Published online Sep 26, 2015. doi: 10.5662/wjm.v5.i3.164
Peer-review started: May 20, 2015
First decision: June 24, 2015
Revised: July 29, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: September 26, 2015
Processing time: 125 Days and 21.5 Hours
AIM: To review previous studies (the last 6 years) about the Helicobacter pylori (H. pylori) antibiotic resistance in order to evaluate the trend in antibiotic resistance.
METHODS: In this study, the PubMed, MEDLINE, Science Direct, Google Scholar and Scielo manuscripts were reviewed from 2009 to 2014.
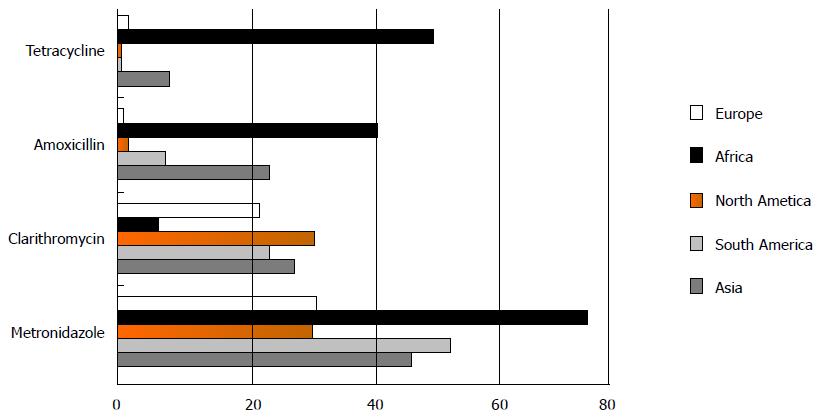
RESULTS: On the whole rates of H. pylori antibiotic resistance were 47.22% (30.5%-75.02%) for metronidazole, 19.74% (5.46%-30.8%) for clarithromycin, 18.94% (14.19%-25.28%) for levofloxacin, and 14.67% (2%-40.87%) for amoxicillin, 11.70% (0%-50%) for tetracycline, 11.5% (0%-23%) for furazolidon and 6.75% (1%-12.45%) for rifabutin. The frequency of tetracycline, metronidazole and amoxicillin resistance was higher in Africa, while clarithromycin and levofloxacin resistance was higher in North America and Asian, respectively.
CONCLUSION: The most sensitive drug is rifabutin and the lowest sensitive drug is metronidazole in the world. The worldwide H. pylori antibiotic resistance to clarithromycin and levofloxacin has increased during the last 6 years. The present systematic review show alarming results and a novel plan is needed for eradication therapy of H. pylori infections.
Core tip: Because of the rising frequency of antimicrobial resistance, management of Helicobacter pylori (H. pylori) infections is a challenge for physicians. We found global frequency rate of resistance is high in Africa. The most sensitive drug is rifabutin and the lowest sensitive drug is metronidazole in the world. The worldwide H. pylori antibiotic resistance to clarithromycin and levofloxacin has increased during the last 6 years. Resistances to antimicrobial agent’s reports describe dramatic decrease of antibiotics efficacy.
-
Citation: Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in
Helicobacter pylori : A recent literature review. World J Methodol 2015; 5(3): 164-174 - URL: https://www.wjgnet.com/2222-0682/full/v5/i3/164.htm
- DOI: https://dx.doi.org/10.5662/wjm.v5.i3.164
Helicobacter pylori (H. pylori) is a motile, curved and Gram negative bacillus[1]. H. pylori certainly is the most prevalent human infection, the frequency of infection due to H. pylori is nearly 50% in the world and in developing country is as high as 80%-90%[2]. This bacterium colonizes the stomach of human and its infection is correlated with gastritis, peptic ulcer disease and extra-digestive diseases[3,4]. H. pylori is also considered as a human carcinogen[5]. Since, H. pylori eradication therapy represents a key clinical essential. Unfortunately, therapy against H. pylori has turned out to be more difficult over the years, principally due to the great decrease of standard eradication therapies efficacy.
Although H. pylori is sensitive to many antibiotics in vitro, just a few antibiotics can be used in vivo to treat infected patients. Management of H. pylori infections are recommended in all suggestive individuals[6]. According to the latest Maastricht Guidelines, in regions of low clarithromycin resistance, clarithromycin-containing treatments are recommended for first-line empirical treatment[7]. In regions of high resistance to clarithromycin, the quadruple treatment including bismuth has been proposed as first-line treatment. In case of unavailability of this therapy, non-bismuth (three antibiotics plus Proton pump inhibitors) quadruple therapy and the so-called “sequential therapy” (that includes five days of PPIs plus amoxicillin followed by five more days of PPIs plus metronidazole and clarithromycin) have been recommended as an alternative[7]. Table 1 is shown mode of actions and resistance mechanisms of antibiotics used for treatment of H. pylori infection.
| Antibiotic | Mode of action | Resistance mechanisms |
| Metronidazole | Electron reduction processes, leads to the formation of nitro-anion radicals and subsequent DNA damage | (1) Poor drug uptake and/or increased drug efflux; (2) enhanced activity of DNA repair enzymes; (3) increased oxygen scavenging abilities; and (4) decreased antibiotic activation arising from changes in metronidazole-reducing enzymes[16] |
| Clarithromycin | The inhibition of protein synthesis by binding and slowing down the activity of the bacterial ribosomal unit[17] | rRNA-point mutations |
| Amoxicillin | The inhibition cell wall synthesis | pbp gene mutations, membrane permeability alterations and efflux pumps[17] |
| Tetracycline | Reversible inhibition protein synthesis | Three contiguous nucleotides mutation in the 16S rRNA gene[17] |
| Fluoroquinolones | Inhibiting DNA gyrase, type II topoisomerase, and topoisomerase IV[17] | Point mutations in the quinolones resistance determining regions |
| Rifabutin | Inhibits the b-subunit of H. pylori DNA-dependent RNA polymerase encoded by the rpoB gene[18] | Mutation of the rpoB gene[18] |
Failure of treatment in H. pylori infections has become an actual subject for physicians. The cause of treatment failure is many that can be grouped into microorganism-related factors, host-related factors and treatment-related factors. H. pylori resistance to antibiotic is widely recognized as the chief reason for treatment failure[1,8]. Furthermore, antibiotic resistance should be considered as a lively idea, since its prevalence can change not only among diverse countries, but also between two different periods in the same area[1,9-11]. The rate of antibiotic resistance in H. pylori has been evaluated worldwide. However, most researches originated from single center, included only a small number of bacteria, were often restricted to selected patients, and used different techniques to evaluate antibiotic susceptibility. Though, the investigation platform is luxurious; and only performed in few countries as: United Kingdom, German, Finland[12-18]. Antibiotic use for infections other than H. pylori is accounting for the extensive raise antibiotic resistance rate in H. pylori[19]. Because of the value of H. pylori therapy, antimicrobial susceptibility testing has been widely done. Since, H. pylori antibiotic resistance is fast growing worldwide, an eradication policy based on pre-treatment susceptibility testing is going to get more attractive than in the past[1,7].
The objective of this paper was to review previous studies about the rates of antimicrobial resistance in H. pylori isolates obtained from worldwide during last 6 years in order to evaluate the trend of antibiotic resistance.
In the present study, different computer-assisted searches were achieved using PubMed, MEDLINE, Science Direct, Google Scholar and Scielo. Separately searches were carried out on all English language literatures published through 2009 to 2014, by the key words: Helicobacter pylori, H. pylori, resistance, metronidazole, levofloxacin, amoxicillin, clarithromycin, tetracycline, and rifabutin. Full articles related searches were saved, and articles written in foreign languages were translated when essential. When more than one publication from the same author was obtainable, only new version, counting the whole population was enrolled. Two investigators (Ebrahimzadeh Leylabadlo H and Mohammadzadeh Asl Y) independently and in a blinded manner assessed the articles using pre-designed data extraction.
The following information was collected: (1) sum of bacteria incorporated; (2) rate of antibiotic resistant; and (3) the geographic area involved. The data were summarized in extraction table and analyzed manually. Finally, Excel 2007 software was used to draw charts.
During 6 years a total of 52008 H. pylori isolates meeting the inclusion criteria were identified. Eighty-seven studies from 2009 to 2014 on H. pylori antimicrobial resistance in the different countries were included; there were 43 Asian[20-62], 10 American[63-72], 5 African[73-77], and 29 European studies[78-106]. On the whole rates of H. pylori antibiotic resistance were 47.22% (30.5%-75.02%) for metronidazole, 19.74% (5.46%-30.8%) for clarithromycin, 18.94% (14.19%-25.28%) for levofloxacin, and 14.67% (2%-40.87%) for amoxicillin, 11.70% (0%-50%) for tetracycline, 11.5% (0%-23%) for furazolidon and 6.75% (1%-12.45%) for rifabutin. The frequency of resistance to antibiotics in various continents and countries are demonstrated in Tables 2 and 3, Figures 1 and 2.
| Region (n) | Cla | Amo | Met | Tet | Lev | Rif | Fur |
| % | % | % | % | % | % | % | |
| Asia (23748) | 27.46 | 23.61 | 46.57 | 7.38 | 25.28 | 12.45 | 23 |
| South America (587) | 12.88 | 6.56 | 52.85 | 0 | 21.23 | NR | 0 |
| North America (818) | 30.8 | 2 | 30.5 | 0 | 19 | NR | NR |
| Europe (26024) | 22.11 | 0.35 | 31.19 | 1.15 | 14.19 | 1 | NR |
| Africa (831) | 5.46 | 40.87 | 75.02 | 50 | 15 | NR | NR |
| Total (52008) | 19.74 | 14.67 | 47.22 | 11.70 | 18.94 | 6.75 | 11.5 |
| Countries | Year | Isolates | Cla | Amo | Met | Tet | Lev | Rif | Fur | Method | Ref. |
| (N) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | ||||
| 2014 | 95 | 33.7 | E-Test | [20] | |||||||
| 2013 | 82 | 17.1 | 9.8 | 64.4 | 0 | DDM | [21] | ||||
| 2013 | 78 | 15.3 | 6.4 | 55.1 | DDM | [22] | |||||
| Iran | 2012 | 150 | 34 | 10 | 78.6 | 9.3 | 5.3 | E-T,ADM | [23] | ||
| 2012 | 112 | 14.3 | 28.6 | 76.8 | 18.7 | 28.6 | DDM | [24] | |||
| 2011 | 197 | 45.2 | 23.9 | 65.5 | 37.1 | 61.4 | DDM | [25] | |||
| 2011 | 42 | 14.3 | 2.4 | 40.5 | 4.8 | ADM | [26] | ||||
| 2010 | 121 | 5 | 20 | 44 | 3 | E-Test | [27] | ||||
| 2010 | 132 | 30 | 6.8 | 73.4 | 9 | E-Test | [28] | ||||
| 2014 | 73 | 80.8 | 0 | 58.9 | 12.3 | E-Test | [29] | ||||
| 2013 | 17731 | 21.5 | 0.1 | 95.4 | 20.6 | 0.1 | ADM | [30] | |||
| China | 2011 | 73 | 84.9 | 0 | 61.6 | 0 | 13.7 | 6.8 | PCR | [31] | |
| 2010 | 374 | 37.2 | 0.3 | 63.9 | 1.2 | 50.3 | E-Test | [32] | |||
| 2009 | 36 | 8.3 | 33.3 | 94.4 | 0 | 16.7 | DDM | [33] | |||
| 2014 | 124 | 36.2 | 0 | 2.1 | E-Test | [34] | |||||
| Japan | 2014 | 135 | 25.9 | 20.7 | E-Test | [35] | |||||
| 2014 | 1073 | 31.1 | 40.2 | ADM | [36] | ||||||
| 2013 | 204 | 86.4 | 8.2 | 71.3 | 57 | ADM | [37] | ||||
| 2011 | 153 | 55.6 | PCR | [38] | |||||||
| 2010 | 61 | 36.1 | 0 | 14.8 | ADM | [39] | |||||
| 2014 | 212 | 8.5 | 9 | 36.3 | 0 | ADM | [40] | ||||
| South Korea | 2013 | 165 | 11.5 | 2.45 | 50.7 | 0 | 24.55 | ADM | [41] | ||
| 2013 | 150 | 6 | ADM | [42] | |||||||
| 2012 | 185 | 10.8 | 2.2 | 30.3 | 0.05 | ADM | [43] | ||||
| 2014 | 161 | 1.2 | 36.6 | E-Test | [44] | ||||||
| Malaysia | 2014 | 102 | 6.8 | 0 | 32.3 | 0 | 6.8 | 0 | E-Test | [45] | |
| 2011 | 90 | 0 | 0 | 75.5 | 0 | 14.4 | E-Test | [46] | |||
| 2011 | 187 | 2.1 | 0 | 37.4 | 0 | 1 | E-Test | [47] | |||
| 2009 | 187 | 2.1 | E-Test | [48] | |||||||
| 2014 | 46 | 47.8 | 54.3 | 73.9 | 4.3 | E-Test | [49] | ||||
| Pakistan | 2012 | 178 | 36 | 37 | 89 | 12 | ADM | [50] | |||
| 2010 | 92 | 33 | 2 | 48 | E-Test | [51] | |||||
| 2014 | 98 | 23.5 | 3.9 | 11.7 | DDM | [52] | |||||
| 2012 | 149 | 18.2 | 0 | 45.5 | 18.2 | E-Test | [53] | ||||
| Turkey | 2012 | 61 | 21.3 | 0 | 42.6 | 9.1 | 3.3 | DDM | [54] | ||
| 2009 | 31 | 41.9 | 3.2 | 41.9 | 3.2 | E-Test | [55] | ||||
| 2009 | 38 | 13.5 | ADM | [56] | |||||||
| Taiwan | 2014 | 61 | 35.3 | 0 | 17.6 | 0 | 23.5 | E-Test | [57] | ||
| 2009 | 180 | 10.6 | 0 | 26.7 | 9.4 | E-Test | [58] | ||||
| Thailand | 2009 | 120 | 29.2 | PCR | [59] | ||||||
| UAE | 2010 | 26 | 19.2 | E-Test | [60] | ||||||
| India | 2014 | 80 | 58.8 | 72.5 | 83.8 | 53.8 | 13.8 | 13.8 | DDM | [61] | |
| Vietnam | 2013 | 103 | 33 | 0 | 69.9 | 5.8 | 18.4 | E-Test | [62] | ||
| South American | |||||||||||
| 2014 | 54 | 11.1 | 1.9 | E-Test | [63] | ||||||
| Brazil | 2013 | 77 | 19.5 | 10.4 | 40 | 0 | 0 | ADM | [64] | ||
| 2011 | 39 | 8 | 0 | 51 | 0 | 23 | 0 | ADM | [65] | ||
| Colombia | 2012 | 203 | 19.8 | 20.5 | ADM | [66] | |||||
| Cuba | 2010 | 40 | 10 | 85 | E-Test | [67] | |||||
| PCR | |||||||||||
| Peru | 2011 | 95 | 36.9 | ADM | [68] | ||||||
| DDM | |||||||||||
| Uruguay | 2009 | 79 | 8.9 | 0 | 35.4 | 0 | 3.8 | E-Test | [69] | ||
| North America | |||||||||||
| Mexico | 2011 | 90 | 5.5 | 19 | E-Test | [70] | |||||
| Canada | 2009 | 42 | 57 | E-Test | [71] | ||||||
| United States | 2011 | 686 | 30 | 2 | 42 | 0 | 19 | E-Test | [72] | ||
| ADM | |||||||||||
| Senegal | 2013 | 108 | 1 | 0 | 85 | 0 | 15 | E-test | [73] | ||
| DDM | |||||||||||
| Nigeria | 2009 | 186 | 66 | 95 | 100 | E-test | [74] | ||||
| Gambia | 2012 | 64 | 0 | 68.8 | ADM | [75] | |||||
| Tunisia | 2010 | 273 | 15.4 | 0 | 51.3 | E-test | [76] | ||||
| South Africa | 2010 | 200 | 97.5 | ADM | [77] | ||||||
| DDM | |||||||||||
| 2014 | 1651 | 6.7 | 29.4 | E-test | [78] | ||||||
| Germany | 2013 | 5296 | 67.1 | 0 | 67.1 | 24.9 | E-test | [79] | |||
| 2013 | 436 | 7.5 | 0 | 32.7 | 11.7 | E-test | [80] | ||||
| Italy | 2012 | 111 | 35.2 | 59.3 | 22.1 | E-test | [81] | ||||
| 2011 | 253 | 9.9 | PCR | [82] | |||||||
| England | 2009 | 255 | 1 | E-test DDM | [83] | ||||||
| 2013 | 343 | 23.5 | 33 | E-test | [84] | ||||||
| Spain | 2011 | 71 | 14.7 | 1.4 | 45.1 | 0 | 14.5 | E-test | [85] | ||
| 2010 | 118 | 35.6 | E-test | [86] | |||||||
| 2009 | 101 | 54.6 | 35.7 | E-test | [87] | ||||||
| Norway | 2012 | 102 | 5.9 | 0 | 22.5 | 0 | E-test | [88] | |||
| Finland | 2010 | 505 | 8 | 0 | 41 | 7 | E-test | [89] | |||
| 2013 | 588 | 20.1 | 34.5 | 2.6 | ADM | [90] | |||||
| Bulgaria | 2011 | 519 | 17.9 | 29.5 | 4 | ADM | [91] | ||||
| 2009 | 1057 | 18.7 | 0.5 | 21.35 | 3.15 | ADM | [92] | ||||
| Croatia | 2012 | 382 | 11.9 | 0.6 | 10.1 | E-test | [93] | ||||
| 2014 | 210 | 8.1 | E-test | [94] | |||||||
| Poland | 2013 | 165 | 10.9 | 32.7 | 1.2 | E-test | [95] | ||||
| 2012 | 51 | 22 | 16 | E-test | [96] | ||||||
| 2011 | 115 | 34 | 0 | 44 | 5 | E-test | [97] | ||||
| Portugal | 2014 | 180 | 50 | 0.6 | 34.4 | 0.6 | 33.9 | E-test | [98] | ||
| 2011 | 1115 | 34.7 | 0 | 13.9 | 0 | E-test | [99] | ||||
| Belgium | 2013 | 189 | 13.3 | 0.8 | 26.1 | [100] | |||||
| 2011 | 10670 | 20.3 | 0 | 27 | ADM | [101] | |||||
| Netherlands | 2014 | 417 | 6.14 | 10.1 | E-test | [102] | |||||
| 2013 | 746 | 20.5 | 0.68 | 19.9 | E-test | [103] | |||||
| Ireland | 2013 | 85 | 0 | 11.7 | 0 | E-test | [104] | ||||
| 2010 | 219 | 13.2 | 31.5 | E-test | [105] | ||||||
| Southern Europe | 2014 | 74 | 34.7 | 16.7 | E-test | [106] |
Monitoring of resistance to antimicrobial agents is important for H. pylori infections therapy in medical practice[17]. Resistance to antimicrobial agents creates at risk H. pylori eradication in the world[10,98]. The most recent recommendations on H. pylori therapy suggested that initially management had better be personalized based on clarithromycin and metronidazole resistance. In fact, fourteen days triple-therapy is recommended in area where resistance to clarithromycin is more than 15% to 20%, if resistance to metronidazole is more than 40%, the association with amoxicillin is preferred[17]. At the present, due to H. pylori antibiotics resistance, eradication therapy appears was not carried out as simple as and we are now founded many failures which make the use of standard therapy unacceptable in many parts of the world[107]. This article systematically studied the latest data on H. pylori resistance to antibiotic.
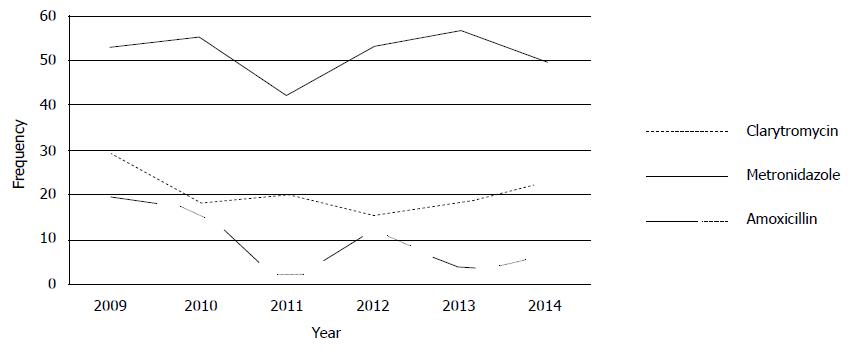
Because clarithromycin is the most potent antibiotic involved in the management of H. pylori infections, resistance to clarithromycin is important[8,17,105]. As presented in Table 2, the rate of clarithromycin resistance was 19.74%, and occurrence of clarithromycin resistance is increasing worldwide (Figure 2). The rate of clarithromycin resistance has been broadly studied, and information are on hand from nearly all areas in the world: it ranges from 5.46% to 30.8% (Figure 1).
In European regions, the lowest clarithromycin resistance was reported from Norway (5.9%), whilst the highest in Spain (32.01%) and Portugal (42.35%). European studies performed at the past 6 years intervals reported that H. pylori resistance decrease from 36.65% in 2009 to 24.38% in 2014. In Asian regions, a surprising clarithromycin resistance frequency was reported from India (58.8%) and China (46.54%), whereas the lowest rate was discovered in Malaysia (2.4%). An increase in clarithromycin resistance has been faced in the Asia, from 15.28% in 2009 to 32.46% in 2014, probably in the Asian countries macrolid drugs used more. In recent years due to widespread use of clarithromycin for respiratory infections in the public especially in children, clarithromycin resistance has augmented in diverse regions, and there is an association between outpatient use of long-acting macrolide and clarithromycin resistance[10,17,108].
In conclusion, the highest clarithromycin resistant area was North America, and this study showed a slight increasing tendency of clarithromycin resistance of H. pylori in the world. Since clarithromycin is the most potent antimicrobial agent involved in the standard treatment protocol as well as the resistance rates were still at the low level, where clarithromycin-containing triple therapies could be used empirically.
Metronidazole is used against H. pylori infections and is one of the few antibacterial agents as drug of choice that is effective in eradicated the microorganism. Some researcher reported that the rate of treatment failure is more than 20% with triple therapy in which metronidazole is the drug of choice, also H. pylori resistance to metronidazole is the chief solitary reason responsible for management failure[109,110].
Metronidazole resistance is the most common antibiotic resistance in H. pylori and overall metronidazole resistance found in 47.22% in descending order in Africa 75.02%, South America 52.85%, Asia 46.57%, Europe 31.19%, to 30.5% in North America. In developed countries about 30% of the H. pylori strains are metronidazole resistant, whereas in developing countries, the occurrence of resistance is very high. This association between metronidazole resistance and socioeconomic state level is maybe due to use of metronidazole and related drugs for gynecological, dental and parasitic related infectious diseases[13,111]. The comparison of results indicated that resistance to metronidazole have remained significantly unchanging in Asian, European and North American countries but is increasing in African countries (51.3% in 2010 to 85% in 2013). Furthermore metronidazole resistance in 2014 has stayed approximately at the similar level as in early 2009 in Europe. So, in accordance with latest guidelines, metronidazole is favored to amoxicillin in first-line therapy in Asian, Europe and North American but not in African patients.
Amoxicillin is suggested for anti-H. pylori triple therapy in region where metronidazole resistance is high. Universal resistance to amoxicillin is uncommon; it was detected in 14.67%. The frequency of amoxicillin resistance extensively differs in Asian regions, ranging from zero in Malaysia, Taiwan and Vietnam to 72.5% in India. The rate of amoxicillin resistance in Africa was 40.87%.
The prevalence of amoxicillin resistance in Europe countries and North American is low from zero in certain area as Finland, Germany, Norway and Poland, 1.4% in Spain to 2% in United States. It seems the government policy possibly to limit the use of antibiotic for infectious diseases in European and North American countries. The incidence of amoxicillin resistance in H. pylori seems to increase specially in Asia and South America, where these antibiotics can be obtained without prescription. H. pylori resistance rates of 97.5%, 72.5%, 66% and 20.5% for amoxicillin have recently been reported in South Africa, India, Nigeria and Colombia, respectively.
Among the 4 most common used antimicrobial agents, tetracycline resistance was the lowest (Table 3). In general H. pylori resistance to tetracycline was detected 11.7% in the world. The total rate of tetracycline resistance did not vary in South America and North America (the resistance was absent), whilst it was relatively high in Africa (50%). In Asia, the resistance was absent in Thailand, and very low in China (0.6%) and South Korea (0.01%). In contrast, increased values were found in India (53.8%), and Iran (11.7%). The prevalence of tetracycline resistance stays very low (less than 7.4%) in almost most parts of the world except for Africa. The comparison of data showed that tetracycline resistance is decreasing in the world, 26.85% in 2009 to 6.11% in 2014.
Tetracycline is a bacteriostatic and broad spectrum antimicrobial agent that is active against H. pylori and tetracycline is the most generally used antibiotic for treatment of H. pylori and other infectious diseases[109]. Tetracycline is extensively used in many countries, but resistance to this antibiotic has not become a great problem yet. Management failure owing to the tetracycline resistant has been reported[112,113], though there is not enough data obtainable until now to determine the impact of this resistance on management success.
However, the study on H. pylori rifabutin resistance is inadequate and in South America, North America and Africa has not been done during previous 6 years. The rate of rifabutin resistance was higher in Asia (12.45%) as compared to Europe (1%). The frequency of rifabutin resistance differs in Asian countries, ranging from 28.6% in Iran to about 7% in China and Malaysia. Rifabutin is structurally related to rifampin group, and it has potential efficacy against H. pylori[114]. Rifabutin is usually used to treat mycobacterium diseases, so the secondary resistance of H. pylori to rifabutin is not currently expected in the healthy people.
Generally, resistance to levofloxacin is low (< 19%) worldwide. The prevalence rate was higher in Asia (25.28%) and South America (21.23%) as compared to Africa and Europe (less than 15%). The frequency of levofloxacin resistance widely differs in Asian regions, about 57% in Japan, 24.55% in South Korea, 5.3% in Iran and 2.6% in Malaysia. In addition the levofloxacin resistance rate differs between European countries, ranging from 7% to 33.9%. The rate of levofloxacin resistance seems to be increasing universal from 4.25% in 2009 to 17.55% in 2014. Furthermore, during the past 3 years levofloxacin resistance rates have even been more increasing.
Due to the dramatic increase in clarithromycin resistance, levofloxacin, a wide spectrum quinolone, has been used as an option of clarithromycin in some regimens. But the frequent use of quinolones for urinary tract infections has increased the incidence of H. pylori resistance in the world[17]. Failure of therapy due to levofloxacin resistance and the emerging development of quinolones resistance, use of levofloxacin as first-line therapy is generally discouraged, and its utilize should be reserved as a second-line or save regimens after failure of a clarithromycin and/or a metronidazole based regimen[7,80].
The study on furazolidon resistance was not widely performed in the world, and in Europe, North America and Africa has not been achieved during past 6 years. The rate of furazolidon resistance was higher in Asia (13.8%) as compared to South America (0%). The rate of furazolidon resistance broadly differs in Asia, from 61.4% in Iran to 16.8% in China and 13.8% in India. Furazolidon is a cheap and synthetic nitrofuran with a wide spectrum activities usually used in the treatment of bacterial and protozoa infections. Since high H. pylori resistance to metronidazole in some region as China and South America, furazolidon sometimes has been used as an option for H. pylori infections[65]. However some researchers were reported that the rate of cure with furazolidon-based regimens is low and a large amount of furazolidon increases the therapy rate but it significantly raises complications[81].
The prevalence of H. pylori metronidazole resistance is at a high level, and resistance to clarithromycin and levofloxacin is increasing worldwide. The most effective drug is rifabutin and the lowest sensitive drug is metronidazole. Resistance to levofloxacin does not show any region difference. There are no studies regarding rifabutin and furazolidon resistance of H. pylori in America and Africa. According to the present findings, the mean resistance rate in H. pylori isolated from European and North American patients is lower than other countries. The rate of tetracycline, metronidazole and amoxicillin resistance is higher in African patients, while clarithromycin and levofloxacin resistance is higher in North America and Asian patients. In conclusion, antibiotic resistance is increasing, so empirical therapy must be based on information of antimicrobial drug resistance, and this paper highlight a steady worldwide surveillance of H. pylori antibiotic resistance.
Helicobacter pylori (H. pylori) is a most important human pathogen associated with significant disease and fatality.
Due to the rising frequency of antimicrobial resistance, management of H. pylori remains a challenge for physicians in most parts of the world.
Search was carried out about H. pylori antimicrobial resistance literatures published through 2009 to 2014.
The frequency of antibiotic resistance is increasing, and this article highlight a steady worldwide surveillance of H. pylori antibiotic resistance.
This is a systematic review article on H. pylori resistance to antibiotics. The manuscript is well written and the topic of interest.
P- Reviewer: Franceschi F, Gao ZJ, Safaei HG, Yuan Y S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Rafeey M, Ghotaslou R, Nikvash S, Hafez AA. Primary resistance in Helicobacter pylori isolated in children from Iran. J Infect Chemother. 2007;13:291-295. [PubMed] |
| 2. | Ghotaslou R, Milani M, Akhi MT, Hejazi MS, Nahaei MR, Hasani A, Sharifi Y. Relationship between drug resistance and cagA Gene in Helicobacter pylori. Jundishapur J Microbiol. 2013;6:8480. |
| 3. | Ghotaslou R, Milani M, Akhi MT, Nahaei MR, Hasani A, Hejazi MS, Meshkini M. Diversity of Helicobacter Pylori cagA and vacA Genes and Its Relationship with Clinical Outcomes in Azerbaijan, Iran. Adv Pharm Bull. 2013;3:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 4. | Gasbarrini G, Racco S, Franceschi F, Miele L, Cammarota G, Grieco A, Gasbarrini A. [Helicobacter pylori infection: from gastric to systemic disease]. Recenti Prog Med. 2010;101:27-33. [PubMed] |
| 5. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] |
| 6. | Smith SM, O’Morain C, McNamara D. Antimicrobial susceptibility testing for Helicobacter pylori in times of increasing antibiotic resistance. World J Gastroenterol. 2014;20:9912-9921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 7. | Cammarota G, Ianiro G, Bibbò S, Di Rienzo TA, Masucci L, Sanguinetti M, Gasbarrini A. Culture-guided treatment approach for Helicobacter pylori infection: review of the literature. World J Gastroenterol. 2014;20:5205-5211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Di Mario F, Cavallaro LG, Scarpignato C. ‘Rescue’ therapies for the management of Helicobacter pylori infection. Dig Dis. 2006;24:113-130. [PubMed] |
| 9. | Seo JH, Jun JS, Yeom JS, Park JS, Youn HS, Ko GH, Baik SC, Lee WK, Cho MJ, Rhee KH. Changing pattern of antibiotic resistance of Helicobacter pylori in children during 20 years in Jinju, South Korea. Pediatr Int. 2013;55:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Boltin D, Ben-Zvi H, Perets TT, Kamenetsky Z, Samra Z, Dickman R, Niv Y. Trends in secondary antibiotic resistance of Helicobacter pylori from 2007 to 2014: has the tide turned? J Clin Microbiol. 2015;53:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Kupcinskas L, Rasmussen L, Jonaitis L, Kiudelis G, Jørgensen M, Urbonaviciene N, Tamosiunas V, Kupcinskas J, Miciuleviciene J, Kadusevicius E. Evolution of Helicobacter pylori susceptibility to antibiotics during a 10-year period in Lithuania. APMIS. 2013;121:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Cameron EA, Powell KU, Baldwin L, Jones P, Bell GD, Williams SG. Helicobacter pylori: antibiotic resistance and eradication rates in Suffolk, UK, 1991-2001. J Med Microbiol. 2004;53:535-538. [PubMed] |
| 13. | Glupczynski Y, Mégraud F, Lopez-Brea M, Andersen LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2001;20:820-823. [PubMed] |
| 14. | Kist M, Glocker E. ResiNet - A nationwide German sentinel study for surveillance and analysis of antimicrobial resistance in Helicobacter pylori. Eurosurveillance. 2004;9:44-46. |
| 15. | Koivisto TT, Rautelin HI, Voutilainen ME, Niemelä SE, Heikkinen M, Sipponen PI, Färkkilä MA. Primary Helicobacter pylori resistance to metronidazole and clarithromycin in the Finnish population. Aliment Pharmacol Ther. 2004;19:1009-1017. [PubMed] |
| 16. | Gerrits MM, van der Wouden EJ, Bax DA, van Zwet AA, van Vliet AH, de Jong A, Kusters JG, Thijs JC, Kuipers EJ. Role of the rdxA and frxA genes in oxygen-dependent metronidazole resistance of Helicobacter pylori. J Med Microbiol. 2004;53:1123-1128. [PubMed] |
| 17. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] |
| 18. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1588] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 19. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Keshavarz Azizi Raftar S, Moniri R, Saffari M, Razavi Zadeh M, Arj A, Mousavi SG, Mirzaei Ghazi Kalayeh H, Dastehgoli K. The Helicobacter pylori resistance rate to clarithromycin in Iran. Microb Drug Resist. 2015;21:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Zendedel A, Moradimoghadam F, Almasi V, Zivarifar H. Antibiotic resistance of Helicobacter pylori in Mashhad, Iran. J Pak Med Assoc. 2013;63:336-339. [PubMed] |
| 22. | Khademi F, Faghri J, Poursina F, Esfahani BN, Moghim S, Fazeli H, Adibi P, Mirzaei N, Akbari M, Safaei HG. Resistance pattern of Helicobacter pylori strains to clarithromycin, metronidazole, and amoxicillin in Isfahan, Iran. J Res Med Sci. 2013;18:1056-1060. [PubMed] |
| 23. | Talebi Bezmin Abadi A, Ghasemzadeh A, Taghvaei T, Mobarez AM. Primary resistance of Helicobacter pylori to levofloxacin and moxifloxacine in Iran. Intern Emerg Med. 2012;7:447-452. [PubMed] |
| 24. | Milani M, Ghotaslou R, Akhi MT, Nahaei MR, Hasani A, Somi MH, Rafeey M, Sharifi Y. The status of antimicrobial resistance of Helicobacter pylori in Eastern Azerbaijan, Iran: comparative study according to demographics. J Infect Chemother. 2012;18:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Abadi AT, Taghvaei T, Mobarez AM, Carpenter BM, Merrell DS. Frequency of antibiotic resistance in Helicobacter pylori strains isolated from the northern population of Iran. J Microbiol. 2011;49:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Shokrzadeh L, Jafari F, Dabiri H, Baghaei K, Zojaji H, Alizadeh AH, Aslani MM, Zali MR. Antibiotic susceptibility profile of Helicobacter pylori isolated from the dyspepsia patients in Tehran, Iran. Saudi J Gastroenterol. 2011;17:261-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Farshad S, Alborzi A, Japoni A, Ranjbar R, Hosseini Asl K, Badiee P, Amin Shahidi M, Hosseini M. Antimicrobial susceptibility of Helicobacter pylori strains isolated from patients in Shiraz, Southern Iran. World J Gastroenterol. 2010;16:5746-5751. [PubMed] |
| 28. | Talebi Bezmin Abadi A, Mobarez AM, Taghvaei T, Wolfram L. Antibiotic resistance of Helicobacter pylori in Mazandaran, North of Iran. Helicobacter. 2010;15:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Guo S, He L, Zhang J, Zhou L, Ding Z, Zhang J, Yu F, Wang G, Zhou J, Guan D. [Antibiotic resistance of Helicobacter pylori in children and macrolide-resistant genotypes in Helicobacter pylori]. Zhonghua Yi Xue Za Zhi. 2014;94:563-566. [PubMed] |
| 30. | Su P, Li Y, Li H, Zhang J, Lin L, Wang Q, Guo F, Ji Z, Mao J, Tang W. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Liu G, Xu X, He L, Ding Z, Gu Y, Zhang J, Zhou L. Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter. 2011;16:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Huang LP, Zhuang ML, Gu CP. [Antimicrobial resistance of 36 strains of Helicobacter pylori in adolescents]. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11:210-212. [PubMed] |
| 34. | Nishizawa T, Maekawa T, Watanabe N, Harada N, Hosoda Y, Yoshinaga M, Yoshio T, Ohta H, Inoue S, Toyokawa T. Clarithromycin Versus Metronidazole as First-line Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Controlled Study in Japan. J Clin Gastroenterol. 2015;49:468-471. [PubMed] |
| 35. | Morimoto N, Takeuchi H, Nishida Y, Morisawa M, Yoshikawa T, Morita T, Morimoto M, Sugimoto C, Matsumura Y, Sugiura T. Clinical Application of the DiversiLab Microbial Typing System Using Repetitive Sequence-Based PCR for Characterization of Helicobacter pylori in Japan. J Clin Lab Anal. 2015;29:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Okamura T, Suga T, Nagaya T, Arakura N, Matsumoto T, Nakayama Y, Tanaka E. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter. 2014;19:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Murakami K, Furuta T, Ando T, Nakajima T, Inui Y, Oshima T, Tomita T, Mabe K, Sasaki M, Suganuma T. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Yamade M, Sugimoto M, Uotani T, Nishino M, Kodaira C, Furuta T. Resistance of Helicobacter pylori to quinolones and clarithromycin assessed by genetic testing in Japan. J Gastroenterol Hepatol. 2011;26:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Kato S, Fujimura S. Primary antimicrobial resistance of Helicobacter pylori in children during the past 9 years. Pediatr Int. 2010;52:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Yoon KH, Park SW, Lee SW, Kim BJ, Kim JG. Clarithromycin-based standard triple therapy can still be effective for Helicobacter pylori eradication in some parts of the Korea. J Korean Med Sci. 2014;29:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | An B, Moon BS, Kim H, Lim HC, Lee YC, Lee G, Kim SH, Park M, Kim JB. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann Lab Med. 2013;33:415-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Kim BJ, Kim JG. Substitutions in penicillin-binding protein 1 in amoxicillin-resistant Helicobacter pylori strains isolated from Korean patients. Gut Liver. 2013;7:655-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Chung JW, Lee GH, Jeong JY, Lee SM, Jung JH, Choi KD, Song HJ, Jung HY, Kim JH. Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol. 2012;27:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Alfizah H, Norazah A, Hamizah R, Ramelah M. Resistotype of Helicobacter pylori isolates: the impact on eradication outcome. J Med Microbiol. 2014;63:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Teh X, Khosravi Y, Lee WC, Leow AH, Loke MF, Vadivelu J, Goh KL. Functional and molecular surveillance of Helicobacter pylori antibiotic resistance in Kuala Lumpur. PLoS One. 2014;9:e101481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Goh KL, Navaratnam P. High Helicobacter pylori resistance to metronidazole but zero or low resistance to clarithromycin, levofloxacin, and other antibiotics in Malaysia. Helicobacter. 2011;16:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Ahmad N, Zakaria WR, Mohamed R. Analysis of antibiotic susceptibility patterns of Helicobacter pylori isolates from Malaysia. Helicobacter. 2011;16:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 48. | Ahmad N, Zakaria WR, Abdullah SA, Mohamed R. Characterization of clarithromycin resistance in Malaysian isolates of Helicobacter pylori. World J Gastroenterol. 2009;15:3161-3165. [PubMed] |
| 49. | Rasheed F, Campbell BJ, Alfizah H, Varro A, Zahra R, Yamaoka Y, Pritchard DM. Analysis of clinical isolates of Helicobacter pylori in Pakistan reveals high degrees of pathogenicity and high frequencies of antibiotic resistance. Helicobacter. 2014;19:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Khan A, Farooqui A, Manzoor H, Akhtar SS, Quraishy MS, Kazmi SU. Antibiotic resistance and cagA gene correlation: a looming crisis of Helicobacter pylori. World J Gastroenterol. 2012;18:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Yakoob J, Abid S, Abbas Z, Jafri SN. Antibiotic susceptibility patterns of Helicobacter pylori and triple therapy in a high-prevalence area. Br J Biomed Sci. 2010;67:197-201. [PubMed] |
| 52. | Karabiber H, Selimoglu MA, Otlu B, Yildirim O, Ozer A. Virulence factors and antibiotic resistance in children with Helicobacter pylori gastritis. J Pediatr Gastroenterol Nutr. 2014;58:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Cağdaş U, Otağ F, Tezcan S, Sezgin O, Aslan G, Emekdaş G. [Detection of Helicobacter pylori and antimicrobial resistance in gastric biopsy specimens]. Mikrobiyol Bul. 2012;46:398-409. [PubMed] |
| 54. | Ozbey G, Bahcecioglu IH, Acik MN. Resistance Rates to Various Antimicrobial Agents of Helicobacter pylori Isolates in Eastern Turkey. IJMCM. 2012;2:148-152. |
| 55. | Bakir Ozbey S, Ozakin C, Keskin M. [Antibiotic resistance rates of Helicobacter pylori isolates and the comparison of E-test and fluorescent in situ hybridization methods for the detection of clarithromycin resistant strains]. Mikrobiyol Bul. 2009;43:227-234. [PubMed] |
| 56. | Safak B, Ciftci IH, Ozdemir M, Kiyildi N, Cetinkaya Z, Aktepe OC, Altindis M, Asik G. In vitro anti-Helicobacter pylori activity of usnic acid. Phytother Res. 2009;23:955-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 57. | Liang CM, Cheng JW, Kuo CM, Chang KC, Wu KL, Tai WC, Chiu KW, Chiou SS, Lin MT, Hu TH. Levofloxacin-containing second-line anti-Helicobacter pylori eradication in Taiwanese real-world practice. Biomed J. 2014;37:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Chang WL, Sheu BS, Cheng HC, Yang YJ, Yang HB, Wu JJ. Resistance to metronidazole, clarithromycin and levofloxacin of Helicobacter pylori before and after clarithromycin-based therapy in Taiwan. J Gastroenterol Hepatol. 2009;24:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Tanuma M, Rimbara E, Noguchi N, Boonyaritichaikij S, Kuwabara K, Fukunaga Y, Sasatsu M. Analysis of clarithromycin resistance and CagA status in Helicobacter pylori by use of feces from children in Thailand. J Clin Microbiol. 2009;47:4144-4145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Alfaresi MS, Elkoush AA. Characterization of clarithromycin resistance in isolates of Helicobacter pylori from the UAE. Indian J Gastroenterol. 2010;29:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Pandya HB, Agravat HH, Patel JS, Sodagar NR. Emerging antimicrobial resistance pattern of Helicobacter pylori in central Gujarat. Indian J Med Microbiol. 2014;32:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Binh TT, Shiota S, Nguyen LT, Ho DD, Hoang HH, Ta L, Trinh DT, Fujioka T, Yamaoka Y. The incidence of primary antibiotic resistance of Helicobacter pylori in Vietnam. J Clin Gastroenterol. 2013;47:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Picoli SU, Mazzoleni LE, Fernández H, De Bona LR, Neuhauss E, Longo L, Prolla JC. Resistance to amoxicillin, clarithromycin and ciprofloxacin of Helicobacter pylori isolated from Southern Brazil patients. Rev Inst Med Trop Sao Paulo. 2014;56:197-200. [PubMed] |
| 64. | Ogata SK, Godoy AP, da Silva Patricio FR, Kawakami E. High Helicobacter pylori resistance to metronidazole and clarithromycin in Brazilian children and adolescents. J Pediatr Gastroenterol Nutr. 2013;56:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Eisig JN, Silva FM, Barbuti RC, Navarro-Rodriguez T, Moraes-Filho JP, Pedrazzoli Jr J. Helicobacter pylori antibiotic resistance in Brazil: clarithromycin is still a good option. Arq Gastroenterol. 2011;48:261-264. [PubMed] |
| 66. | Figueroa M, Cortés A, Pazos Á, Bravo LE. [Antimicrobial susceptibility of Helicobacter pylori with chronic gastritis]. Biomedica. 2012;32:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 67. | Llanes R, Soria C, Nagashima S, Kobayashi N, Gala A, Guzmán D, Feliciano O, Valdés L, Gutiérrez O, Fernández H. Phenotypic and genetic characterization of antimicrobial profiles of Helicobacter pylori strains in Cuba. J Health Popul Nutr. 2010;28:124-129. [PubMed] |
| 68. | Mochizuki Tamayo H, Noriega Aldave AP. [Antimicrobial susceptibility of helicobacter pylori to levofloxacin determined in a miniwell format and disk diffusion tests using egg yolk agar]. Rev Gastroenterol Peru. 2011;31:224-229. [PubMed] |
| 69. | Torres-Debat ME, Pérez-Pérez G, Olivares A, Fernández L, Raisler K, González N, Stein S, Bazet MC, Alallón W, Cohen H. Antimicrobial susceptibility of Helicobacter pylori and mechanisms of clarithromycin resistance in strains isolated from patients in Uruguay. Rev Esp Enferm Dig. 2009;101:757-762. [PubMed] |
| 70. | Ayala G, Galván-Portillo M, Chihu L, Fierros G, Sánchez A, Carrillo B, Román A, López-Carrillo L, Silva-Sánchez J. Resistance to antibiotics and characterization of Helicobacter pylori strains isolated from antrum and body from adults in Mexico. Microb Drug Resist. 2011;17:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Slinger R, Yan L, Chan F, Forward K, Cooper-Lesins G, Best L, Haldane D, Veldhuyzen van Zanten S. Pyrosequencing assay to rapidly detect clarithromycin resistance mutations in Canadian Helicobacter pylori isolates. Can J Gastroenterol. 2009;23:609-612. [PubMed] |
| 72. | Tveit AH, Bruce MG, Bruden DL, Morris J, Reasonover A, Hurlburt DA, Hennessy TW, McMahon B. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska Native persons from 2000 to 2008. J Clin Microbiol. 2011;49:3638-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Seck A, Burucoa C, Dia D, Mbengue M, Onambele M, Raymond J, Breurec S. Primary antibiotic resistance and associated mechanisms in Helicobacter pylori isolates from Senegalese patients. Ann Clin Microbiol Antimicrob. 2013;12:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Oyedeji KS, Smith SI, Coker AO, Arigbabu AO. Antibiotic susceptibility patterns in Helicobacter pylori strains from patients with upper gastrointestinal pathology in western Nigeria. Br J Biomed Sci. 2009;66:10-13. [PubMed] |
| 75. | Secka O, Berg DE, Antonio M, Corrah T, Tapgun M, Walton R, Thomas V, Galano JJ, Sancho J, Adegbola RA. Antimicrobial susceptibility and resistance patterns among Helicobacter pylori strains from The Gambia, West Africa. Antimicrob Agents Chemother. 2013;57:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Ben Mansour K, Burucoa C, Zribi M, Masmoudi A, Karoui S, Kallel L, Chouaib S, Matri S, Fekih M, Zarrouk S. Primary resistance to clarithromycin, metronidazole and amoxicillin of Helicobacter pylori isolated from Tunisian patients with peptic ulcers and gastritis: a prospective multicentre study. Ann Clin Microbiol Antimicrob. 2010;9:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Tanih NF, Okeleye BI, Naidoo N, Clarke AM, Mkwetshana N, Green E, Ndip LM, Ndip RN. Marked susceptibility of South African Helicobacter pylori strains to ciprofloxacin and amoxicillin: clinical implications. S Afr Med J. 2010;100:49-52. [PubMed] |
| 78. | Wüppenhorst N, Draeger S, Stüger HP, Hobmaier B, Vorreiter J, Kist M, Glocker EO. Prospective multicentre study on antimicrobial resistance of Helicobacter pylori in Germany. J Antimicrob Chemother. 2014;69:3127-3133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Wueppenhorst N, Stueger HP, Kist M, Glocker EO. High secondary resistance to quinolones in German Helicobacter pylori clinical isolates. J Antimicrob Chemother. 2013;68:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Selgrad M, Meissle J, Bornschein J, Kandulski A, Langner C, Varbanova M, Wex T, Tammer I, Schlüter D, Malfertheiner P. Antibiotic susceptibility of Helicobacter pylori in central Germany and its relationship with the number of eradication therapies. Eur J Gastroenterol Hepatol. 2013;25:1257-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Saracino IM, Zullo A, Holton J, Castelli V, Fiorini G, Zaccaro C, Ridola L, Ricci C, Gatta L, Vaira D. High prevalence of primary antibiotic resistance in Helicobacter pylori isolates in Italy. J Gastrointestin Liver Dis. 2012;21:363-365. [PubMed] |
| 82. | De Francesco V, Giorgio F, Ierardi E, Zotti M, Neri M, Milano A, Varasano V, Luzza F, Suraci E, Marmo R. Primary clarithromycin resistance in Helicobacter pylori: the Multicentric Italian Clarithromycin Resistance Observational (MICRO) study. J Gastrointestin Liver Dis. 2011;20:235-239. [PubMed] |
| 83. | Chisholm SA, Owen RJ. Frequency and molecular characteristics of ciprofloxacin- and rifampicin-resistant Helicobacter pylori from gastric infections in the UK. J Med Microbiol. 2009;58:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 85. | Cuadrado-Lavín A, Salcines-Caviedes JR, Carrascosa MF, Mellado P, Monteagudo I, Llorca J, Cobo M, Campos MR, Ayestarán B, Fernández-Pousa A. Antimicrobial susceptibility of Helicobacter pylori to six antibiotics currently used in Spain. J Antimicrob Chemother. 2012;67:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 86. | Agudo S, Pérez-Pérez G, Alarcón T, López-Brea M. High prevalence of clarithromycin-resistant Helicobacter pylori strains and risk factors associated with resistance in Madrid, Spain. J Clin Microbiol. 2010;48:3703-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 87. | Agudo S, Alarcón T, Cibrelus L, Urruzuno P, Martínez MJ, López-Brea M. [High percentage of clarithromycin and metronidazole resistance in Helicobacter pylori clinical isolates obtained from Spanish children]. Rev Esp Quimioter. 2009;22:88-92. [PubMed] |
| 88. | Larsen AL, Ragnhildstveit E, Moayeri B, Eliassen L, Melby KK. Resistance rates of metronidazole and other antibacterials in Helicobacter pylori from previously untreated patients in Norway. APMIS. 2013;121:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Kostamo P, Veijola L, Oksanen A, Sarna S, Rautelin H. Recent trends in primary antimicrobial resistance of Helicobacter pylori in Finland. Int J Antimicrob Agents. 2011;37:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Boyanova L, Ilieva J, Gergova G, Evstatiev I, Nikolov R, Mitov I. Living in Sofia is associated with a risk for antibiotic resistance in Helicobacter pylori: a Bulgarian study. Folia Microbiol (Praha). 2013;58:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Boyanova L, Ilieva J, Gergova G, Davidkov L, Spassova Z, Kamburov V, Katsarov N, Mitov I. Numerous risk factors for Helicobacter pylori antibiotic resistance revealed by extended anamnesis: a Bulgarian study. J Med Microbiol. 2012;61:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Boyanova L. Prevalence of multidrug-resistant Helicobacter pylori in Bulgaria. J Med Microbiol. 2009;58:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Hojsak I, Kos T, Dumančić J, Mišak Z, Jadrešin O, Jaklin Kekez A, Lukić Grlić A, Kolaček S. Antibiotic resistance of Helicobacter pylori in pediatric patients -- 10 years’ experience. Eur J Pediatr. 2012;171:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Karczewska E, Klesiewicz K, Wojtas-Bonior I, Skiba I, Sito E, Czajecki K, Zwolińska-Wcisło M, Budak A. Levofloxacin resistance of Helicobacter pylori strains isolated from patients in southern Poland, between 2006-2012. Acta Pol Pharm. 2014;71:477-483. [PubMed] |
| 95. | Gościniak G, Biernat M, Grabińska J, Bińkowska A, Poniewierka E, Iwańczak B. The antimicrobial susceptibility of Helicobacter pylori strains isolated from children and adults with primary infection in the Lower Silesia Region, Poland. Pol J Microbiol. 2014;63:57-61. [PubMed] |
| 96. | Karczewska E, Klesiewicz K, Skiba I, Wojtas-Bonior I, Sito E, Czajecki K, Zwolińska-Wcisło M, Budak A. Variability in Prevalence of Helicobacter pylori Strains Resistant to Clarithromycin and Levofloxacin in Southern Poland. Gastroenterol Res Pract. 2012;2012:418010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Karczewska E, Wojtas-Bonior I, Sito E, Zwolińska-Wcisło M, Budak A. Primary and secondary clarithromycin, metronidazole, amoxicillin and levofloxacin resistance to Helicobacter pylori in southern Poland. Pharmacol Rep. 2011;63:799-807. [PubMed] |
| 98. | Almeida N, Romãozinho JM, Donato MM, Luxo C, Cardoso O, Cipriano MA, Marinho C, Fernandes A, Calhau C, Sofia C. Helicobacter pylori antimicrobial resistance rates in the central region of Portugal. Clin Microbiol Infect. 2014;20:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 99. | Oleastro M, Cabral J, Ramalho PM, Lemos PS, Paixão E, Benoliel J, Santos A, Lopes AI. Primary antibiotic resistance of Helicobacter pylori strains isolated from Portuguese children: a prospective multicentre study over a 10 year period. J Antimicrob Chemother. 2011;66:2308-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Vekens K, Vandebosch S, De Bel A, Urbain D, Mana F. Primary antimicrobial resistance of Helicobacter pylori in Belgium. Acta Clin Belg. 2013;68:183-187. [PubMed] |
| 101. | Miendje Deyi VY, Bontems P, Vanderpas J, De Koster E, Ntounda R, Van den Borre C, Cadranel S, Burette A. Multicenter survey of routine determinations of resistance of Helicobacter pylori to antimicrobials over the last 20 years (1990 to 2009) in Belgium. J Clin Microbiol. 2011;49:2200-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | de Boer EM, Schneeberger PM, de Boer WA. [Antibiotic resistance of Helicobacter pylori: prevalence in one region in the southern Netherlands and implications for treatment]. Ned Tijdschr Geneeskd. 2014;158:A7501. [PubMed] |
| 103. | Loffeld RJ, Werdmuller BF. Changes in Antibiotic Susceptibility of Helicobacter pylori in the Course of Eight Years in the Zaanstreek Region in The Netherlands. Gastroenterol Res Pract. 2013;2013:625937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 104. | O'Connor A, Taneike I, Nami A, Fitzgerald N, Ryan B, Breslin N, O’Connor H, McNamara D, Murphy P, O’Morain C. Helicobacter pylori resistance rates for levofloxacin, tetracycline and rifabutin among Irish isolates at a reference centre. Ir J Med Sci. 2013;182:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | O'connor A, Taneike I, Nami A, Fitzgerald N, Murphy P, Ryan B, O’connor H, Qasim A, Breslin N, O’moráin C. Helicobacter pylori resistance to metronidazole and clarithromycin in Ireland. Eur J Gastroenterol Hepatol. 2010;22:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Montes M, Villalon FN, Eizaguirre FJ, Delgado M, Muñoz-Seca IM, Fernández-Reyes M, Pérez-Trallero E. Helicobacter pylori Infection in Children. Antimicrobial Resistance and Treatment Response. Helicobacter. 2015;20:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Mégraud F. Current recommendations for Helicobacter pylori therapies in a world of evolving resistance. Gut Microbes. 2013;4:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 632] [Article Influence: 52.7] [Reference Citation Analysis (3)] |
| 109. | Mégraud F, Lamouliatte H. Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1333-1343. [PubMed] |
| 110. | Dammann HG, Fölsch UR, Hahn EG, von Kleist DH, Klör HU, Kirchner T, Strobel S, Kist M. Eradication of H. pylori with pantoprazole, clarithromycin, and metronidazole in duodenal ulcer patients: a head-to-head comparison between two regimens of different duration. Helicobacter. 2000;5:41-51. [PubMed] |
| 111. | Frenck RW, Clemens J. Helicobacter in the developing world. Microbes Infect. 2003;5:705-713. [PubMed] |
| 112. | Ecclissato C, Marchioretto MA, Mendonça S, Godoy AP, Guersoni RA, Deguer M, Piovesan H, Ferraz JG, Pedrazzoli J. Increased primary resistance to recommended antibiotics negatively affects Helicobacter pylori eradication. Helicobacter. 2002;7:53-59. [PubMed] |
| 113. | Realdi G, Dore MP, Piana A, Atzei A, Carta M, Cugia L, Manca A, Are BM, Massarelli G, Mura I. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter. 1999;4:106-112. [PubMed] |
| 114. | Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |