load while P. aeruginosa PAO1 infected group showed heavy bacterial load of (4.3 ± 0.12) × 106, (1.1 ± 0.01) × 106 and (2.2 ± 0.22) × 106 per gram of lungs, liver and spleen respectively. Bacterial loads in terms of CFU/g of lungs, spleen and liver of the mice group immunized with Bap were (1.2 ± 0.06) × 107, (11.1 ± 0.041) × 105 and (3.6 ± 0.42) × 106 respectively. Unimmunized mice exhibited bacterial load of (3.20 ± 0.11) × 107, (1.90 ± 0.14) × 106 and (2.6 ± 0.11) × 106 CFU per gram of lungs, liver and spleen respectively in challenge with A. baumannii and (2.17 ± 0.2) × 107, (2.1 ± 0.1) × 106 and (5.4 ± 0.11) × 106 per gram of lungs, liver and spleen respectively in challenge with P. aeruginosa PAO1 (Figures 5 and 6).
Protectivity of immunized mice sera against bacterial challenge was determined by neutralization assay. There was an increased antibody titer against Bap and Bap-flagellin in the vaccinated group. As shown in Figures 4 and 5, all the experimental groups, except for Flagellin administered group were significantly (P < 0.05) protected against A. baumannii.
Despite development of new generation antibiotics, the wide expansion of multi drug resistant A. baumannii is still considered as a potent threat[20]. Because of it is remarkable resistance to an extensive range of antibiotics[21], A. baumannii is regarded as a problematic pathogen. Its colonization ability, survival on nutrient-limited surfaces and resistance to antimicrobial situation made it as a difficult-to-treat nosocomial pathogen[5]. Combination of biofilm forming and MDR of A. baumanni contribute in importance of this pathogen in Hospital - acquired infections[5]. Moreover recent researches confirm beside biofilm forming, multi drug resistance of embedded bacteria in matrix of biofilm is showed[22,23]. Using different subunits of Bap as a recombinant subunit vaccines[24], clarify the expansion of infections caused by A. baumannii. In silico studies described intercellular adhesion of BapA. baumannii in maturation of biofilm[10]. Twenty or more antigenic determinants and 55 discontinuous B-Cell epitopes were predicted for Bap subunits[11].
Adjuvant as a chemical catalyst without any considering, specific antigenic effect mount the response to a vaccine[25]. The results (Figure 2A and D) show elevated antibody level triggered against Bap indicating that N flagellin contributed to enhancement of antigen efficacy by playing a role as an adjuvant. Physical conjugation of a vaccine antigen to a TLR ligand brings about discrimination of macromolecule from self-apoptotic bodies by the antigen presenting cells which ultimately leads to enhancement of immunogenicity and subsequently antigen presentation by MHC molecules[26]. Vance et al[27] investigated dissemination of P. aeruginosa in neutropenic mice via pneumonic challenge model. Bacteremia, dissemination, and eventual death of P. aeruginosa PAO1 challenged mice was noted upon Cy administration to the infected mice. In this work the efficacy of A. baumannii immunogen was studied in immunocompromised mice. Active vaccination with Bap increased survival rate and LD50 of Cy-treated mice to i.n. challenge with A. baumannii. Many studies described interaction of flagellin and TLR5 as a signaling of flagellin result in releasing of inflammatory mediator[28]. In silico studies describe the interaction between D1 domain of flagellin as an important region of it and TLR5[28,29]. Our study on the mice group administered only with Bap + flagellin subunit (Figure 2E) without using Freund’s adjuvant showed higher antibody titer than other groups immunized with the recombinant proteins using Freund’s adjuvant (Figure 2E). These results further support the adjuvant role of N-flagellin. The role of Salmonella Flagellin as an adjuvant has been studied. Particular interest was paid in flagellum structural subunit as an adjuvant imparting elevated immunogenicity to soluble proteins or peptides, to activate antibody as well as cell immune responses[30]. Vaccination with chimeric flagellin provided mice with significant protection against H. pylori[31]. Use of EtIMP1-flagellin fusion protein has been suggested as an effective immunogen against Eimeria infection[32]. An experimental report on the immunogenicity of a 19 kDa merozoite surface protein-1 [MSP1(19)] from Plasmodium vivax C-terminal fragment against malaria and an innate immunity against the Salmonella enterica serovar Typhimurium flagellin (FliC) is available[33]. There is a report on the enhancement of FimH protective immunity against UPEC infection where the ability of FliC a Toll-like receptor 5 flagellin, a UPEC strain agonist, has been compared with Montanide ISA 206, a conventional adjuvant[34]. A TLR5 flagellin binding site at 88-97 residues located within the constant domain D1[35] coincides with that found experimentally by other researchers[28]. The importance of flagellin side chains was also described in interact with surface side chains of TLR5[35]. In this study, a 10 amino acid stretch was predicted in the N-terminal of P. aeruginosa PAO1 flagellin to have importance for binding to TLR5 and acting as an adjuvant[36]. Despite significant (P < 0.05) increase in IgG titers of the mice groups immunized with Flagellin and Bap in groups C, D and E, better protection was exhibited by Bap immunized group against lethal dose of A. baumannii (Figure 4). Such a phenomenon could be attributed to the vaccination dose of group E which was half of the Bap quantity used in other groups. This is further supported by the absence of initial administration of Freund’s adjuvant. The adjuvant role of Flagellin subunit is well documented in groups C and D where either the initial dose of Flagellin followed by the subsequent doses of Bap or initial dose of subsequently followed by administration of Flagellin subunit significantly elevated the anti Bap titer. Vaccination of mice prior to immunocompromisation and subsequent infection of mice decreases the susceptibility on the basis of lower bacterial load and better survival rate. Significant (P < 0.001) rise in antibody titers of groups C (received 20 μg of recombinant Bap in the first vaccination followed by administration of N-flagellin in subsequent vaccinations) and D (received 20 μg of recombinant N Flagellin in the first vaccination followed by administration of Bap in subsequent vaccinations) and the 80% survival of group D challenged with either A. baumannii or P. aeruginosa, indicate the efficiency of Flagellin as an adjuvant and protective immunity against A. baumannii. The survival against P. aeruginosa could be due to the immunogenicity of the recombinant Flagellin.
Although there is no significant difference among groups A, C, D and E, higher load of bacteria was found in the liver and spleen of group E as compared to other immunized groups (Figures 5 and 6) which could explain the 20% mortality in this group (Figure 4).
In conclusion, because of the complications and difficulties in treating A. baumannii infections, and with respect to the significant role of Bap on one hand and the efficacy of bacterial proteins such as Flagellin as an adjuvant on the other, this model could be useful to evaluate new vaccine regimens against A. baumannii infections.
We thank Center for Molecular Microbiology, Shahed University for their financial support toward his work.
Acinetobacter baumannii (A. baumannii) is an opportunistic pathogen of major concerns. Biofilm associated protein is the most important factor in A. baumannii biofilm formation. Seven tandem repeats are the major functional and conserved regions components of Bap. Pattern recognition receptors, i.e., “Toll like receptors (TLRs)” are found on various microbial cells. A recombinant fusion protein strategy was used with TLR5s like Flagellin to show its role in the development of vaccine. N-terminal region amino acids of Pseudomonas aeruginosa (P. aeruginosa) PAO1 flagellin was administrated to play significant binding role to TLR5. Researches show significant role of N-flagellin terminal region in immunization and can play as well as whole flagellin. The present study was designed to examine the role of Pseudomonas flagellin as an adjuvant to biofilm associated protein (Bap) against infections caused by A. baumannii.
Flagellin of P. aeruginosa is an important vaccine candidate. N-terminal domains are highly conserved in both type A and type B flagellins. The efficacy of gold nanoparticles (AuNPs) conjugated to N-terminal domains of P. aeruginosa flagellin [flagellin(1-161)], as an immunogen in mice, has been assessed by Farida. Flagellin(1-161), AuNP-flagellin(1-161), and flagellin(1-161) emulsified in Freund’s adjuvant were administered subcutaneously to BALB/c mice. Mice given AuNP-flagellin(1-161) elicited high titers of anti-flagellin(1-161) antibodies compared with non-immune group and/or mice which received flagellin(1-161) without adjuvant. Recently, Delphine demonstrated that the adaptive responses stimulated by intranasal administration of flagellin and antigen were linked to TLR5 signaling in the lung epithelium. They sought to identify the antigen presenting cells involved in this adjuvant activity. They first found that the lung dendritic cells captured antigen very efficiently in a process independent of TLR5. However, TLR5-mediated signaling specifically enhanced the maturation of lung dendritic cells. Afterward, the number of antigen-bound and activated conventional dendritic cells (both CD11b+ and CD103+) increased in the mediastinal lymph nodes in contrast to monocyte-derived dendritic cells. Their data suggested that flagellin-activated lung conventional dendritic cells migrate to the draining lymph nodes. The results demonstrated that indirect TLR5-dependent stimulation of airway conventional dendritic cells is essential to flagellin’s mucosal adjuvant activity.
In this study the authors have used a conserved region of Bap instead of the entire protein. Bap is a very large protein and its expression is near to impossible in recombinant form. There are many such proteins involved in pathogenesis of various micro-organisms. This study can be a clue to the researchers to use immunogenic conserved regions of proteins involved in pathogenesis and enhance their immunogenicity by natural adjuvants such as flagellin.
The findings could be applied in development of novel vaccines against disease causing micro-organisms.
TLR: “Toll like receptors” are pattern recognition receptors present on various types of microbial cells; Bap: Biofilm associated protein. Bap A. baumannii is one of the biggest and the most acidic bacterial proteins with a predicted pI -3 is composed of about 8620 amino acids (aa). Seven tandem repeats are the major functional and conserved regions components of Bap.
The authors present the extent which Pseudomonas flagellin can play a role as an adjuvant for Bap A. baumannii and the model could be useful to evaluate new vaccine regimens against A. baumannii. This manuscript is interesting. It’s just a suggestion, the N-flagellin and recombinant Bap could be fused to express.
Manuscript source: Invited manuscript
Specialty type: Clinical medicine
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bai CQ, Yuan J S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Bergogne-Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148-165. [PubMed] |
| 2. | Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77:3150-3160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 372] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Rodríguez-Baño J, Bonomo RA. Multidrug-resistant Acinetobacter baumannii: eyes wide shut? Enferm Infecc Microbiol Clin. 2008;26:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938-1941. [PubMed] |
| 6. | Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473-3484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 442] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 7. | Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953-5963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 8. | Monds RD, O’Toole GA. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 2009;17:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 9. | Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penadés JR. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol. 2001;183:2888-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 587] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Rahbar MR, Rasooli I, Mousavi Gargari SL, Amani J, Fattahian Y. In silico analysis of antibody triggering biofilm associated protein in Acinetobacter baumannii. J Theor Biol. 2010;266:275-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20 Suppl 3:S56-S64. [PubMed] |
| 13. | O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 290] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, Song L, Nakaar V, Powell TJ. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 610] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 17. | Fattahian Y, Rasooli I, Mousavi Gargari SL, Rahbar MR, Darvish Alipour Astaneh S, Amani J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb Pathog. 2011;51:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Bentancor LV, O’Malley JM, Bozkurt-Guzel C, Pier GB, Maira-Litrán T. Poly-N-acetyl-β-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect Immun. 2012;80:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Scarff JM, Goldberg JB. Vaccination against Pseudomonas aeruginosa pneumonia in immunocompromised mice. Clin Vaccine Immunol. 2008;15:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Gordon NC, Wareham DW. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents. 2010;35:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784-3788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3309] [Cited by in RCA: 3683] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 22. | Rao RS, Karthika RU, Singh SP, Shashikala P, Kanungo R, Jayachandran S, Prashanth K. Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Indian J Med Microbiol. 2008;26:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Wendt C, Dietze B, Dietz E, Rüden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394-1397. [PubMed] |
| 24. | Liljeqvist S, Ståhl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J Biotechnol. 1999;73:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 3054] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 26. | Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 737] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 27. | Vance RE, Rietsch A, Mekalanos JJ. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun. 2005;73:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Donnelly MA, Steiner TS. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J Biol Chem. 2002;277:40456-40461. [PubMed] |
| 29. | Eaves-Pyles T, Murthy K, Liaudet L, Virág L, Ross G, Soriano FG, Szabó C, Salzman AL. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol. 2001;166:1248-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Braga CJ, Massis LM, Sbrogio-Almeida ME, Alencar BC, Bargieri DY, Boscardin SB, Rodrigues MM, Ferreira LC. CD8+ T cell adjuvant effects of Salmonella FliCd flagellin in live vaccine vectors or as purified protein. Vaccine. 2010;28:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Mori J, Vranac T, Smrekar B, Cernilec M, Serbec VČ, Horvat S, Ihan A, Benčina M, Jerala R. Chimeric flagellin as the self-adjuvanting antigen for the activation of immune response against Helicobacter pylori. Vaccine. 2012;30:5856-5863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Yin G, Qin M, Liu X, Suo J, Tang X, Tao G, Han Q, Suo X, Wu W. An Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and the TLR-5 agonist Salmonella typhimurium FliC flagellin. Biochem Biophys Res Commun. 2013;440:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Bargieri DY, Leite JA, Lopes SC, Sbrogio-Almeida ME, Braga CJ, Ferreira LC, Soares IS, Costa FT, Rodrigues MM. Immunogenic properties of a recombinant fusion protein containing the C-terminal 19 kDa of Plasmodium falciparum merozoite surface protein-1 and the innate immunity agonist FliC flagellin of Salmonella typhimurium. Vaccine. 2010;28:2818-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Asadi Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S. Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine. 2013;31:1210-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Jacchieri SG, Torquato R, Brentani RR. Structural study of binding of flagellin by Toll-like receptor 5. J Bacteriol. 2003;185:4243-4247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Lahiri A, Das P, Chakravortty D. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine. 2008;26:6777-6783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |