Published online Sep 20, 2025. doi: 10.5662/wjm.v15.i3.97694
Revised: November 18, 2024
Accepted: December 3, 2024
Published online: September 20, 2025
Processing time: 273 Days and 23.8 Hours
Pituitary macroadenomas represent a significant challenge in clinical mana
To summarize the literature on pituitary macroadenoma and outline the possible multidisciplinary approach in the diagnosis, management, and rehabilitation of individuals with pituitary adenomas, to add to already preexisting knowledge, in managing these cases enhancing better ocular and systemic outcomes.
A search was conducted on an online publication database (PubMed) using the term “pituitary adenoma” including all results published over twenty years (2004-2024). Results were sorted for relevance, language, and completeness.
A total of 176 records were returned. The guidelines of the PRISMA 2020 sta
Pituitary macroadenomas pose substantial clinical challenges due to their size and potential for significant hormonal and neurological impact, modern therapeutic strategies offer effective management options. Early detection and comprehensive treatment are essential for optimizing patient outcomes and maintaining quality of life. Continued research and advancements in medical technology are likely to further enhance the management and prognosis of this condition in the future
Core Tip: The review encompasses recent advancements in diagnostic imaging techniques, surgical approaches, hormonal management, and adjuvant therapies. Furthermore, it discusses the importance of a collaborative framework involving specialists from various disciplines to optimize patient outcomes and quality of life. By synthesizing current evidence and clinical experiences, this manuscript aims to provide insights into the comprehensive management of pituitary macroadenomas and stimulate further research.
- Citation: Aluyi-Osa G, Suleman A, Salati C, Spadea L, Gagliano C, Musa M, Zeppieri M. Multidisciplinary management of pituitary macroadenoma. World J Methodol 2025; 15(3): 97694
- URL: https://www.wjgnet.com/2222-0682/full/v15/i3/97694.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i3.97694
Pituitary adenoma, whether micro or macro depending on size, is a group of benign tumors within the skull, around the pituitary gland, which can extend to extra pituitary areas. A pituitary adenoma is referred to as micro when the size is less than 10mm, and when the size is more than 10mm it is referred to as Macroadenoma. Sizes larger than 40mm are referred to as giant tumors[1].
Pituitary adenomas effect on the visual system is dependent on several factors including, size, location, and whether it is functional or not, it is still not a fact if there is any relationship between the effect of functional and non-functional pituitary adenoma[2], with little or no effect on the motor function. Although it is a benign condition it can significantly affect vision and other systemic functions, because the pituitary gland also referred to as the master gland, helps regulate the function of other endocrine glands (glands whose secretion flows directly into the bloodstream), for the onward manifestation of its effect at effector organ or tissue or even system.
Pituitary adenoma can be said to be functional or non-functional depending on its effect on hormonal secretion; non-functional adenoma can affect vision, as seen in about 58% of cases, and the person involved may not know immediately because there is a compensation from the unaffected eye[3].
The pituitary gland is made up of two lobes, the anterior pituitary and the posterior pituitary. The anterior pituitary, constitutes a major portion of the pituitary gland, hence it is also responsible for the triggering effect on other glands within the body, including those anatomically far from it. Pituitary adenoma can cause varying levels of visual impairment, its manifestations in the eyes depending on size and location, including but not limited to optic atrophy, visual field defect, specifically bitemporal hemianopia in both eyes, due to possible compression at the chiasma level, swollen disc, reduced visual acuity, when the optic nerve (CNII) is involved, sluggish pupillary reaction and possibly raised intracranial pressure. This condition's differential includes Brain stem glioma, Arachnoid cyst, Ependymoma, Glioblastoma multiforme, etc.[4]. Diagnosis is strictly by matching visual or systemic findings with neuroimaging, the systemic component of it comes into play when the tumor is functional as earlier stated. Computerized tomography scan, or magnetic resonance imaging (MRI), requires a multidisciplinary approach to not only diagnosis but also care in the long run, if the affected individual must have a better quality of life[5].
As earlier said, Pituitary adenomas are one of the most common intracranial tumors, the non-functional type ordinarily will not cause or show any signs, unless there is a mass effect on structures surrounding the pituitary gland[6].
Due to the complex systemic sequelae presented in patients with pituitary adenoma, there is a need for a holistic approach to care, this includes the help of the ophthalmologist, Neurologist, Neuroopthalmologist, Radiologist, and Endocrinologist. Each of these specialties has a role they play if the management of a pituitary macroadenoma is regarded as holistic and clinical in approach. Although pituitary macroadenomas are referred to as benign tumors, they can exhibit some significant effects, both in the visual system, and the overall health of the patient, hence the importance of a multidisciplinary approach cannot be overemphasized. This will not only potentiate the management regimen but will also hasten it, with high chances of better recovery or prognosis. In cases where pituitary macroadenomas affect the secretion of hormones, for example, the thyroid hormone secretion macroadenomas, metabolic activities like heart rate, sweating, and the like might become significantly altered[7], the ophthalmologist under this circumstance initiate the need for the patient to see the endocrinologist as soon as possible, also in individuals who have pituitary macroadenoma affecting prolactin hormone, there is bound to be sexual/reproductive changes be it male or a female[8]. Furthermore, some pituitary tumors might present like a pituitary macroadenoma, but in the real sense, it is a more metastatic condition, the help of the radiologist and neurosurgeon, comes into play here, not just for better prognosis, but sometimes to even save the life of the individual, so at the end of the day all tumors involving the pituitary gland, whether quiet or active require the action of the various above-listed professionals for proper management now and in the future. Over the years there have been various workups done in seeing to a better pituitary macroadenoma treatment from the aspect of immunity[8], to the aspect of radiotherapy[9], surgical approach[10-12], with the sole aim of better outcome, so a look at the preexisting management plan for pituitary macroadenomas and its systemic sequelae is very important. Transient visual obscuration (TVO) is rare in pituitary adenoma, there is evidence of resolved TVOs after resection of pituitary macroadenoma that was compressive[12].
This review was done using a systematic search string on PubMed, as stated below, (("eye" [MeSH Terms] OR "eye" [All Fields]) AND ("pituitary neoplasms" [MeSH Terms] OR ("pituitary" [All Fields] AND "neoplasms" [All Fields]) OR "pituitary neoplasms" [All Fields] OR ("pituitary" [All Fields] AND "adenoma" [All Fields]) OR "pituitary adenoma" [All Fields])) AND ((ffrft [Filter]) AND (2004: 2024 [pdat])). Two authors (AG and AS) scrutinized all records for relevance. The search strategy followed the PRISMA[13] guidelines as shown in the flowchart below as Figure 1. The results were further stratified to only include relevant work between 2004 and 2024. This is shown in Figure 1 below.
A total of 176 records were returned. 23 records were excluded due to being out of scope while a further 13 records were duplicates. Another 17 records were not available as full-length articles and were also excluded. A further 18 articles were harvested from the references of the 123 records so stratified. A total of 141 records were therefore used in this minireview.
Importance of a multidisciplinary approach: In the management of pituitary macroadenoma and tumors in general, there is a need for the utilization of a multidisciplinary approach, in the form of shared care, whether the tumor is benign or metastatic, a constant observation by various specialties involved in neoplasia, depending on the location and cause is constantly being sought for. Before now Multidisciplinary approach to care was based on consultation as requested, but recently it has very paramount for better outcomes in the long run. An important component of an effective multidisciplinary team includes better communication, standardized coordination, and improved interdisciplinary decision-making, all of this must include the patient in the care process[14,15]. Although there are barriers to effective inter-professional collaboration in the management of pituitary macroadenoma, another importance of this review is to suggest possible mechanisms of action to be taken to enhance effective shared care regimen in the management of this emerging condition, which is sometimes masqueraded in form of other conditions. Furthermore, outcomes of pituitary surgery as a result of various analyses, have been shown to demonstrate significant positive outcomes following the implementation of a multidisciplinary approach. The outcome can be measured in terms of lower complications, shorter duration of stay in health centers, and increased curative resection[16].
Post-operative management would require a multidisciplinary approach, each doctor should be brought to the status quo regarding the performed procedure to avoid complications involved in managing the patient[17].
Pathophysiology of pituitary macroadenoma: Pathophysiology of Pituitary adenoma seems not be straight forward, hence prompt recognition especially in patient presenting with cavernous sinus syndrome, particularly in an hemodialysis clinic, is important for better management outcome[18]. In patients with pituitary adenoma, early detection of a dysfunctional visual pathway, can give insight into treatment modality and reduce the risk of permanent vision loss[19]. Tumors involving the sellar and parasellar region usually present with optic nerve head neuropathy similar to those found in glaucoma patients[20].
Pituitary adenoma can occur side by side with other tumors like the clear cell meningioma, due to their close similarities in radiological assessment, these are known as collision tumors e.g. the Clear cell meningioma which happens to be very aggressive[21]. The phenomenon by which pituitary apoplexy occurs is connected to either a decreased blood supply or by hemorrhagic mechanism[22,23]. There have been reported cases of Pituitary adenoma being confused with other syndromes, like the Tolosa Hunt Syndrome[24]. Changes in vascular perfusion of the pituitary gland can be affected by uncontrolled diabetes[25]. The presence of a pituitary lesion or disease usually points to the possibility of an existing malignancy[26]. Pituitary macroadenoma should be considered in cases presenting with typical clinical signs of Foster-Kennedy syndrome[27,28].
Though rare there has been a report of metastatic gastric cancer found in the pituitary (MGCP)[29]. Acute ischemic stroke though rare has been identified as a complication of pituitary apoplexy secondary to pituitary adenoma[30]. Stable chiasma lesions have been shown to have similar visual field defects as the patient grows older hence ruling out any statistical difference in visual field defect as a result of age[31].
Tumor biology and growth patterns: The height of a pituitary adenoma from the genu of the cavernous internal carotid artery correlates better with visual outcome compared with gross tumor height[32]. Results from previous studies showed biological evidence to support, the use of specific proteostasis modulation in the management of solid tumors, with syndromic sequelae, especially tumors that involves misfolding of proteins[33].
Diagnosing cases of collision tumor is sometimes challenging, but it poses a great importance regarding post-operative care of the individual, in terms of using radiation therapy, it also helps to detect recurrence nature of tumors[34].
There is the possibility of gradual resolution of the residue of a pituitary adenoma, within the intra-cavernous sinus space, as a result of possible necrosis[35]. Involvement of orbital regions in cases involving the sinonasal anatomy usually is a sign[36].
Tumor size has been found to correlate positively with the mean deviation and correlates negatively with average sensitivity in both cases of functional and non-functional pituitary adenomas[2].
Orbital involvement in most of the sinonasal diseases indicates the extensive and aggressive nature of the pathology and many of these, even if they are not malignancies are difficult to treat[37]. This is especially true for acute fulminant and chronic invasive fungal rhinosinusitis[38].
Decisions in the early detection and management of pituitary carcinoma, it is important to establish systems that help with genomic profiling and also determination of molecular biomarkers[39]. The relationship between the existence of adrenocorticotropic hormone (ACTH) pheochromocytoma on its own or as part of the multiple endocrine Neoplasia type 1[40]. Rapidly developing headache which are accompanied by visual impairment in the female gender with sellar tumors is usually of the sellar atypical teratoid/rhabdoid tumors[41]. Tumor growth and volume have been implicated in symptoms reoccurring from time to time, In cases of highly vascularized lesions in the suprasellar space, this should give a high index of suspicion for possible Hemangioblastoma[42,43]. The E2F3 transcription factor has been fingered as one of the players in tumorgenicity, although the mechanism is still undergoing a lot of research, as seen in the work of Parisi et al[44], it is not a common phenomenon for pituitary infarction or hemorrhage to occur around the parasellar region[45].
Pituitary adenoma without chiasma compression but with lateral extension into the cavernous sinus can present with normal visual fields and cranial nerve three palsy with pupillary involvement[46]. Pituitary macroadenoma presenting with ophthalmoplegia without visual field defect has been shown to have a good prognosis provided it is attended to on time[47]. Metastatic progression of pituitary macroadenoma post radiotherapy has been reported although it incidence is low[48]. The growth of adenoma during pregnancy and subsequent regression post-partum has been reported[49,50]. Pituitary apoplexy should be considered as a differential in patients suspected to have bacterial meningitis, this should be done due to the associated mortality and morbidity if missed[51].
Hormonal dysregulation and clinical manifestations: The pituitary gland secretes various hormones that help to regulate various bodily functions. Changes in the hypophyseal structure would therefore result in a lot of systemic manifestations (hormonal) such as hypothyroidism, hyperthyroidism, hypokalemia, etc. Persistent hypokalemia could result in ocular manifestations such as central retina vein occlusion[52]. Hyponatremia resistant to conventional therapy should point to pituitary gland macroadenoma[53]. Pituitary prolactinoma secreting growth hormone (GH) has been reported as a variant of the macroadenomas[54]. Also, suppressed immunity leading to intraocular complications has been reported in individuals with pituitary macroadenoma[55]. In cases of Hypothalamic-pituitary tumors, as seen in some genetic conditions, there is the possibility of the individual subsequently having severe visual deterioration as the person ages, which has been reported in Klinefelter's syndrome[56].
Changes seen in the trabecular meshwork in cases of excessive secretion of the adrenocorticotrophic hormone (hypercortisolism) can be reversed subsequently to its normal physiological state[57]. Pituitary macroadenoma with apoplexy has been seen in cases of coronavirus disease 2019 (COVID-19) infection, with patients presenting with sudden bilateral vision loss and fever, so this should be looked out for when attending to patients with respiratory infection and unexplained vision loss[58]. Diplopia as a result of restrictive extraocular muscle myopathy is a possible sign of acro
Imaging techniques (MRI, computed tomography, etc.): Early detection of visual field pathway dysfunction, may help in treatment regimen modification for patients with pituitary macroadenoma and also help to reduce the occurrence of irreversible optic neuropathy[62]. Ganglion cell layer-internal plexiform layer assessment using optical coherence tomography (OCT) is a very important tool, with a high degree of sensitivity, in detecting lesions in the anterior portion of the visual pathway, especially in cases where chiasmatic compression exists as a result of pituitary adenoma[63]. Changes in the structure of retinal tissues have a close relationship to retinal nerve fiber thickness and the outcome of the management regimen[64,65]. It is possible for Cushing’s syndrome to occur as a result of hypersecretion of ACTH, but this time it is stimulated from an orbital neuroendocrine tumor, rather than the typical Pituitary gland tumor or associated tumor[66]. The parapapillary zone of atrophy is worse and more frequent in those with large intrasellar or perisellar pituitary tumors[67].
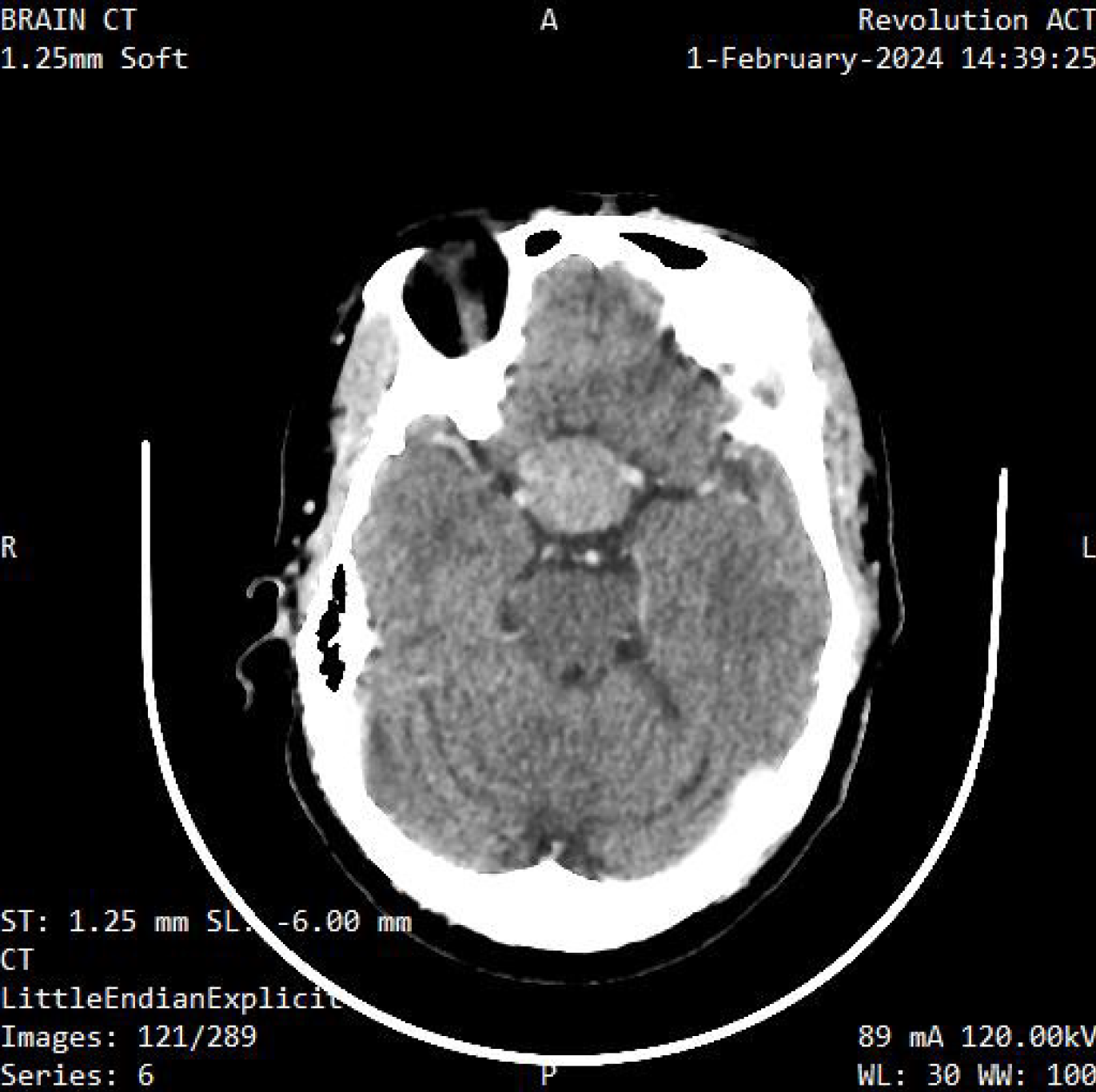
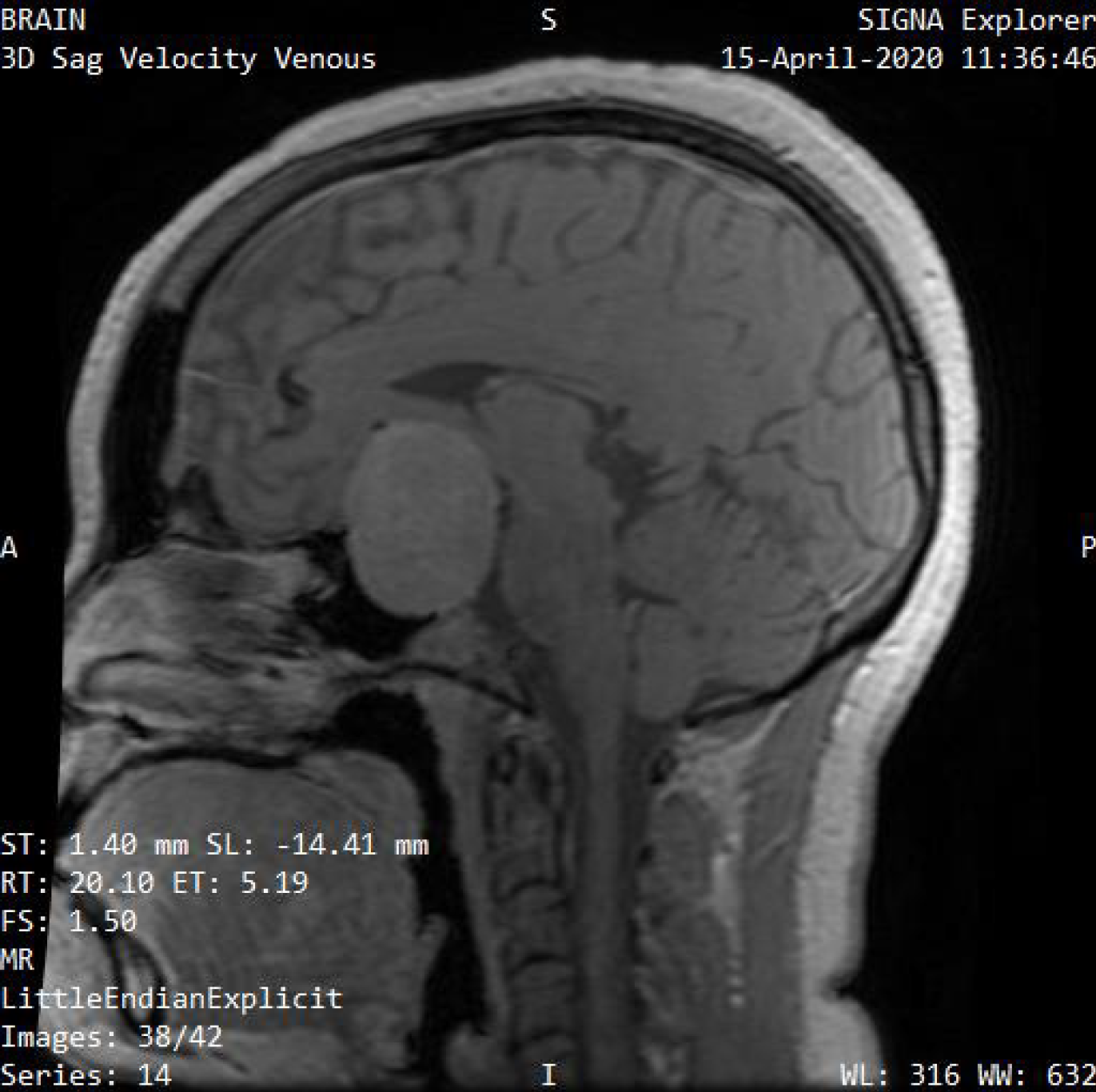
Fast changes in the pattern electroretinogram in patients with glaucoma should spur the conduction of an MRI or computed tomography (CT) scan to rule out intra-cranial space occupying lesions[68,69]. Figure 2 and Figure 3 show examples of imaging scans. Defects in the retinal nerve fiber layer (RNFL) and macula ganglion cell complex (GCC) are seen in patients with chronic chiasma compression earlier than visual field defects. The macula GCC correlates with visual outcomes[69-72]. The assessment of contrast sensitivity can be very important in diagnosing changes at the chiasmatic level in cases of pituitary adenoma[73], Disorders of the anterior visual pathway capable of causing secondary visual disorders of the visual cortex, this can be detected through the use of MRI[74,75]. MRI is often used over CT for diagnosing pituitary adenomas because it excels in characterizing tiny pituitary sellar lesions and improving anatomical delineation prior to surgery. Additionally, MRI is recommended for post-operative monitoring[76].
Apart from the visual field changes in chiasma pathology, inattention to the temporal side on monocular testing should prompt the clinician's attention to possible chiasma pathology[77]. Cases of retinal alterations that are uncharacteristic, should be carefully evaluated for the possibility of chiasma lesions[78].
Stratus OCT has been shown useful in the diagnosis of band atrophy caused by chiasma lesions[79]. In the absence of a visual field assessment, a domain OCT can be used to differentiate lesions of the ganglion cell layer, from that typical of glaucomatous changes[80,81]. Information gathered from the OCT angiography, can be used as a prognostic factor, in determining visual outcome following surgical intervention[82,83]. Although sometimes the extent of visual field loss is related to the tumor size, but not in all cases[84]. The use of OCT in the detection of the degree of RNFL loss and associated visual field changes might have some limitations to its use[85].
The use of simple temporal depression in visual field results has been shown to aid in the diagnosis of compressive optic lesions affecting the optic chiasm which is affected by pituitary gland tumor[86,87]. Having good knowledge about identifying isolated nerve palsy can play a major role in helping to diagnose Pituitary gland-related anomalies, and it will also help direct treatment to the appropriate quarters[88].
Endocrine testing (hormonal assays): Hormonal testing can help to confirm or refute diagnoses of pituitary adenomas in a timely and minimally invasive manner.
Insulin growth factor testing is usually the first port of call. This hormone is usually secreted in times of increased GH in the bloodstream. The presence of elevated levels of both these hormones can confirm the presence of a pituitary adenoma[89]. These hormones are also useful for monitoring the progression of management for pituitary adenomas as a return to normal levels may indicate a remission[89].
Adrenocorticotropic hormone helps to regulate the adrenal steroid in the body. A raised ACTH may indicate the presence of an ACTH-secreting adenoma[90]. These types of pituitary hormones are usually more aggressive[90]. In such patients, there can be a regression into Cushing’s disease[91]. Patients who present with headaches, visual changes, and reduced libido should undergo prolactin levels test, ACTH levels, and testosterone[92]. The dexamethasone/corticotrophin-releasing-hormone test is classically used to test for this. Following a 48-hour regime of 0.5 mg/kg of dexamethasone, a loading dose of 1 ug/kg body weight is given exactly 2 hours after the last dexamethasone dose followed by a 15 minutes wait before the level of plasma cortisol is measured[93]. Cortisol levels higher than 1.8 µg/dL subsequently confirm abnormally increased ACTH levels which is a sequelae of ACTH releasing pituitary adenoma[93].
Other assays that can help in the differential diagnoses of pituitary macroadenomas include gonadotroph secreting hormone assay, lactotroph secreting hormone, somatotroph secreting hormone, and thyroid-secreting hormone assays[94,95].
Histopathological evaluation: In cases of pediatric and juvenile craniopharyngioma, changes in vascular structure, are closely related to structural and functional outcomes[96,97]. Expert knowledge in the area of the anatomy of the brain and histopathologic assessment of imaging findings, are very important in differentiating various pathologies and aid in better management[98]. Proper analysis is needed to differentiate pituitary macroadenoma from sellar chondrosarcoma due to similar systemic effects[99].
Vasospasms and fluctuation in blood pressure intraoperatively have been indicted and correlated to the existence of pituitary adenoma[100]. There is a synergistic improvement in terms of visual outcome and recovery in cases where both surgical resection and additional radiological intervention are carried out[101].
In cases where individuals present with similar pathognomic signs with other pathological entities, a multidisciplinary approach should be instituted[102], Autoimmune workup is necessary in cases of xanthogranulomatous hypohysitis[103]. Management can be multifaceted depending on the presenting signs and diagnostic findings[104], Been able to carry out extensive systemic workup and keeping the mind open is important in managing conditions that might be very radical, but will present initially as a calm lesion[105].
Patients presenting with prolactinoma secondary to macroadenoma could benefit from the use of pharmaceutical agents like cabergoline[106]. Different patients require different approaches, pregnant women require multidisciplinary consideration, and pregnant women taking bromocriptine for macroprolactinoma secondary to pituitary macroadenoma would require surgery due to the low data of the safety of bromocriptine to the fetus[107].
Neurosurgical intervention provides a better prognosis for pituitary macroadenomas as compared to purely conservative medical management[108]. Debulking of tumor size usually leads to improvement in the patient's symptoms and signs[109]. However, a high degree of suspicion is required to screen for other coexisting disorders of the central nervous system, in cases of optic disc melanocytoma which is presenting with disproportionate signs or symptoms[110]. Hypothalomopituitary involvement in cases of neurosarcoidosis is rare, which leads to complications that seem to occur more frequently, than those seen in other neurological and or systemic syndromes[111,112]. The thickness of the RNFL has been found to not be a very important factor in determining post-surgical outcome, after a decompressive surgery[113], It is important to recognize pituitary apoplexy as a major cause of cranial nerve anomaly, as it has been fingered as a rare presenting sign of pituitary adenoma[113]. In cases of pituicytoma where there is a partial removal of the tumor, management can be completed with the help of radiotherapy[114,115]. In cases of collision tumors, a craniotomy is preferred over the transsphenoidal approach[116].
The size of the tumor plays a key role in determining the treatment regimen[117]. The minimally invasive nature of transsphenoidal and endoscopic procedures in the management of pituitary adenoma or tumors of the sellar turcica makes them the go-to procedure[118].
Timing for surgical intervention in cases of pituitary apoplexy resulting from internal carotid artery should be prompt, due to the effect of this condition on visual acuity[119]. Neuroimaging should be considered in patients with conjunctival chemosis, as Yamamuro et al[120], presented a case where a pituitary neuroendocrine tumor masqueraded as severe conjunctiva chemosis[120].
Transphenoidal surgeries go through the nose to reach the pituitary adenoma as against accessing via the skull. Visual field recovery following transsphenoidal tumor resection surgery is correlated with the absence of preoperative central visual field anomaly or even bilateral visual field defects, in patients with pituitary adenoma[121], the use of tran
Transsphenoidal surgery has been shown to cause progressive improvement in visual outcomes in patients with non-functional pituitary macroadenoma[127]. There is a report that transsphenoidal surgery for the removal of pituitary macroadenoma is associated with transient refractive error changes with the postulation that hyponatremia induced by tumor removal causes changes in the aqueous humor which in turn causes changes in the lens via osmosis[128]. Early neurosurgical intervention is crucial in patients with pituitary apoplexy[129]. Pituitary apoplexy is a complication of pituitary adenoma in most cases[130]. Decreased perfusion to the optic disc and retina caused by compressive lesions has been shown to possibly further progress after decompression procedures[131].
Extended intracavernous surgery using endoscopy and a measured, selective resection of the medial wall of the cavernous sinus was reported to be viable for the management of invasive secreting pituitary adenomas[132]. In another case, no tumor recurrence and the total removal of the aneurysm was completed at about 6 months of follow-up, using the endoscopic endonasal surgery technique[133]. Following Endoscopic surgery assessment of OCT angiography showed improvement in the density of retinal fibers[134-136]. Endoscopic endonasal approaches to the removal of pituitary macroadenoma or tuberculum sellae tumors are safe and less invasive than craniotomy[137,138].
Radiological therapy involves the use of ionizing radiation to manage diseases. These are commonly used against cancer cells to kill or restrict their multiplication. There seems to be no significant relationship between the amount of radiation delivered and its effect on some ocular parameters like the RNFL and the Endothelial cell density, otherwise, changes are usually related to the tumor suppression mechanism[139]. The use of Gamma knife Radiosurgery has been shown to come out with lesser side effects and the patient can return to their normal daily activities soon afterward[140].
Outcomes of proton therapy have been linked to the possible technique in unlocking the effectiveness or benefits of proton stereotactic radiosurgery[141]. Aneurysm can be a complication following stereotactic surgery, and it can happen many years post-surgery[142]. Fractionated stereotactic therapy has been shown to reduce tumor size without toxicity[143] There have been incidences of the development of central nervous system lymphoma post-irradiation[144]. Stereotactic radiotherapy has been proven to give better doses to the surrounding extra cranium than conventional radiotherapy[145].
Physiologic pituitary gland enlargement following pregnancy has been treated using bromocriptine[146]. Fan et al[147] reported on a case of pituitary adenoma which regressed and completely shrunk after management with bromocriptine[147]. Bromocriptine is a dopamine receptor agonist that helps to reduce the amount of prolactin in the body, thereby mitigating the progression of prolactin-linked-adenomas[148].
One of the major life-threatening complications of using the gonadotropin-releasing hormone agonist, is Pituitary apoplexy[149,150], Pasireotide which is a somatostatin analogue has been shown to help in the reduction of pituitary mass as seen in a case of Cushing syndrome[151], where a 14-year-old girl presented with headache, unilateral right eye ptosis and secondary amenorrhea. Baagar et al[151] also successfully managed a case of pituitary adenoma using Pasireotide[151].
Excluding thyroiditis, the majority of the endocrine dysfunction or dysregulation usually becomes permanent regardless of the use of immune checkpoint inhibitors[152,153].
The effect of high prolactin levels on the tear film is duration-dependent[154]. Studies have shown that the eye might become very sensitive, due to prolonged exposure to GH and the insulin-like growth factor-1[155], Studies have shown that the effect of retinoic acid anabolic androgenic steroids and their relationship with the prolactin-secreting pituitary adenoma needs to be studied more in other to get an idea of what links these parameters together[156]. Carbagoline use can be very effective in terms of visual improvement and reduction in tumor size in cases of some pituitary adenomas, that are functional[157]. Prompt use of Carbagoline resulted in swift recovery of visual acuity[158].
A drop in visual acuity may be the first sign or symptom noticed in cases of pituitary adenomas. Visual acuity usually improves spontaneously post-operatively, although rates may differ[159,160]. Basic tests such as frequency doubling technique have been shown effective in pituitary macroadenoma diagnosis[161].
Contrast sensitivity has been found to vary with various factors, including luminance level, neural mechanism, grating motion, and shape. It is worthy of note that contrast sensitivity can be influenced by factors such as refractive status, surgeries e.g. cataract surgery or even refractive surgery, diabetic eye changes, and Pituitary adenomas[162], RNFL thickness and optic disc can be very important indicators in terms of prognosis, Severe scotoma is found more in the upper quadrant of the temporal field, and also in the lower nasal hemifield[163]. Patients with tumors affecting the extraocular muscle may present with complaints at the first visit to an eye hospital, quickly identifying these conditions is necessary for their management, both in the short and long run[164] for visual outcome evaluation, following pituitary adenoma surgical procedure[165]. Eyecare practitioners should have a very high level of suspicion for internal carotid artery aneurism, as a differential for any form of sellar mass[166]. Evidence support the fact that mutation in specific genes can cause early onset Cushing disease, with invariably increased size of adrenocorticotrophic pituitary adenoma[166]. Radial peripapillary capillary density is a stronger indicator for determining outcome in terms of visual field recovery[167]. Visual field constriction that appears to be rapid even in the presence of other ocular morbidity, should prompt immediate radiological assessment. A high index of suspicion must be correlated with appropriate and prompt radiological assessment[168], The GCC and ganglion cell layer thickness parameters can help predict the integrity of the central visual field of patients with pituitary tumors[169,170]. Care and due diligence must be ensured to avoid misdiagnosis, in their case series Choudhari et al[171], showed the misdiagnosing of pituitary adenoma as NTG[171].
More work on the aspect of the effect of the COVID-19 vaccine on the effect of pituitary adenoma needs to be done, The COVID-19 vaccine has been implicated in the possible cause for the increase in the size of the pituitary gland, although more work needs to be done[172]. The use of BRAF and MEK inhibitors represent or stand a chance in the future, in the treatment of capillary craniopharyngioma[173]. Differentiating some forms of sellar region tumor possesses some challenges, hence newer methods such as the use of randomics and deep learning(machine learning are the newer methods to give better differentiating outcomes, with subsequent effects on the management plan and expected outcome[174]. The use of intraoperative MRI together with a transsphenoidal approach is an emerging technique found to be associated with significantly reduced complications[175]. Some records could not be retrieved as they did not meet the selection criteria
In conclusion, the management of pituitary macroadenomas exemplifies the necessity of a multidisciplinary approach in modern medicine. These tumors, due to their potential to cause significant endocrine dysfunction and compress adjacent neural structures, demand comprehensive and coordinated care. Collaboration among endocrinologists, neurosurgeons, ophthalmologists, radiologists, and pathologists is pivotal for accurate diagnosis, effective treatment planning, and monitoring of patient outcomes. Advanced imaging techniques and tailored surgical strategies, combined with targeted pharmacotherapy, have significantly enhanced the prognosis for patients with pituitary macroadenomas. However, ongoing research is essential to refine these interventions, reduce recurrence rates, and better understand the molecular and genetic underpinnings of these tumors. By embracing a multidisciplinary framework, healthcare professionals can ensure holistic and patient-centered care, ultimately improving the quality of life for individuals affected by this condition.
We want to thank Dr. G. O. Okoye (MD, PhD), Founder of Africa Eye Laser Centre, Benin City, Edo State, Nigeria, who provided us with the figures used in this paper.
| 1. | Russ S, Anastasopoulou C, Shafiq I. Pituitary Adenoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 2. | Qin J, Li K, Wang X, Bao Y. A comparative study of functioning and non-functioning pituitary adenomas. Medicine (Baltimore). 2021;100:e25306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Hlaváč M, Sommer F, Karpel-Massler G, Wirtz R, Hoffmann T, Paľa A. [Differential diagnosis and treatment of pituitary adenomas]. HNO. 2019;67:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Chapman PR, Singhal A, Gaddamanugu S, Prattipati V. Neuroimaging of the Pituitary Gland: Practical Anatomy and Pathology. Radiol Clin North Am. 2020;58:1115-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Waqar F, Arif A, Muazzam A, Khan A. Pituitary Adenoma With Apoplexy Presenting As Unilateral Third Nerve Palsy. Cureus. 2023;15:e40555. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Trukhina DA, Przhiyalkovskaya EG, Belaya ZE, Grigoriev AY, Azizyan VN, Mamedova EO, Rozhinskaya LY, Lapshina AM, Pigarova EA, Dzeranova LK, Platonova NM, Troshina EA, Melnichenko GA. [Thyrotropin-secreting pituitary adenomas: clinical features and results of treatment in 45 patients]. Probl Endokrinol (Mosk). 2023;70:23-36. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, Higham CE, Lupi I, Paschou SA, Toth M, van der Kooij M, Dekkers OM. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol. 2022;187:G1-G21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Xia Y, Ma X, Griffiths BB, Luo Y. Neurosurgical anesthesia for a pregnant woman with macroprolactinoma: A case report. Medicine (Baltimore). 2018;97:e12360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Wilk A, Zielinski G, Witek P, Koziarski A. Outcome Assessment After Surgical Treatment of Tuberculum Sellae Meningiomas- A Preliminary Report. Turk Neurosurg. 2016;26:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Tanriverdi F, Karaca Z, Oner A, Durak AC, Selcuklu A, Unluhizarci K, Kelestimur F. Complete surgical resolution of bilateral total opthalmoplegia without visual field defect in an acromegalic patient presented with pituitary apoplexy. Endocr J. 2007;54:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Ryden NA, Lam H, Judge C, Venteicher AS, Lee MS. Transient Visual Obscurations Without Papilloedema as the Heralding Symptom of Chiasmal Compression. Neuroophthalmology. 2023;47:106-109. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Hansford HJ, Richards GC, Page MJ, Sharp MK, Lee H, Cashin AG. Reporting health and medical research. BMJ Evid Based Med. 2024;29:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | White A, Junior de Andrade E, Kshettry VR, Sindwani R, Recinos PF. Preoperative Workup for Patients with Pituitary Lesions. Otolaryngol Clin North Am. 2022;55:233-246. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Haliloglu O, Kuruoglu E, Ozkaya HM, Keskin FE, Gunaldi O, Oz B, Gazioglu N, Kadioglu P, Tanriover N. Multidisciplinary Approach for Acromegaly: A Single Tertiary Center's Experience. World Neurosurg. 2016;88:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Colliander R, Sharma S, Shlobin NA, Fernandez LG, LoPresti MA, Lam S, DeCuypere M. Visual outcomes after treatment of craniopharyngioma in children: A systematic review. Childs Nerv Syst. 2024;40:1641-1659. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Nioi M, Napoli PE, Ferreli F. Fatal Iatrogenic Pituitary Apoplexy after Surgery for Neuroophthalmological Disorder. Anesthesiology. 2019;130:822. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Jamal Y, Camacho Y, Hanft S, Chiarolanzio P, Goldberg MD, Mullally JA. A Case of Pituitary Apoplexy and Cavernous Sinus Syndrome during Hemodialysis. Case Rep Endocrinol. 2023;2023:3183088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Lachowicz E, Lubiński W. The clinical value of the multi-channel PVEP and PERG in the diagnosis and management of the patient with pituitary adenoma: a case report. Doc Ophthalmol. 2018;137:37-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Qu Y, Wang YX, Xu L, Zhang L, Zhang J, Zhang J, Wang L, Yang L, Yang A, Wang J, Jonas JB. Glaucoma-like optic neuropathy in patients with intracranial tumours. Acta Ophthalmol. 2011;89:e428-e433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Chatain GP, Chee K, Driscoll M, Kleinschmidt-DeMasters BK, Lillehei KO. Pituitary Adenoma Coexistent with Sellar Clear Cell Meningioma Unattached to the Dura: Case Report and Treatment Considerations. J Neurol Surg Rep. 2024;85:e1-e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Tanios G, Mungo NA, Kapila A, Bajaj K. Pituitary apoplexy: a rare complication of leuprolide therapy in prostate cancer treatment. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Özçetin M, Karacı M, Toroslu E, Edebali N. A pediatric case of pituitary macroadenoma presenting with pituitary apoplexy and cranial nerve involvement: case report. Turk Pediatri Ars. 2016;51:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Świątkowska-Stodulska R, Stodulski D, Babińska A, Piskunowicz M, Sworczak K. Bilateral Tolosa-Hunt syndrome mimicking pituitary adenoma. Endocrine. 2017;58:582-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Mittal A, Mishra S, Yadav K, Rajput R. Uncontrolled diabetes as a rare presenting cause of pituitary apoplexy. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Mansoor Q, Carey PE, Adams W. A rare ophthalmic presentation of pituitary metastases. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Ayele B, Mengesha A, Wotiye A, Alemayehu Y. Giant Pituitary Adenoma Presenting with Foster-Kennedy Syndrome in a 21-Year Old Ethiopian Patient: A Rarely Reported Phenomenon: A Case Report. Ethiop J Health Sci. 2020;30:311-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Musa M, Aluyi-Osa G, Zeppieri M. Foster Kennedy Syndrome (FKS): A Case Report. Clin Pract. 2022;12:527-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 29. | Yang C, Zhang H, Zhang S, Liu L, Ma B, Lou J, Sun X, Zhang B. Oculomotor Paralysis, Postorbital Pain, and Hypopituitarism as First Presentations of Metastatic Gastric Cancer in the Pituitary Flourished by Internal Carotid Aneurysm: A Case Report. Medicine (Baltimore). 2015;94:e2317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Pasha SA, Ranganthan LN, Setty VK, Reddy R, Ponnuru DA. Acute Ischaemic Stroke as a Manifestation of Pituitary Apoplexy in a Young Lady. J Clin Diagn Res. 2017;11:OD03-OD05. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Rudolph T, Frisén L. Influence of ageing on visual field defects due to stable lesions. Br J Ophthalmol. 2007;91:1276-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Ng BCF, Mak CH, Steffi CSY, Wing SK, Shing TT, Ching CF. A Factorial Analysis on Visual Outcomes of Transsphenoidal Surgery for Pituitary Macroadenoma. Asian J Neurosurg. 2022;17:280-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Chittiboina P, Mandal D, Bugarini A, Asuzu DT, Mullaney D, Mastorakos P, Stoica S, Alvarez R, Scott G, Maric D, Elkahloun A, Zhuang Z, Chew EY, Yang C, Linehan M, Lonser RR. Proteostasis Modulation in Germline Missense von Hippel Lindau Disease. Clin Cancer Res. 2023;29:2199-2209. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Aydin MV, Yangi K, Toptas E, Aydin S. Skull Base Collision Tumors: Giant Non-functioning Pituitary Adenoma and Olfactory Groove Meningioma. Cureus. 2023;15:e44710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Nadkarni T, Desai K, Goel A. Spontaneous resolution of residual pituitary adenoma. Case report. Neurol Med Chir (Tokyo). 2005;45:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Bulte CA, Hoegler KM, Khachemoune A. Collision tumors: A review of their types, pathogenesis, and diagnostic challenges. Dermatol Ther. 2020;33:e14236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Fan X, Liu T, Zhang Z, Sun J, Niu N, Mao C, Wang F, Li J, Zhou D, Cao X, Jin Z, Feng F. Comparison of neuroimaging features of histiocytic neoplasms with central nervous system involvement: a retrospective study of 121 adult patients. Eur Radiol. 2023;33:8031-8042. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Zheng XQ, Zhou X, Yao Y, Deng K, You H, Duan L, Zhu HJ. Acromegaly complicated with fulminant pituitary apoplexy: clinical characteristic analysis and review of literature. Endocrine. 2023;81:160-167. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Xu L, Khaddour K, Chen J, Rich KM, Perrin RJ, Campian JL. Pituitary carcinoma: Two case reports and review of literature. World J Clin Oncol. 2020;11:91-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Rebrova DV, Grigorova SI, Vorokhobina NV, Zgoda EA, Novokshonov KY, Feofanova SG, Rusakov VF, Krasnov LM, Fedorov EA, Chinchuk IK, Shikhmagomedov SS, Pushkaruk AA, Sleptsov IV. [Corticotropin-producing pheochromocytoma in multiple endocrine neoplasia type 1]. Probl Endokrinol (Mosk). 2023;69:55-64. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Yu R. Sellar Mass in 2 Patients With Acute-Onset Headache and Visual Symptoms: Not Your Usual Pituitary Adenoma. AACE Clin Case Rep. 2023;9:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Alvarez R, Mastorakos P, Hogan E, Scott G, Lonser RR, Wiley HE, Chew EY, Chittiboina P. Retrobulbar Hemangioblastomas in von Hippel-Lindau Disease: Clinical Course and Management. Neurosurgery. 2021;88:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Li Z, Feng T, Teng H, Hu Y, Yao Y, Liu Y. Suprasellar hemangioblastoma without von Hippel-Lindau disease: a case report and literature review. Int J Clin Exp Pathol. 2015;8:7553-7558. [PubMed] |
| 44. | Parisi T, Yuan TL, Faust AM, Caron AM, Bronson R, Lees JA. Selective requirements for E2f3 in the development and tumorigenicity of Rb-deficient chimeric tissues. Mol Cell Biol. 2007;27:2283-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Sivaraju L, Hegde VS, Kiran NA, Ghosal N, Hegde AS. Pituitary apoplexy presenting as a peripheral rim enhancing parasellar mass lesion with dural enhancement along the tentorium. Neuroradiol J. 2017;30:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Natarajan D, Tatineni S, Ponnapalli SP, Sachdeva V. Pituitary adenoma presenting as acute onset isolated complete third cranial nerve palsy without vision changes. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Zoli M, Milanese L, Faustini-Fustini M, Guaraldi F, Asioli S, Zenesini C, Righi A, Frank G, Foschini MP, Sturiale C, Pasquini E, Mazzatenta D. Endoscopic Endonasal Surgery for Pituitary Apoplexy: Evidence On a 75-Case Series From a Tertiary Care Center. World Neurosurg. 2017;106:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Lall RR, Shafizadeh SF, Lee KH, Mao Q, Mehta M, Raizer J, Bendok BR, Chandler JP. Orbital metastasis of pituitary growth hormone secreting carcinoma causing lateral gaze palsy. Surg Neurol Int. 2013;4:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Lee HR, Song JE, Lee KY. Developed diplopia and ptosis due to a nonfunctioning pituitary macroadenoma during pregnancy. Obstet Gynecol Sci. 2014;57:66-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Vosoughi AR, Tyndel F, Suthiphosuwan S, Micieli JA. Post-partum Resolution of Bitemporal Hemianopia with Persisting Pituitary Adenoma. Can J Neurol Sci. 2024;51:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 51. | Wong SH, Das K, Javadpour M. Pituitary apoplexy initially mistaken for bacterial meningitis. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Kalaria TR, Chopra R, Ayuk J, Buch H. Retinal vein occlusion as the presenting feature of Cushing's syndrome. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Madhusudhan S, Madhusudhan TR, Haslett RS, Sinha A. Pituitary apoplexy following shoulder arthroplasty: a case report. J Med Case Rep. 2011;5:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Besouw MT, Levtchenko EN, Willemsen MA, Noordam K. Growth hormone producing prolactinoma in juvenile cystinosis: a simple coincidence? Pediatr Nephrol. 2008;23:307-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Lee EK, Kim JH, Yu HG. Candida albicans endophthalmitis in a patient with a non-functioning pituitary adenoma evolving into Cushing׳s disease: A case report. Med Mycol Case Rep. 2014;6:37-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Beisti Ortego A, De Arriba Muñoz A, Ferrer Lozano M, Martínez de Zabarte Fernández JM, Calvo Escribano C, Labarta Aizpún JI. [Hypogonadotropic hypogonadism in Klinefelter syndrome and hypothalamic-pituitary tumor]. Arch Argent Pediatr. 2015;113:e6-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Griffin S, Boyce T, Edmunds B, Hills W, Grafe M, Tehrani S. Endogenous hypercortisolism inducing reversible ocular hypertension. Am J Ophthalmol Case Rep. 2019;16:100573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Katti V, Ramamurthy LB, Kanakpur S, Shet SD, Dhoot M. Neuro-ophthalmic presentation of COVID-19 disease: A case report. Indian J Ophthalmol. 2021;69:992-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Heireman S, Delaey C, Claerhout I, Decock CE. Restrictive extraocular myopathy: a presenting feature of acromegaly. Indian J Ophthalmol. 2011;59:517-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Gaballa S, Lindsay J, AlJaf A, Hlaing KM, Patel K. Acute Unilateral Oculomotor Nerve Palsy as the Initial Presenting Sign of Nonfunctioning Apoplectic Gonadotroph Adenoma. Cureus. 2020;12:e8819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 61. | Zahedi M, Hizomi Arani R, Tohidi M, Haghighi S, Mehrpour M, Hadaegh F. Nasopharyngeal B-cell lymphoma with pan-hypopituitarism and oculomotor nerve palsy: a case report and review of the literature. BMC Endocr Disord. 2020;20:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Lachowicz E, Lubiński W. The importance of the electrophysiological tests in the early diagnosis of ganglion cells and/or optic nerve dysfunction coexisting with pituitary adenoma: an overview. Doc Ophthalmol. 2018;137:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Agarwal R, Jain VK, Singh S, Charlotte A, Kanaujia V, Mishra P, Sharma K. Segmented retinal analysis in pituitary adenoma with chiasmal compression: A prospective comparative study. Indian J Ophthalmol. 2021;69:2378-2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Wang X, Chou Y, Zhu H, Xing B, Yao Y, Lu L, You H, Gan L, Wang M, Ma J, Zhong Y. Retinal Microvascular Alterations Detected by Optical Coherence Tomography Angiography in Nonfunctioning Pituitary Adenomas. Transl Vis Sci Technol. 2022;11:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Lee GI, Park KA, Oh SY, Kong DS, Hong SD. Inner and outer retinal layer thickness alterations in pediatric and juvenile craniopharyngioma. Sci Rep. 2021;11:2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Tan H, Chen D, Yu Y, Yu K, He W, Cai B, Jiang S, Tang Y, Tong N, An Z. Unusual ectopic ACTH syndrome in a patient with orbital neuroendocrine tumor, resulted false-positive outcome of BIPSS:a case report. BMC Endocr Disord. 2020;20:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Wang YX, Xu L, Lu W, Liu FJ, Qu YZ, Wang J, Jonas JB. Parapapillary atrophy in patients with intracranial tumours. Acta Ophthalmol. 2013;91:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Ventura LM, Venzara FX 3rd, Porciatti V. Reversible dysfunction of retinal ganglion cells in non-secreting pituitary tumors. Doc Ophthalmol. 2009;118:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Molitch ME. Nonfunctioning pituitary tumors. Handb Clin Neurol. 2014;124:167-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Lukewich MK, Micieli JA. Chronic chiasmal compression and persistent visual field defect without detectable changes in optical coherence tomography of the macular ganglion cell complex. Am J Ophthalmol Case Rep. 2019;16:100533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Yum HR, Park SH, Park HY, Shin SY. Macular Ganglion Cell Analysis Determined by Cirrus HD Optical Coherence Tomography for Early Detecting Chiasmal Compression. PLoS One. 2016;11:e0153064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Cennamo G, Auriemma RS, Cardone D, Grasso LF, Velotti N, Simeoli C, Di Somma C, Pivonello R, Colao A, de Crecchio G. Evaluation of the retinal nerve fibre layer and ganglion cell complex thickness in pituitary macroadenomas without optic chiasmal compression. Eye (Lond). 2015;29:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Kasputytė R, Slatkevičienė G, Liutkevičienė R, Glebauskienė B, Bernotas G, Tamašauskas A. Changes of visual functions in patients with pituitary adenoma. Medicina (Kaunas). 2013;49:132-137. [PubMed] |
| 74. | Sun M, Zhang Z, Ma C, Chen S, Chen X. Quantitative analysis of retinal layers on three-dimensional spectral-domain optical coherence tomography for pituitary adenoma. PLoS One. 2017;12:e0179532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Song X, Wang G, Zhang T, Feng L, An P, Zhu Y. Functional magnetic resonance imaging evaluation of visual cortex activation in patients with anterior visual pathway lesions. Neural Regen Res. 2012;7:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 76. | Guy RL, Benn JJ, Ayers AB, Bingham JB, Lowy C, Cox TC, Sonksen PH. A comparison of CT and MRI in the assessment of the pituitary and parasellar region. Clin Radiol. 1991;43:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Fledelius HC. Temporal visual field defects are associated with monocular inattention in chiasmal pathology. Acta Ophthalmol. 2009;87:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Lavaque AJ, Yilmaz T, Cordero-Coma M. Localized bi-nasal macular edema in optic chiasmal syndrome. Indian J Ophthalmol. 2013;61:351-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 79. | Leal BC, Moura FC, Monteiro ML. Retinal nerve fiber layer loss documented by Stratus OCT in patients with pituitary adenoma: case report. Arq Bras Oftalmol. 2006;69:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Yang L, Qu Y, Lu W, Liu F. Evaluation of Macular Ganglion Cell Complex and Peripapillary Retinal Nerve Fiber Layer in Primary Craniopharyngioma by Fourier-Domain Optical Coherence Tomography. Med Sci Monit. 2016;22:2309-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Shon K, Sung KR. Assessment of macular ganglion cell loss patterns in neurologic lesions that mimic glaucoma. Korean J Ophthalmol. 2014;28:314-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Ergen A, Kaya Ergen S, Gunduz B, Subasi S, Caklili M, Cabuk B, Anik I, Ceylan S. Retinal vascular and structural recovery analysis by optical coherence tomography angiography after endoscopic decompression in sellar/parasellar tumors. Sci Rep. 2023;13:14371. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Cennamo G, Solari D, Montorio D, Scala MR, D'Andrea L, Tranfa F, Cavallo LM. The role of OCT- angiography in predicting anatomical and functional recovery after endoscopic endonasal pituitary surgery: A 1-year longitudinal study. PLoS One. 2021;16:e0260029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Boland MV, Lee IH, Zan E, Yousem DM, Miller NR. Quantitative Analysis of the Displacement of the Anterior Visual Pathway by Pituitary Lesions and the Associated Visual Field Loss. Invest Ophthalmol Vis Sci. 2016;57:3576-3580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Johansson C, Lindblom B. The role of optical coherence tomography in the detection of pituitary adenoma. Acta Ophthalmol. 2009;87:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Takahashi M, Goseki T, Ishikawa H, Hiroyasu G, Hirasawa K, Shoji N. Compressive Lesions of the Optic Chiasm: Subjective Symptoms and Visual Field Diagnostic Criteria. Neuroophthalmology. 2018;42:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 87. | Kummararaj G, Balaji V, Kummararaj S, Venugopal NP. Full-field perimetry for evaluation of glaucomatous (presumed) cup. Indian J Ophthalmol. 2012;60:581-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 88. | Koylu B, Firlatan B, Sendur SN, Oguz SH, Dagdelen S, Erbas T. Giant growth hormone-secreting pituitary adenomas from the endocrinologist's perspective. Endocrine. 2023;79:545-553. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 89. | Graffeo CS, Donegan D, Erickson D, Brown PD, Perry A, Link MJ, Young WF, Pollock BE. The Impact of Insulin-Like Growth Factor Index and Biologically Effective Dose on Outcomes After Stereotactic Radiosurgery for Acromegaly: Cohort Study. Neurosurgery. 2020;87:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Zoli M, Faustini-Fustini M, Mazzatenta D, Marucci G, De Carlo E, Bacci A, Pasquini E, Lanzino G, Frank G. ACTH adenomas transforming their clinical expression: report of 5 cases. Neurosurg Focus. 2015;38:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Sun X, Lu L, Feng M, Fan Y, Bao X, Dai C, Deng K, Guo D, Yao Y, Zhu H, Wang R. Cushing Syndrome Caused by Ectopic Adrenocorticotropic Hormone-Secreting Pituitary Adenomas: Case Report and Literature Review. World Neurosurg. 2020;142:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Phillips J, East HE, French SE, Melcescu E, Hamilton RD, Nicholas WC, Fratkin JF, Parent AD, Luzardo G, Koch CA. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones (Athens). 2012;11:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing's disease from normal physiology. J Clin Endocrinol Metab. 1998;83:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Tsukaguchi R, Hasebe M, Honjo S, Hamasaki A. Ovarian Hyperstimulation Syndrome Caused by Functional Gonadotroph Pituitary Adenoma. JCEM Case Rep. 2023;1:luad087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 95. | Horiguchi K. The molecular biology of thyrotroph pituitary neuroendocrine tumors. Endocr J. 2023;70:135-139. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 96. | Lee GI, Kim Y, Park KA, Oh SY, Kong DS, Hong SD. Parafoveal and peripapillary vessel density in pediatric and juvenile craniopharyngioma patients. Sci Rep. 2022;12:5355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 97. | Liu G, Su L, Xiang Y, Liu Y, Zhang S. Coexistence of craniopharyngioma and meningioma: Two rare cases and literature review. Medicine (Baltimore). 2020;99:e23183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 98. | Larkin S, Ansorge O. Pathology And Pathogenesis Of Pituitary Adenomas And Other Sellar Lesions. 2017 Feb 15. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 99. | Cao J, Li G, Sun Y, Hong X, Huang H. Sellar chondrosarcoma presenting with amenorrhea: A case report. Medicine (Baltimore). 2018;97:e11274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Joo C, Ha G, Jang Y. Pituitary apoplexy following lumbar fusion surgery in prone position: A case report. Medicine (Baltimore). 2018;97:e0676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 101. | Patel KR, Zheng J, Tabar V, Cohen MA, Girotra M. Extended Survival After Surgical Resection for Pituitary Metastases: Clinical Features, Management, and Outcomes of Metastatic Disease to the Sella. Oncologist. 2020;25:e789-e797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Paschou SA, Tzioras K, Trianti V, Lyra S, Lioutas VA, Seretis A, Vryonidou A. Young adult patient with headache, fever and blurred vision. Hormones (Athens). 2016;15:548-550. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 103. | Salhi S, Oueslati I, Mouelhi Y, Zehani A, Kchir N, Kamoun E, Yazidi M, Chihaoui M. Secondary xanthogranulomatous hypophysitis mimicking a pituitary macroadenoma: a case report. J Int Med Res. 2024;52:3000605231223033. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 104. | Clapp AN, DePold Hohler A. A case of parasympathetic hyperactivity and associated Parry-Romberg syndrome. SAGE Open Med Case Rep. 2021;9:2050313X211034351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 105. | Gao H, Wu S, Zhang X, Xie T. Minimally invasive follicular thyroid carcinoma mimicking pituitary adenoma: a case report. Int J Clin Exp Pathol. 2019;12:3949-3952. [PubMed] |
| 106. | Dutta D, Ahuja A, Sharma L, Bhardwaj M, Kulshreshtha B. Macular amyloidosis complicating macroprolactinoma--a novel clinical association. Endokrynol Pol. 2015;66:555-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 107. | Gondim J, Ramos Júnior F, Pinheiro I, Schops M, Tella Júnior OI. Minimally invasive pituitary surgery in a hemorrhagic necrosis of adenoma during pregnancy. Minim Invasive Neurosurg. 2003;46:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 108. | Saktiwarawat K, Tunthanathip T, Oearsakul T, Taweesomboonyat C. Comparing neuroendocrine recovery between surgical and conservative management in pituitary apoplexy patients: a propensity score-matched analysis. Neurosurg Rev. 2024;47:236. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 109. | Huynh N, Stemmer-Rachamimov AO, Swearingen B, Cestari DM. Decreased vision and junctional scotoma from pituicytoma. Case Rep Ophthalmol. 2012;3:190-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Attiku Y, Rishi P, Bassi S. Coexisting Optic Disc Melanocytoma and Pituitary Adenoma. Ocul Oncol Pathol. 2019;5:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 111. | Hassani FD, Fadli M, El Abbadi N. [Pituitary sarcoidosis mimicking pituitary adenoma: case report and literature review]. Pan Afr Med J. 2019;33:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 112. | Póczoš P, Kremláček J, Česák T, Macháčková M, Jirásková N. The use of optical coherence tomography in chiasmal compression. Cesk Slov Oftalmol. 2019;75:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Cho WJ, Joo SP, Kim TS, Seo BR. Pituitary apoplexy presenting as isolated third cranial nerve palsy with ptosis : two case reports. J Korean Neurosurg Soc. 2009;45:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Zaki U, Shakeel AS, Rauf Y, Raza M. Pituicytoma: A rare tumor of the sella. A case report and review of literature for diagnosis and management. Surg Neurol Int. 2023;14:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 115. | Yin S, Zhou P, Li Q, Jiang S. Intrasellar Clear Cell Meningioma Mimicking Invasive Pituitary Adenoma: A Case Report and Review of the Literature. Turk Neurosurg. 2015;25:976-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Jin G, Hao S, Xie J, Mi R, Liu F. Collision tumors of the sella: coexistence of pituitary adenoma and craniopharyngioma in the sellar region. World J Surg Oncol. 2013;11:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Lynch GL, Broome MR, Scagliotti RH. What is your diagnosis? Mass originating from the pituitary fossa. J Am Vet Med Assoc. 2006;228:1681-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 118. | Jugović D, Spazzapan P, Porčnik A, Prestor B. TRANS-ENDOSCOPIC TREATMENT OF CRANIOPHARYNGIOMA AND RECOVERY FROM BLINDNESS IN ADULT PATIENT - A CASE REPORT. Acta Clin Croat. 2020;59:549-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 119. | Chokyu I, Tsuyuguchi N, Goto T, Chokyu K, Chokyu M, Ohata K. Pituitary apoplexy causing internal carotid artery occlusion--case report. Neurol Med Chir (Tokyo). 2011;51:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Yamamuro S, Yoshino A, Nishide T, Negishi H, Kumagawa T. A case report of pituitary neuroendocrine tumor manifesting as severe conjunctival chemosis. BMC Ophthalmol. 2023;23:479. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Lee DK, Sung MS, Park SW. Factors Influencing Visual Field Recovery after Transsphenoidal Resection of a Pituitary Adenoma. Korean J Ophthalmol. 2018;32:488-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 122. | Hug NF, Purger DA, Moss HE, Dodd RL. Pituitary macroadenoma causing vision loss in Wyburn-Mason syndrome: illustrative case. J Neurosurg Case Lessons. 2022;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 123. | Santos CDSE, Filho LMDCL, Santos CAT, Neill JS, Vale HF, Kurnutala LN. Pituitary tumor resection in a patient with SARS-CoV-2 (COVID-19) infection. A case report and suggested airway management guidelines. Braz J Anesthesiol. 2020;70:165-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Tagoe NN, Essuman VA, Bankah P, Dakurah T, Hewlett VK, Akpalu J, Ndanu TA. Visual Outcome of Patients with Pituitary Adenomas Following Surgery and Its Contributory Factors at a Tertiary Hospital in Ghana. Ethiop J Health Sci. 2019;29:895-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 125. | Wang J, Song DL, Deng L, Sun SY, Liu C, Gong DS, Wang Y, Xu QW. Extraventricular neurocytoma of the sellar region: case report and literature review. Springerplus. 2016;5:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Choi KY, Choi S, Jeong S, Won TB. Successful Endoscopic Transsphenoidal Approach Treatment of Sphenoid Sinus Organized Hematoma Causing Visual Deficit: A Case Report. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 127. | Dekkers OM, de Keizer RJ, Roelfsema F, Vd Klaauw AA, Honkoop PJ, van Dulken H, Smit JW, Romijn JA, Pereira AM. Progressive improvement of impaired visual acuity during the first year after transsphenoidal surgery for non-functioning pituitary macroadenoma. Pituitary. 2007;10:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 128. | Ishikawa H, Akura J, Uchida K, Ikeda N, Ikeda T, Borlongan CV, Mimura O. A case with transient refractive change after removal of pituitary tumor. BMC Ophthalmol. 2013;13:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 129. | Seuk JW, Kim CH, Yang MS, Cheong JH, Kim JM. Visual outcome after transsphenoidal surgery in patients with pituitary apoplexy. J Korean Neurosurg Soc. 2011;49:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Pokhrel B, Khanal S, Chapagain P, Sedain G. Pituitary Apoplexy Complicated by Cerebral Infarction: A Case Report. JNMA J Nepal Med Assoc. 2021;59:723-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 131. | Lee GI, Park KA, Oh SY, Kong DS. Changes in parafoveal and peripapillary perfusion after decompression surgery in chiasmal compression due to pituitary tumors. Sci Rep. 2021;11:3464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Lefevre E, Chasseloup F, Hage M, Chanson P, Buchfelder M, Kamenický P. Clinical and therapeutic implications of cavernous sinus invasion in pituitary adenomas. Endocrine. 2024;85:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 133. | Gu Y, Zhong X, Gao Y, He L. Endoscopic endonasal approach for simultaneously treating a pituitary adenoma coexisting with a paraclinoid aneurysm: illustrative case. J Neurosurg Case Lessons. 2022;3:CASE22130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 134. | Wei P, Falardeau J, Chen A, Wang J, Liu L, Jia Y, Huang D. Optical coherence tomographic angiography detects retinal vascular changes associated with pituitary adenoma. Am J Ophthalmol Case Rep. 2022;28:101711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 135. | Kurian DE, V R, Horo S, Chacko AG, Prabhu K, Mahasampath G, Korah S. Predictive value of retinal nerve fibre layer thickness for postoperative visual improvement in patients with pituitary macroadenoma. BMJ Open Ophthalmol. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 136. | Cennamo G, Solari D, Montorio D, Scala MR, Melenzane A, Fossataro F, Somma T, Tranfa F, Cavallo LM. Early vascular modifications after endoscopic endonasal pituitary surgery: The role of OCT-angiography. PLoS One. 2020;15:e0241295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 137. | Thirumala PD. Visual evoked potentials for visual function monitoring during endoscopic sphenoidal surgery: Advancement and challenges. Neurol India. 2018;66:958-959. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 138. | Mahvash M, Igressa A, Pechlivanis I, Weber F, Charalampaki P. Endoscopic endonasal transsphenoidal approach for resection of a coexistent pituitary macroadenoma and a tuberculum sellae meningioma. Asian J Neurosurg. 2014;9:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 139. | La Rosa A, Wroe A, Fellows Z, Kotecha R. Proton Radiosurgery: Current Concepts and Limitations for CNS Radiosurgery. Neurol India. 2023;71:S174-S182. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 140. | Orski M, Tarnawski R, Wylęgała E, Tarnawska D. The Impact of Robotic Fractionated Radiotherapy for Benign Tumors of Parasellar Region on the Eye Structure and Function. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 141. | Chao ST, Thakkar VV, Barnett GH, Vogelbaum MA, Angelov L, Weil RJ, Rasmussen P, Reuther AM, Jamison B, Neyman G, Suh JH. Prospective study of the short-term adverse effects of gamma knife radiosurgery. Technol Cancer Res Treat. 2012;11:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 142. | Wang PW, Chung MH, Feng SW, Liao HC, Wu YC, Hueng DY, Yang YJ, Ju DT. Case report: Ruptured internal carotid artery fusiform aneurysm mimicking pituitary apoplexy after stereotactic radiosurgery. Front Neurol. 2023;14:1219372. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 143. | Puataweepong P, Dhanachai M. Rapid and significant reduction in size of pituitary adenoma in children treated with fractionated stereotactic radiation therapy: a case report. Case Rep Endocrinol. 2011;2011:187839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 144. | Papanastasiou L, Pappa T, Dasou A, Kyrodimou E, Kontogeorgos G, Samara C, Bacaracos P, Galanopoulos A, Piaditis G. Case report: Primary pituitary non-Hodgkin's lymphoma developed following surgery and radiation of a pituitary macroadenoma. Hormones (Athens). 2012;11:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 145. | Ram TS, Ravindran PB, Viswanathan FR, Viswanathan PN, Pavamani SP. Extracranial doses in stereotactic and conventional radiotherapy for pituitary adenomas. J Appl Clin Med Phys. 2006;7:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 146. | Ennaifer H, Jemel M, Kandar H, Grira W, Kammoun I, Salem LB. Developed diplopia due to a pituitary macroadenoma during pregnancy. Pan Afr Med J. 2018;29:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 147. | Fan J, Shen H, Mo J, Zhang J. Complete Shrinking of Mixed Growth Hormone and Prolactin-Secreting Pituitary Adenoma With Bromocriptine Therapy Alone. J Craniofac Surg. 2024;35:e620-e622. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 148. | Chen Z, Shou X, Ji L, Cheng H, Shen M, Ma Z, He W, Ye Z, Zhang Y, Qiao N, Zhang Q, Wang Y. Presurgical Medical Treatment in Prolactinomas: Surgical Implications and Pathological Characteristics From 290 Cases. J Clin Endocrinol Metab. 2024;109:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 149. | Triviño V, Fidalgo O, Juane A, Pombo J, Cordido F. Gonadotrophin-releasing hormone agonist-induced pituitary adenoma apoplexy and casual finding of a parathyroid carcinoma: A case report and review of literature. World J Clin Cases. 2019;7:3259-3265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Kim H, Lee JH, Choi SJ, Kim WK, Lee JS, Lee KH. Analysis of fatal intracranial hemorrhage in 792 acute leukemia patients. Haematologica. 2004;89:622-624. [PubMed] |
| 151. | Baagar KA, Sadiq A, Khan AA, Dabbous Z, Rohani Z. Successful medical management of a pituitary macroadenoma with features of resistant acromegaly and hyperprolactinemia using pasireotide. Qatar Med J. 2024;2024:17. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 152. | Regazzo D, Avallone S, MacSweeney CP, Sergeev E, Howe D, Godwood A, Bennett KA, Brown AJH, Barnes M, Occhi G, Barbot M, Faggian D, Tropeano MP, Losa M, Lasio G, Scaroni C, Pecori Giraldi F. A novel somatostatin receptor ligand for human ACTH - and GH -secreting pituitary adenomas. Eur J Endocrinol. 2024;190:K8-K16. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 153. | Kotwal A, Kennedy R, Kikani N, Thosani S, Goldner W, Shariff A. Endocrinopathies Associated With Immune Checkpoint Inhibitor Use. Endocr Pract. 2024;30:584-591. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 154. | Doğan C, Güleser ÜY, Kılıçarslan O, Mergen B, Açbay Ö, İskeleli G. The Effect of Prolactinoma on Tear Film Function. Turk J Ophthalmol. 2022;52:374-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 155. | Skrzypiec I, Wierzbowska J, Sobol M, Zieliński G. Corneal Tonometric and Morphological Changes in Patients with Acromegaly. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 156. | Barbosa AP, Oliveira FR, Rocha FJD, Muglia VF, Rocha EM. Lacrimal gland atrophy and dry eye related to isotretinoin, androgen, and prolactin: differential diagnosis for Sjögren's syndrome. Arq Bras Oftalmol. 2021;84:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 157. | Kanj U, Lee SS, Wattegama M, Chavda S, Karavitaki N, Batra R. Foster Kennedy syndrome secondary to a giant prolactinoma with a remarkable response to cabergoline. Endocrinol Diabetes Metab Case Rep. 2022;2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 158. | Shibue K, Yamakawa M, Nishida N, Hamasaki A. Resolution of Visual Field Defect in Macroprolactinoma After Treatment With Cabergoline. Cureus. 2022;14:e25548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 159. | Uvelius E, Valdemarsson S, Bengzon J, Hammar B, Siesjö P. Visual acuity in patients with non-functioning pituitary adenoma: Prognostic factors and long-term outcome after surgery. Brain Spine. 2023;3:102667. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 160. | Butenschoen VM, Schwendinger N, von Werder A, Bette S, Wienke M, Meyer B, Gempt J. Visual acuity and its postoperative outcome after transsphenoidal adenoma resection. Neurosurg Rev. 2021;44:2245-2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 161. | Monteiro ML, Moura FC, Cunha LP. Frequency doubling perimetry in patients with mild and moderate pituitary tumor-associated visual field defects detected by conventional perimetry. Arq Bras Oftalmol. 2007;70:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 162. | Kaur K, Gurnani B. Contrast Sensitivity. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2024. [PubMed] |
| 163. | Kotoda Y, Kotoda M, Ogiwara M, Kinouchi H, Iijima H. Left-Right and Upper-Lower Light Sensitivity Asymmetry in Visual Field Defects Caused by Pituitary Adenoma: A Retrospective Observational Study. Clin Ophthalmol. 2020;14:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 164. | Zheng J, Chen W, Huang D, Wang Y, Zheng D, Zhou L, Brelén ME, Huang Z. Ocular symptoms as the initial clinical manifestations in patients with extraocular tumors. Ann Transl Med. 2021;9:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 165. | Iqbal M, Irfan S, Goyal JL, Singh D, Singh H, Dutta G. An Analysis of Retinal Nerve Fiber Layer Thickness before and after Pituitary Adenoma Surgery and its Correlation with Visual Acuity. Neurol India. 2020;68:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 166. | Bao XD, Lu L, Zhu HJ, Yao Y, Feng M, Wang RZ, Zhai X, Fu Y, Gong FY, Lu ZL. Concurrent mutations of germline GPR101 and somatic USP8 in a pediatric giant pituitary ACTH adenoma: a case report. BMC Endocr Disord. 2022;22:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 167. | Yoneoka Y, Hatase T, Watanabe N, Jinguji S, Okada M, Takagi M, Fujii Y. Early morphological recovery of the optic chiasm is associated with excellent visual outcome in patients with compressive chiasmal syndrome caused by pituitary tumors. Neurol Res. 2015;37:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 168. | Nganga HK, Lubanga RP. Pituitary macroadenoma presenting with pituitary apoplexy, acromegaly and secondary diabetes mellitus - a case report. Pan Afr Med J. 2013;15:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 169. | Santorini M, Ferreira De Moura T, Barraud S, Litré CF, Brugniart C, Denoyer A, Djerada Z, Arndt C. Comparative Evaluation of Two SD-OCT Macular Parameters (GCC, GCL) and RNFL in Chiasmal Compression. Eye Brain. 2022;14:35-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 170. | Koçer AM, İlhan B, Güngör A. Intracranial Mass Lesion in a Patient Being Followed up for Amblyopia. Turk J Ophthalmol. 2020;50:317-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 171. | Choudhari NS, Neog A, Fudnawala V, George R. Cupped disc with normal intraocular pressure: the long road to avoid misdiagnosis. Indian J Ophthalmol. 2011;59:491-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 172. | Srimanan W, Panyakorn S. COVID-19 Vaccine-Induced Expansion of Pituitary Adenoma: A Case Report. Cureus. 2023;15:e50685. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 173. | Shah SN, Kaki PC, Shah SS, Shah SA. Concurrent Radiation and Targeted Therapy for Papillary Craniopharyngioma: A Case Report. Cureus. 2023;15:e40190. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 174. | Jiang C, Zhang W, Wang H, Jiao Y, Fang Y, Feng F, Feng M, Wang R. Machine Learning Approaches to Differentiate Sellar-Suprasellar Cystic Lesions on Magnetic Resonance Imaging. Bioengineering (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 175. | Baumann F, Schmid C, Bernays RL. Intraoperative magnetic resonance imaging-guided transsphenoidal surgery for giant pituitary adenomas. Neurosurg Rev. 2010;33:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |