Published online Jun 20, 2025. doi: 10.5662/wjm.v15.i2.101458
Revised: November 7, 2024
Accepted: November 20, 2024
Published online: June 20, 2025
Processing time: 73 Days and 16.2 Hours
Knee osteoarthritis (OA) is a debilitating condition with limited long-term treatment options. The therapeutic potential of mesenchymal stem cells (MSCs), particularly those derived from bone marrow aspirate concentrate, has garnered attention for cartilage repair in OA. While the iliac crest is the traditional site for bone marrow harvesting (BMH), associated morbidity has prompted the exploration of alternative sites such as the proximal tibia, distal femur, and proximal humerus. This paper reviews the impact of different harvesting sites on mesenchymal stem cell (MSC) yield, viability, and regenerative potential, emphasizing their relevance in knee OA treatment. The iliac crest consistently offers the highest MSC yield, but alternative sites within the surgical field of knee procedures offer comparable MSC characteristics with reduced morbidity. The integration of harvesting techniques into existing knee surgeries, such as total knee arthroplasty, provides a less invasive approach while maintaining thera
Core Tip: Knee osteoarthritis (OA) has limited long-term treatments. Mesenchymal stem cells from bone marrow aspirate concentrate show promise for cartilage repair. However, variability in mesenchymal stem cell (MSC) yield from the sites necessitates further research to standardize techniques and optimize outcomes. Future directions include large-scale studies and personalized harvesting strategies to enhance MSC-based therapies for knee OA.
- Citation: Nallakumarasamy A, Shrivastava S, Rangarajan RV, Jeyaraman N, Devadas AG, Ramasubramanian S, Jeyaraman M. Optimizing bone marrow harvesting sites for enhanced mesenchymal stem cell yield and efficacy in knee osteoarthritis treatment. World J Methodol 2025; 15(2): 101458
- URL: https://www.wjgnet.com/2222-0682/full/v15/i2/101458.htm
- DOI: https://dx.doi.org/10.5662/wjm.v15.i2.101458
Knee osteoarthritis (OA) is a prevalent and debilitating condition, contributing significantly to the global burden of disease and affecting millions of individuals worldwide[1,2]. As one of the leading causes of disability, knee OA is associated with chronic pain, reduced mobility, and a diminished quality of life. Current treatment modalities, including pharmacological interventions, physical therapy, and surgical procedures, such as total knee arthroplasty, often provide only temporary relief or are limited in their effectiveness, especially in the long term[3,4]. The limitations of these treatments have driven the exploration of regenerative medicine, particularly the use of mesenchymal stem cells (MSCs), as a promising alternative for cartilage repair and the management of OA[5]. Mesenchymal stem cell (MSC)-based therapies have garnered attention due to the cells' ability to differentiate into chondrocytes, their immune-modulatory properties, and their potential to release trophic factors that promote tissue regeneration. Bone marrow aspirate concentrate (BMAC), which contains a high concentration of MSCs, is commonly harvested from the iliac crest and is considered the gold standard for regenerative procedures[6]. However, the procedure is associated with significant donor-site morbidity, which has prompted research into alternative bone marrow harvesting (BMH) sites that could provide an adequate yield of MSCs with reduced morbidity[7].
Emerging evidence suggests that bone marrow harvested from sites such as the proximal humerus, tibia, and femur could offer similar or even superior yields of MSCs compared to the iliac crest[8]. This is particularly relevant in knee OA treatments, where local harvesting sites within the surgical field could not only reduce morbidity but also enhance the efficiency of the procedure by eliminating the need for a secondary harvest site. Despite these potential benefits, there remains a paucity of comprehensive studies comparing the yield, viability, and regenerative potential of MSCs obtained from different anatomical sites within the same patient. While the iliac crest remains the most commonly utilized source for MSCs due to its well-documented efficacy in providing a high yield of progenitor cells, the associated morbidity of this harvest site necessitates the exploration of alternative sites[8]. The potential of other anatomical locations, such as the femur, tibia, and proximal humerus, to provide MSCs with comparable regenerative capacity is yet to be fully understood. Although some studies have investigated the yield and characteristics of MSCs from these alternative sites, results have been inconsistent and often limited by small sample sizes or the lack of intra-subject comparisons.
The impact of anatomical variability on MSC yield and the functional abilities of these cells across different harvesting sites is not well established. The current understanding of MSC functionality, particularly about their chondrogenic potential and the expression of surface markers, is primarily based on studies using cells from the iliac crest, with limited comparative data from other bone marrow sites[9]. Moreover, the clinical outcomes of using MSCs from alternative harvest sites in regenerative procedures for knee OA remain underexplored, highlighting the need for rigorous, standardized studies that evaluate the efficacy and safety of these approaches[10]. This review aims to discuss the optimal BMH site for obtaining MSCs with the highest yield and regenerative potential for use in knee OA treatment.
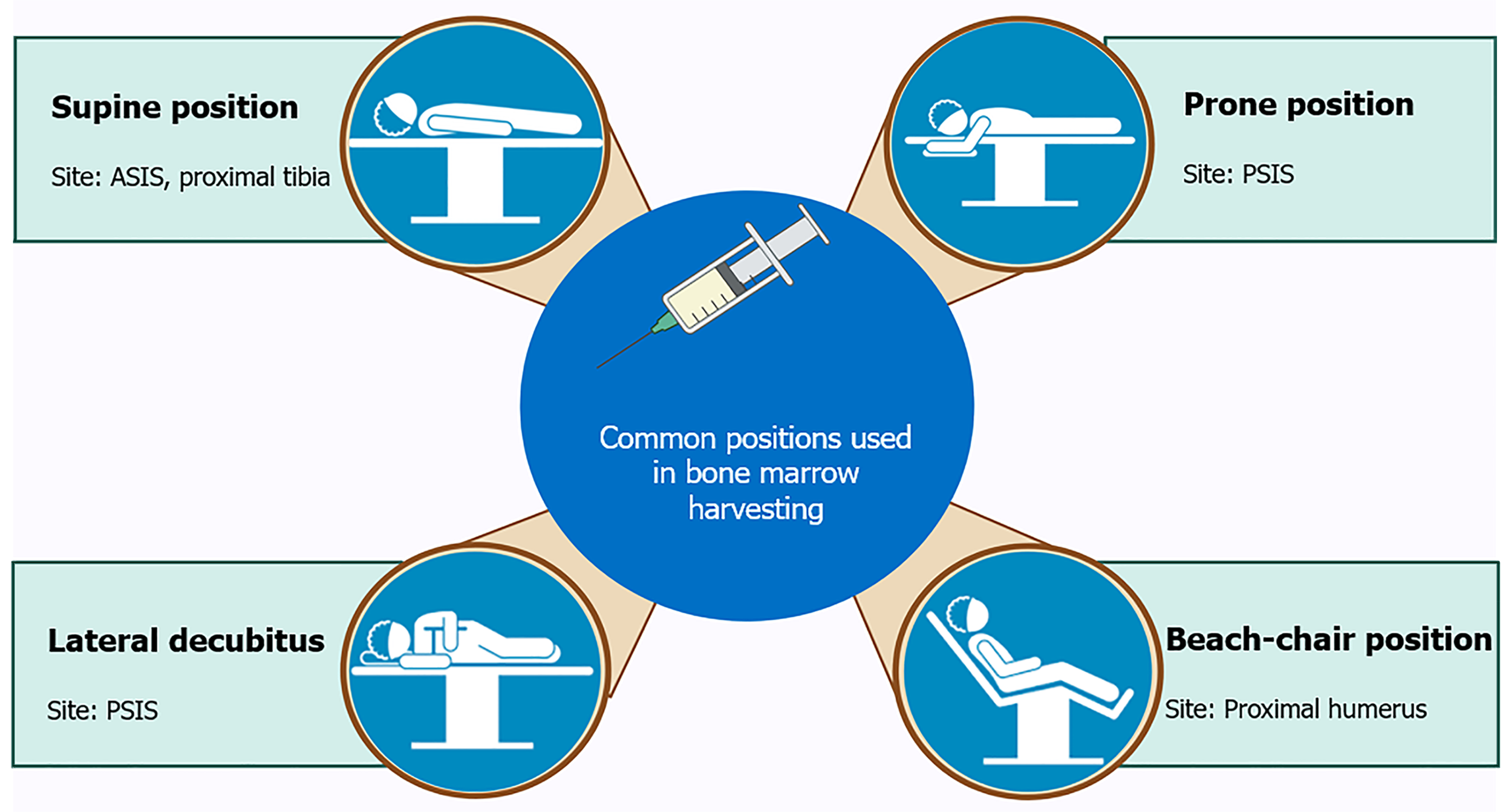
Patient positioning is a crucial aspect of BMH techniques, particularly in procedures intended to optimize the characteristics and purity of the harvested cells for use in knee OA treatment. Proper positioning not only enhances the efficacy of the procedure but also minimizes complications, thereby improving patient outcomes. This section discusses the various aspects of patient positioning relevant to BMH, drawing on insights from multiple studies and technical guidelines (Figure 1).
The correct positioning of the patient during BMH is vital for several reasons. Firstly, it ensures that the harvesting needle is accurately guided to the optimal site, such as the iliac crest or other accessible locations like the proximal tibia or humerus. Secondly, it helps in reducing the risk of injury to surrounding tissues, nerves, and blood vessels. Thirdly, proper positioning facilitates the ease and efficiency of the procedure, which is crucial when large volumes of bone marrow are required for concentration and subsequent therapeutic use[11]. Patient comfort and safety are also paramount considerations during positioning. Incorrect positioning can lead to complications such as nerve damage, excessive bleeding, or suboptimal cell yield, which can compromise the effectiveness of the treatment. Therefore, a thorough understanding of anatomical landmarks and the patient’s specific anatomy is essential for optimal positioning.
Supine position: The supine position is one of the most commonly used positions in BMH, especially when the anterior superior iliac spine or proximal tibia is the site of aspiration[5]. This position is favored for its accessibility and ease of patient monitoring during the procedure. It is particularly useful in procedures where the bone marrow needs to be harvested from the anterior iliac crest or proximal tibia, adjacent to the knee joint. In the supine position, the patient lies flat on their back with their legs extended. For harvesting from the proximal tibia, the knee may be slightly flexed, and a leg holder can be used to stabilize the limb. The tibial tuberosity and posteromedial border of the tibia serve as important landmarks, and the needle is inserted at an angle directed toward the fibular head. This position provides a stable and secure access point, which is crucial for the successful aspiration of bone marrow.
Prone position: The prone position is typically used when accessing the posterior iliac crest, which is considered one of the gold standard sites for BMH due to its high yield of cell-dense marrow[12]. In this position, the patient lies face down on the operating table, with their hips slightly elevated and the area around the iliac crest exposed. Pillows or foam pads are often placed under the patient’s abdomen and pelvis to reduce pressure on the chest and abdomen, thus minimizing discomfort and facilitating respiration during the procedure. The prone position allows for a more direct approach to the posterior iliac crest, which can result in a higher quality and quantity of bone marrow aspirate. The use of this position, however, requires careful consideration of the patient’s overall condition, particularly in those with respiratory or cardiovascular issues, as lying face down for an extended period can exacerbate these conditions.
Lateral decubitus position: In some cases, particularly when accessing the posterior iliac spine, the lateral decubitus position may be employed[11]. In this position, the patient lies on their side, with the side of interest facing up. The legs are often bent at the knees, and pillows are placed between them to maintain spinal alignment and reduce pressure on the lower back. This position provides good access to the posterior iliac crest while allowing for easier patient breathing compared to the prone position. The lateral decubitus position is advantageous in situations where the prone position is contraindicated, such as in patients with respiratory compromise. It also allows for simultaneous access to both the anterior and posterior aspects of the iliac crest if needed, thereby increasing the flexibility and efficiency of the procedure.
Beach-chair position: For procedures involving the proximal humerus, particularly in shoulder-related harvesting, the beach-chair position is commonly used[6]. This position involves the patient sitting in a semi-reclined position, with the backrest of the operating table elevated to about 45-60 degrees. The arms are positioned at the sides or slightly abducted to allow access to the humeral head. The beach chair position is particularly useful for harvesting bone marrow for shoulder-related conditions. It allows for easy access to the proximal humerus, where the lateral acromial border and the greater tuberosity serve as important anatomical landmarks. The position also facilitates the surgeon's ability to rotate and manipulate the limb as needed to optimize needle placement and marrow extraction.
The use of positioning aids such as leg holders, foam pads, and pillows is critical in maintaining the correct posture and stability of the patient throughout the BMH procedure. These aids help minimize movement, reducing patient discomfort, and ensuring that the anatomical landmarks are consistently accessible during the procedure.
Leg holders: In procedures involving the lower extremities, such as those targeting the proximal tibia, leg holders are often used to stabilize the limb and maintain the desired flexion or extension angle[13]. This stability is crucial for ensuring that the needle is accurately positioned and that the harvesting process is efficient.
Pillows and foam pads: Pillows and foam pads are commonly used to provide additional support and comfort, particularly in the prone and lateral decubitus positions[14]. These aids help in relieving pressure on certain body parts, thus reducing the risk of pressure sores and enhancing overall patient comfort.
Intraoperative imaging: Intraoperative imaging techniques such as fluoroscopy or ultrasound can also be employed to guide needle placement, particularly in challenging cases where anatomical variations or previous surgical interventions might obscure standard landmarks[15]. These imaging techniques can help in confirming the correct needle placement within the bone marrow cavity, thus optimizing the yield and quality of the aspirate.
The choice of patient positioning must also take into account the type of anesthesia being used[7]. General anesthesia is commonly employed for BMH, particularly when larger volumes are required, as it allows for complete muscle relaxation and patient immobility[16]. However, in cases where local or regional anesthesia is used, positioning becomes even more critical, as the patient may still have some degree of muscle tone or reflex movement. Under general anesthesia, the patient’s position must be carefully monitored to avoid complications such as nerve compression or impaired circulation. For example, in the prone position, care must be taken to ensure that the head and neck are properly aligned to prevent airway obstruction or cervical spine injury. Similarly, in the supine position, attention must be paid to the patient’s lower back and sacrum, areas that are prone to pressure-related injuries if not adequately supported.
The procedure for bone marrow aspiration (BMA) typically involves the extraction of bone marrow from the iliac crest, a common site due to its accessibility and the richness of the marrow. The process begins with the identification and preparation of the aspiration site, usually the posterior superior iliac spine (PSIS), under sterile conditions. Local anesthesia is administered to minimize patient discomfort during the procedure.
Two primary techniques are utilized for BMA: (1) Single-site aspiration; and (2) Multiple-site aspiration. Each technique has its implications for cell yield and patient outcomes. For instance, in single-site aspiration, a larger volume is drawn from one puncture site, which may result in a higher yield of MSCs from that specific location but can also lead to increased contamination with peripheral blood[17]. Conversely, multiple-site aspiration involves collecting smaller volumes from several sites, potentially reducing peripheral blood dilution and improving the overall purity and quality of the MSCs obtained[17].
Optimal aspiration volumes: The volume of marrow aspirated during each pull is a critical determinant of the yield and purity of MSCs. Research indicates that larger aspiration volumes tend to dilute the marrow with peripheral blood, which contains significantly fewer MSCs and can lower the overall cellular concentration of the aspirate[18]. Studies recommend limiting each aspiration to approximately 5-8 mL to maintain a higher concentration of MSCs and minimize peripheral blood contamination[19]. When more bone marrow is needed for therapeutic purposes, instead of increasing the volume from a single site, which risks excessive dilution, it is advisable to perform multiple small-volume aspirations from different sites. This approach, often referred to as “multi-site small volume aspiration” or “re-orientation technique” ensures that each pull is less contaminated and remains rich in MSCs, which are essential for subsequent therapeutic applications[20–22].
Single vs multiple site aspiration: Single-site aspiration involves drawing a larger volume of marrow from one site, which can lead to higher MSC yields initially but also increases the risk of peripheral blood contamination as the marrow cavity becomes depleted[17]. In contrast, multiple-site aspiration, where smaller volumes are drawn from different sites, can reduce this risk[17]. This technique ensures that the marrow drawn is less likely to be diluted with peripheral blood, thus maintaining a higher purity of MSCs across the samples. The choice between these techniques often depends on the specific clinical requirements, including the volume of MSCs needed and the patient’s tolerance to multiple puncture sites[17].
Needle types: The choice of needle for bone marrow aspiration significantly impacts both the quantity and quality of the aspirated material. The traditional Jamshidi needle is commonly used due to its reliability and effectiveness[23]. However, more advanced needles, such as the Marrow Cellution™ (AMC) needle[21,24], have been developed to optimize the process further. The AMC needle features a unique design with multiple lateral openings along its shaft. These openings allow the needle to aspirate marrow from various points within the bone cavity, rather than from a single location, reducing the likelihood of peripheral blood contamination. Additionally, the needle is designed to be gradually retracted during aspiration, which increases the number of marrow pockets accessed and improves the yield of MSCs. This design innovation represents a significant improvement over traditional needles, particularly in its ability to enhance the purity and quantity of MSCs without requiring additional punctures.
Needle insertion techniques: The technique used to insert the needle into the bone marrow cavity is another crucial factor. A commonly used method is the perpendicular or vertical insertion, which directly targets the marrow cavity[12,25]. However, this method might limit the area of marrow accessed and could lead to quicker depletion of the local marrow, increasing the risk of peripheral blood contamination in subsequent aspirations. An alternative approach is the divergent or angled insertion technique[26]. In this method, the needle is inserted at an angle, allowing it to traverse a broader area of the marrow cavity. This technique can access multiple inter-trabecular spaces, which are rich in MSCs, thereby increasing the heterogeneity and overall yield of the aspirated cells. Divergent insertion also minimizes the depletion of any single marrow pocket, maintaining a more consistent cellular concentration throughout the procedure.
Aspiration rate: The rate at which bone marrow is aspirated plays a pivotal role in the quality of the sample obtained. Rapid aspiration, which involves quickly drawing the marrow into the syringe, creates a high differential pressure that can inadvertently increase the amount of peripheral blood in the sample[27]. This rapid influx of peripheral blood not only dilutes the MSC concentration but may also cause discomfort to the patient due to the sudden change in pressure. In contrast, a slower aspiration technique involves gently drawing the marrow at a controlled pace, typically over 5 seconds to 15 seconds. This method reduces the likelihood of peripheral blood mixing with the marrow, thereby enhancing the purity and concentration of MSCs. Slow aspiration is particularly beneficial in procedures where high-quality cellular material is critical, such as in regenerative therapies for knee OA[27].
Pressure control mechanisms: Controlling the pressure during aspiration is another important consideration. Manual aspiration techniques, where the operator controls the syringe pressure, can vary significantly depending on the practitioner’s experience and technique[28]. This variability can affect the consistency of the aspirate’s quality. To address this, some advanced systems include mechanisms that maintain a consistent differential pressure, regardless of the operator’s manual input. These systems can help standardize the procedure, reducing the variability in MSC yield and improving overall sample quality.
Rotational aspiration devices: Recent innovations include the development of rotational aspiration devices, which use a powered mechanism to rotate the needle during aspiration. This rotation helps the needle traverse more marrow spaces, potentially increasing the volume and quality of the cells collected[28,29]. These devices can reduce the time required for the procedure and may improve patient comfort by decreasing the manual effort needed during aspiration.
OnControl powered bone marrow biopsy system: The OnControl system is one such device that has shown promise in clinical settings. It combines a powered rotary drill with a biopsy needle, which can be used to both aspirate marrow and obtain a core biopsy. Studies have shown that the OnControl system can increase the yield of bone marrow cells while reducing the procedure time[30]. Moreover, the device’s ability to minimize patient discomfort, particularly in challenging cases where manual aspiration might be difficult, makes it an attractive option in clinical practice.
Slow suction techniques: Another innovative approach is the use of slow suction techniques with controlled pressure systems, such as the ones studied in the randomized controlled trials mentioned earlier. These systems are designed to optimize the balance between sufficient pressure to draw marrow and minimizing peripheral blood contamination. In clinical trials, such as those conducted by Grønkjær et al[27], slow suction techniques have demonstrated superior sample quality compared to rapid suction, with the added benefit of being less painful for the patient.
Impact of technique on MSC viability: The techniques employed during bone marrow aspiration can also influence the viability of the MSCs harvested. High shear forces, which may occur during rapid aspiration or improper needle handling, can damage cell membranes, reducing the viability of the cells and their subsequent proliferative and differentiation capacities[31]. Therefore, it is crucial that the aspiration technique not only focuses on maximizing yield but also preserves the functional integrity of the MSCs. To enhance cell viability, techniques that reduce mechanical stress on the cells are preferred. These include slow aspiration, which minimizes shear forces, and the use of specially designed needles that reduce turbulence within the syringe[22,32]. Additionally, maintaining the sample at physiological temperatures and processing it promptly post-aspiration are essential steps in preserving cell viability.
Post-aspiration handling: Once the bone marrow is aspirated, the sample must be handled with care to maintain the viability of the MSCs. This includes avoiding excessive agitation, which can induce apoptosis or necrosis in sensitive cell populations[11]. Immediate cooling of the sample and quick processing in a controlled environment are recommended to ensure that the cells retain their functional characteristics for therapeutic use.
Age and health status: The selection of patients for BMA plays a critical role in the success of the procedure, particularly in the context of regenerative therapies for knee OA. Age is a significant factor, as the quantity and quality of MSCs in bone marrow tend to decrease with age[33]. Studies have shown that older patients, especially those over the age of 60 years, often have a lower density of MSCs in their bone marrow, which can affect the therapeutic efficacy of the harvested cells[34]. Therefore, when selecting patients, clinicians must consider the patient's age and overall health status. Moreover, the presence of comorbid conditions such as osteoporosis or other bone-related diseases can further complicate BMA[35]. In patients with osteoporosis, for instance, the bone marrow cavity may be less dense, potentially leading to a lower yield of MSCs. Pre-procedure assessments, including bone density scans and possibly magnetic resonance imaging (MRI) or computed tomography (CT) imaging, are essential to evaluate the suitability of the bone marrow site for aspiration. These assessments help in identifying the best possible site for marrow extraction, maximizing the likelihood of obtaining a high-quality sample.
Exclusion criteria: Certain conditions may contraindicate BMA or reduce its effectiveness. Patients with hematological disorders that affect bone marrow quality, such as leukemia or myelodysplastic syndromes, are typically excluded from MSC harvesting procedures unless the bone marrow is being specifically assessed or treated for these conditions[36]. Additionally, patients with active infections, especially in the area of the aspiration site, should not undergo BMA due to the risk of spreading infection and contaminating the sample[37]. Patients on anticoagulant therapy or those with coagulation disorders also require careful consideration. These individuals are at an increased risk of bleeding during and after the procedure, which can lead to complications. For such patients, it may be necessary to adjust or temporarily discontinue anticoagulation therapy under close medical supervision before proceeding with BMA[38].
In older adults, the decline in MSC quantity and regenerative capacity requires modifications in harvesting strategies. To maximize MSC yield, employing multiple small-volume aspirations from different sites can enhance concentration while reducing peripheral blood contamination[7]. Harvesting from alternative sites, such as the proximal tibia or distal femur during knee surgeries, can also minimize morbidity associated with iliac crest aspiration[7]. Advanced aspiration devices, like the Marrow Cellution™ needle, may help optimize cell harvest with minimal manipulation[24]. For patients with osteoporosis, considerations related to bone density are crucial. Pre-procedural imaging, including DEXA scan and MRI, helps assess bone quality and determine optimal harvesting sites[39]. Using needles specifically designed for osteoporotic bone, such as those with sharper tips and adjustable depths, minimizes fracture risk[7]. Gentle aspiration techniques, applying controlled pressure, further reduce the chances of cortical breaches. In patients with comorbid conditions such as diabetes or vascular disease, a comprehensive evaluation of how these conditions impact MSC function and healing potential is needed[35]. Anesthesia and sedation protocols should be tailored to the patient’s cardiovascular or metabolic status to reduce procedural risks[7]. Collaborative care, involving specialists like endocrinologists and cardiologists, helps in optimizing patient outcomes. Optimizing aspiration techniques, such as using slow aspiration rates and limiting the volume per pull, can improve MSC purity[7]. Ensuring strict aseptic conditions throughout the procedure is crucial to prevent contamination and maintain cell viability. Educating patients regarding the procedure, its risks, benefits, and post-procedural care can significantly enhance compliance and overall satisfaction.
Personalized approaches based on patient-specific factors are essential for tailoring BMH and MSC therapy. Age-related factors, such as reduced proliferative capacity and differentiation potential of MSCs in older patients, should be considered[7]. While the iliac crest often provides the highest MSC yield, alternative sites like the proximal tibia may be preferred to reduce morbidity during specific surgeries. Adjunct therapies, such as combining MSCs with growth factors or scaffolds, can be used to boost efficacy in older populations. In patients with decreased bone density, imaging modalities help evaluate bone integrity and determine suitable harvesting sites. Adjustments in needle insertion angles and aspiration pressures can reduce the risk of fractures[7]. For individuals with severe osteoporosis, alternative MSC sources like adipose-derived stem cells may be considered. Comorbid conditions, such as diabetes, necessitate cus
Maintaining a sterile environment during the BMA procedure is crucial to prevent infections, which can have severe consequences for both the patient and the quality of the harvested MSCs[40]. The procedure should be performed in a controlled, sterile environment, such as an operating room or a dedicated procedure room equipped with appropriate facilities for sterilization. All equipment, including needles, syringes, and aspiration devices, must be sterile. The skin at the aspiration site should be thoroughly disinfected using antiseptic solutions like chlorhexidine or iodine. Sterile drapes should be used to isolate the procedure area from potential contaminants. Additionally, the clinician performing the aspiration should wear sterile gloves, gowns, and masks to minimize the risk of contamination.
The choice of equipment for BMA is integral to the success of the procedure. High-quality needles, such as the Jamshidi[23] or AMC[21] needles, should be used to ensure effective penetration of the bone and optimal aspiration of marrow. These needles are designed to minimize trauma to the bone and surrounding tissues, reducing patient discomfort and improving the yield of MSCs. Advanced aspiration devices that control the pressure and rate of aspiration are also recommended. These devices can help standardize the procedure, ensuring consistent results across different patients and operators. For example, powered bone marrow aspiration devices like the OnControl system provide a more controlled and efficient method of marrow extraction, which can enhance the quality and quantity of the harvested cells[30]. In addition to the aspiration equipment, facilities for immediate processing of the bone marrow sample are necessary. This includes centrifuges for separating MSCs from other cellular components and flow cytometry equipment for assessing cell viability and concentration. In regions where regulatory requirements permit, in-room processing of the aspirate may be performed to concentrate the MSCs before they are used in therapy.
Informed consent and patient education: Obtaining informed consent is a legal and ethical requirement before performing BMA[6]. The patient must be fully informed about the procedure, including its purpose, risks, benefits, and potential complications. This discussion should also cover what the patient can expect during and after the procedure, including the possibility of pain or discomfort, the time required for recovery, and any specific post-procedure care instructions. Patient education is also critical. Educating patients about the procedure helps to alleviate anxiety, which can significantly impact their overall experience and even the physiological outcomes of the procedure. Patients should be informed about the importance of following pre-procedure instructions, such as fasting or avoiding certain medications, to ensure the best possible outcome.
Pre-procedural medications: Depending on the patient’s condition and the expected level of discomfort, pre-procedural medications may be administered[6]. Local anesthesia is typically used to numb the aspiration site, reducing pain during the procedure. In some cases, especially in patients with high anxiety or those undergoing multiple aspirations, additional anxiolytics or mild sedatives may be administered to help the patient relax. For patients with a history of significant pain during previous BMAs, or those who express considerable concern about pain, stronger analgesics or even conscious sedation may be considered. However, these decisions must be made on a case-by-case basis, weighing the benefits of pain control against the risks of sedation.
Imaging and site selection: Pre-procedural imaging can be beneficial in selecting the optimal site for marrow aspiration. While the PSIS is the most common site for BMA, alternative sites may be considered based on the patient’s anatomy and bone density[25]. Imaging techniques such as MRI or CT scans provide detailed views of the bone and marrow cavity, allowing for more precise targeting of rich marrow areas and avoiding areas with poor cellularity or high-fat content[41,42].
Sample transport and handling: After the bone marrow is aspirated, the sample must be handled with utmost care to maintain the viability and quality of the MSCs. The sample should be immediately transferred to a sterile, pre-chilled container to minimize cell degradation. Rapid transport to the processing laboratory is essential, especially if the aspirate is being used for immediate therapeutic purposes. During transport, the sample should be kept at a controlled temperature, ideally between 2 °C and 8 °C, to preserve cell viability[43,44]. It is also crucial to minimize agitation during transport, as excessive movement can damage the delicate MSCs.
After the successful aspiration of bone marrow, the handling and processing of the BMA are crucial steps that determine the quality, concentration, and efficacy of the BMAC. This section provides a detailed overview of the best practices and critical considerations involved in the post-aspiration handling and processing of BMAC. Once the bone marrow has been aspirated, it must be promptly handled to prevent cellular degradation and clot formation, which could compromise the viability of the stem cells and other bioactive components. The aspirate is typically collected in syringes preloaded with anticoagulants such as heparin to prevent clotting[5]. Anticoagulation is essential because the formation of clots within the aspirate can significantly reduce the yield of viable cells and other critical components necessary for therapeutic use[7]. The aspirated bone marrow should be immediately filtered through a sterile, mesh filter (typically 200-micron) to remove bone fragments and other debris[12]. This filtration step is critical to ensure that the aspirate is free from particulate matter that could interfere with subsequent processing or introduce complications during the injection phase.
Centrifugation is the cornerstone of BMAC processing, allowing for the concentration of stem cells, platelets, and growth factors while removing unwanted components such as red blood cells and plasma. The process typically involves a two-step centrifugation protocol.
First centrifugation (separation step): The filtered bone marrow aspirate is transferred into sterile 50 mL conical tubes and subjected to an initial centrifugation at approximately 3200 rpm for 15 minutes[5]. This step separates the blood components into distinct layers, with the buffy coat (containing mononuclear cells, including MSCs) situated between the red blood cell layer and the plasma.
Buffy coat isolation: After the first centrifugation, the buffy coat layer, which is rich in MSCs and platelets, is carefully extracted. This layer is crucial as it contains the highest concentration of the desired therapeutic cells and factors. The buffy coat is typically transferred into new conical tubes for further processing.
Second centrifugation (concentration step): The isolated buffy coat undergoes a second round of centrifugation at a higher speed, typically around 4800 rpm for 15 minutes[5]. This step further concentrates the mononuclear cells and platelets, resulting in a denser pellet at the bottom of the tube. The supernatant, which contains platelet-poor plasma, is carefully removed, leaving the concentrated BMAC.
Resuspension of the concentrate: The final step in the centrifugation process involves resuspending the concentrated cell pellet in a small volume of platelet-poor plasma or another suitable carrier solution. This resuspension is essential to achieve a consistent and injectable BMAC product, ensuring that the stem cells and growth factors are evenly distributed throughout the solution.
Following centrifugation, it is imperative to perform quality control checks on the BMAC to ensure its suitability for clinical use. This typically involves performing a complete blood count with differential and a hemacytometer analysis to assess the concentration and viability of the MSCs and other mononuclear cells within the BMAC[35,45,46]. The analysis includes the following parameters.
Total nucleated cell count: A higher total nucleated cell count indicates a more concentrated BMAC, which is generally desirable for therapeutic applications.
Mononuclear cell count: This specific cell count is crucial as it includes MSCs, which are the primary therapeutic agents in BMAC.
Cell viability: Assessing cell viability is essential to ensure that the BMAC contains a high proportion of live, functional cells capable of contributing to tissue repair and regeneration.
The results of these analyses guide the decision-making process regarding the adequacy of the BMAC for clinical application. If the cell counts or viability are below acceptable thresholds, additional processing steps or adjustments may be required.
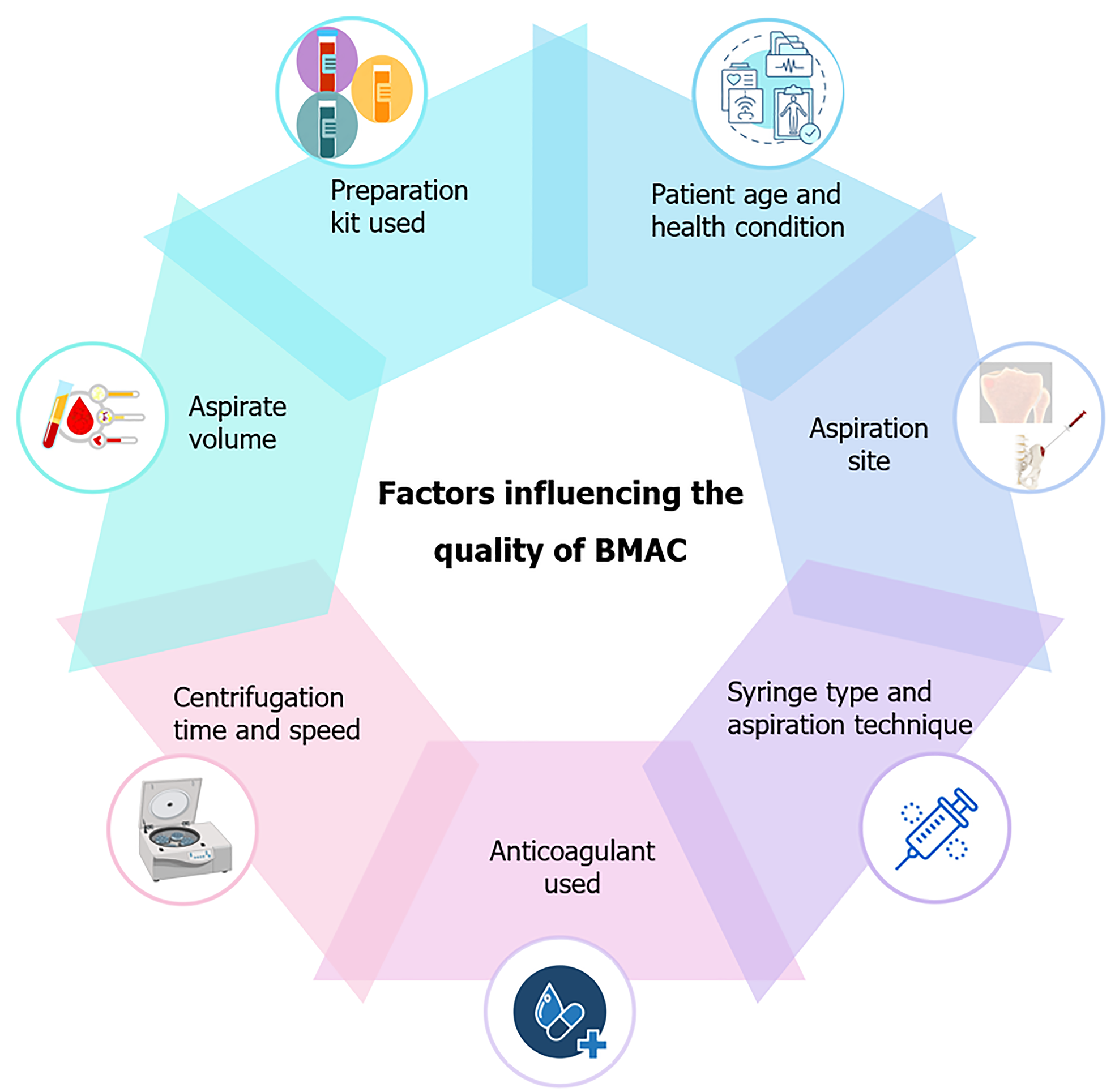
Several factors can influence the quality and therapeutic efficacy of BMAC, and careful attention must be paid to these during the processing phase.
Temperature control: Maintaining the aspirate and BMAC at appropriate temperatures (typically around 4 °C to 8 °C) throughout the processing is crucial to preserving cell viability. Prolonged exposure to suboptimal temperatures can lead to cellular apoptosis or necrosis, reducing the efficacy of the BMAC.
Minimizing mechanical stress: The handling of BMAC during processing should be done gently to avoid mechanical stress that could damage the cells. Excessive agitation or forceful pipetting can result in cell lysis, decreasing the overall cell count and viability.
Processing time: The time between aspiration and final processing should be minimized. Prolonged delays can lead to cell death and reduced growth factor activity, ultimately compromising the therapeutic potential of the BMAC.
Sterility: Throughout the handling and processing of BMAC, maintaining a sterile environment is paramount to prevent contamination. This includes using sterile equipment, working within a laminar flow hood when possible, and employing aseptic techniques at all stages.
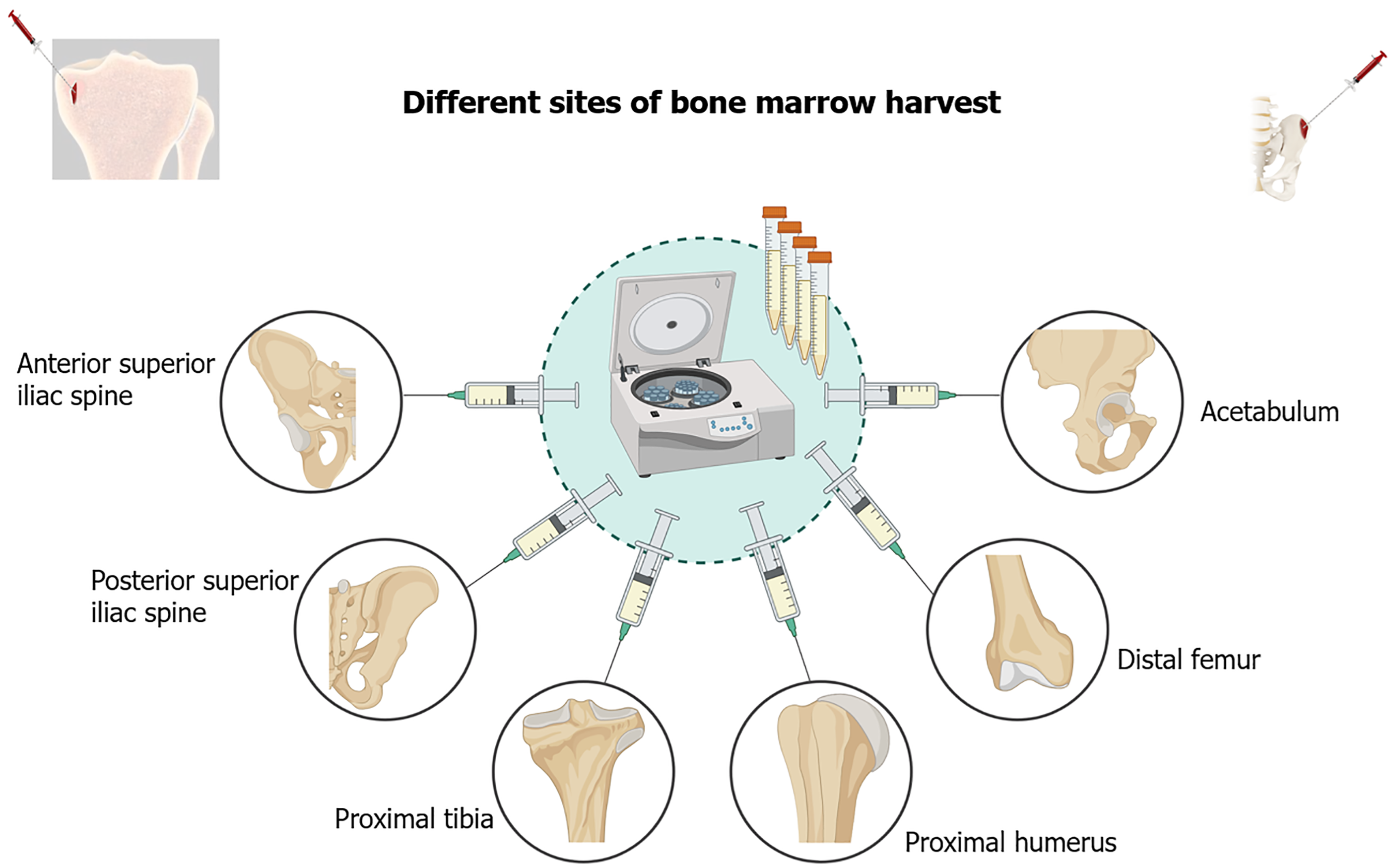
The cellular characteristics of bone marrow from various anatomical locations are tabulated in Table 1[5,6,46] and Figure 2. The clinical application of bone marrow from various anatomical locations are tabulated in Table 2. The merits and de-merits of bone marrow from various anatomical locations are tabulated in Table 3. The factors influencing the quality of BMAC is depicted in Figure 3.
| Anatomical site | Common use | MSC characteristics | Differentiation potential | Homing and engraftment potential | Therapeutic use |
| Iliac crest[5] | Most commonly used site for bone marrow harvesting due to its high yield and easy access | Robust proliferative capacity, multi-lineage differentiation potential. High expression of CD105, CD73, CD90, CD146, and CD271 | Potent osteogenic differentiation; suitable for bone regeneration. High expression of alkaline phosphatase and osteocalcin | Higher due to the high expression of adhesion molecules like CD146 and CD271 | Ideal for bone regeneration due to osteogenic potential may support hematopoiesis |
| Tibial bone marrow[6] | Alternative site for MSC harvesting, especially for knee OA treatment | Potentially enhanced chondrogenic differentiation. Higher expression of chondrogenic markers like Sox9 and aggrecan | Enhanced chondrogenic potential; produces glycosaminoglycans and type II collagen. Suited for cartilage repair | Potentially higher chondrogenic activity due to enhanced niche for cartilage repair | Better suited for cartilage regeneration in OA due to chondrogenic differentiation potential |
| Femoral bone marrow[46] | Commonly accessed during orthopedic procedures such as total knee arthroplasty | High osteogenic potential, expressed higher levels of Runt-related transcription factor-2 and bone sialoprotein but lower proliferation rates compared to iliac crest | Superior osteogenic potential, higher mineralization capacity. High calcium deposition during osteogenic differentiation | Lower proliferation rates but high osteogenic commitment | Best suited for bone repair applications like non-union fractures or large bone defects |
| Anatomical site | Clinical application | Advantages | Drawbacks | Clinical considerations |
| Iliac crest | Gold standard site for BMAC harvesting, widely used in regenerative therapies for knee OA due to high progenitor cell yield. High culture success rate for MSCs | High nucleated cell and clone forming unit yield, extensive clinical experience, and literature supporting efficacy. MSC yield is superior and the culture success rate can reach up to 90% | Associated with donor-site morbidity, including pain, hematoma, and nerve injury. Requires a secondary surgical site, increasing invasiveness | Preferred in cases where maximum progenitor cell yield is critical. Established protocols and extensive use in knee OA treatment. Potential for postoperative complications |
| Proximal humerus | Emerging alternative, commonly used in shoulder surgeries such as rotator cuff repair. Offers high-quality BMAC without a secondary incision | No need for separate incisions during shoulder procedures, and high progenitor cell yield even after large volume aspirations. Reliable across patient age groups | Primarily useful in shoulder surgeries, less studied compared to the iliac crest, though efficacy is promising | Best for minimizing patient morbidity in shoulder surgeries, with comparable efficacy to iliac crest BMAC. Convenient for combined procedures |
| Acetabulum | Primarily used in hip surgeries, where BMAC harvesting can occur within the same surgical field, offering dual-purpose potential. Useful for hip-related therapies | Convenient for hip-related procedures, high progenitor cell counts comparable to the iliac crest. Single-session harvesting and BMAC preparation | Limited to hip-related procedures, indirect application in knee OA treatment | Suited for scenarios where a dual-purpose approach is needed, particularly in hip surgeries. Produces high-quality BMAC but is limited to specific surgeries |
| Distal femur | Anatomically accessible during knee surgeries, particularly TKA. Can be seamlessly integrated with the procedure for autologous therapies | Easy anatomical access during knee surgeries, minimally invasive, lower complication risk, and integrated into surgical workflow. MSCs show similar differentiation potential to those from the iliac crest | Lower MSC yield compared to iliac crest (0.67 million cells/mL vs 10.05 million cells/mL). Slightly lower MSC culture success rate | A viable alternative when iliac crest access is limited or undesirable. Moderate MSC culture success rate (approximately 71%) but lower yield. Beneficial in knee OA treatments integrated with TKA |
| Proximal tibia | Similar to the distal femur, the proximal tibia can be harvested during knee surgeries like TKA. Lower MSC yield compared to the iliac crest but viable for knee OA therapy | Reduces invasiveness, less risk of complications. Easier access in knee surgeries. MSCs exhibit robust differentiation capacity, although yield is lower than iliac crest | Lower MSC yield than iliac crest (1.70 million cells/mL vs 10.05 million cells/mL). MSC culture success rate is around 47% | Suitable alternative for patients contraindicated for iliac crest harvesting. Moderate MSC yield and culture success rate (approximately 47%). Useful in knee-focused procedures |
| Anatomical site | Merits | Demerits |
| Anterior superior iliac spine | Ease of access: Superficially located and easy to palpate, facilitating quicker and less invasive procedures | Lower cell purity: Increased risk of blood dilution due to fatty tissue, potentially reducing MSC concentration |
| Adequate yield: Provides a good volume of aspirate with acceptable TNC and CFU-f yields | Variable CFU-f yield: Typically lower CFU-f counts compared to the posterior superior iliac spine, which may affect the therapeutic potential of the aspirate | |
| Lower complication rate: Reduced risk of neurovascular injury and other complications when performed with proper technique | Patient discomfort: Proximity to muscle attachments can cause discomfort during and after the procedure | |
| High culture success rate: High success rate in MSC culture, indicating reliable cell viability and expansion potential | Postoperative pain: Potential for significant postoperative pain and hematoma formation | |
| Posterior superior iliac spine | High cell yield: Provides a high concentration of TNCs and CFU-fs, making it the preferred site for harvesting high-quality aspirates | Increased technical difficulty: Less accessible, particularly in patients with high BMI or anatomical variations, requiring more complex positioning and technique |
| Reduced blood dilution: Lower fatty infiltration results in higher cell purity and reduced blood contamination | Higher risk of complications: Proximity to the sacroiliac joint and gluteal neurovascular bundle increases the risk of neurovascular injury | |
| Consistency in results: Yields consistent results with less variability in cell counts across different patients | Patient discomfort: A deeper location and the need to traverse more tissue can cause significant post-procedural pain | |
| Gold standard for MSC yield: Considered the gold standard for bone marrow harvesting due to its high MSC yield and well-established protocols | Donor-site morbidity: Associated with significant morbidity, including pain, hematoma, and nerve injury, which may deter its use in certain populations | |
| Proximal tibia | Convenience in certain surgeries: Proximity to the knee joint makes it convenient during knee-related surgeries, reducing procedure time | Lower MSC concentration: Typically provides a lower concentration of MSCs compared to the iliac crest, which may limit its effectiveness in regenerative therapies |
| Adequate cell yield in some cases: Can produce a reasonable volume of aspirate, especially when large volumes are needed | Greater variability in yield: High variability in cell yield depending on factors such as patient age, BMI, and bone density, leading to inconsistent results | |
| Reduced risk of major complications: Stable site with a lower risk of major complications like neurovascular injury, making it a safer choice in some contexts | Difficulty in aspiration technique: Requires careful technique to avoid complications such as cortical bone fracture, particularly in osteoporotic patients | |
| Integrated into knee surgeries: Easily integrated into knee surgeries like TKA, adding minimal additional risk and reducing invasiveness | Lower culture success rate: MSC culture success rate is lower compared to the iliac crest, which may limit its utility in certain therapeutic applications | |
| Proximal humerus | Convenience in shoulder surgeries: Located within the surgical field during shoulder procedures, reducing the need for an additional surgical site | Limited data: While promising, there is limited data compared to the iliac crest, and long-term outcomes need further study |
| High MSC yield: Can yield a comparable number of progenitor cells to the iliac crest, making it a viable alternative for bone marrow aspirate concentrate preparation | Variability with age: Potential variability in MSC yield with age, although studies suggest this site may still be reliable across different age groups | |
| Reduced morbidity: Less invasive compared to iliac crest harvesting, with a lower risk of complications and patient discomfort | Not standard practice: Not as widely used or studied as the iliac crest, leading to less familiarity and potentially greater variability in outcomes | |
| Distal femur | Ease of access during knee surgeries: Easily accessible during knee surgeries such as TKA, reducing the need for additional procedures | Lower MSC concentration: Significantly lower MSC concentration compared to the iliac crest, potentially limiting its effectiveness in high-demand applications |
| Lower postoperative complications: Reduced invasiveness with potentially fewer postoperative complications, particularly in patients with previous pelvic surgeries | Lower culture success rate: The culture success rate for MSCs is lower than that of the iliac crest, which may affect the feasibility of its use in large-scale therapeutic applications | |
| Potential for integration into existing surgeries: Can be seamlessly integrated into existing knee procedures, adding minimal risk and enhancing therapeutic options | Inconsistent yield: Variability in cell yield can lead to inconsistent outcomes, which may affect the reliability of the site for routine use in MSC harvesting | |
| Acetabulum | Dual-purpose during hip surgeries: Accessible during hip surgeries, allowing simultaneous bone marrow harvesting without additional surgical risks | Limited to hip procedures: Primarily applicable in the context of hip surgeries, limiting its broader use in other orthopedic applications such as knee OA |
| Comparable yield to iliac crest: Studies suggest a comparable progenitor cell yield to the iliac crest, making it a feasible alternative in certain contexts | Not a primary choice for knee OA: While effective for hip procedures, its role in knee OA treatment is more indirect and not commonly pursued as a first choice |
BMH from alternative sites, such as the distal femur and proximal tibia, presents notable limitations, primarily due to lower MSC yields compared to the gold standard iliac crest. This lower yield often necessitates the use of larger bone marrow volumes or in vitro cell expansion, increasing both time and cost. Additionally, MSC yields are highly variable due to factors like patient age, bone quality, and harvesting techniques, which further complicate the process. The absence of standardized protocols for bone marrow aspiration across different sites adds to this challenge, resulting in inconsistent outcomes and difficulties in comparing clinical studies. Moreover, donor-site morbidity, especially at the iliac crest, raises concerns about postoperative complications such as pain, hematomas, or nerve damage, prompting the exploration of alternative sites. However, these alternatives also carry risks, such as cortical bone fractures during harvesting from the tibia or femur. Personalized approaches, considering patient-specific factors like age, comorbidities, and bone health, are becoming more relevant to optimizing therapeutic outcomes. Future research should focus on improving aspiration techniques, refining tools for harvesting, and developing standardized protocols for various anatomical sites. Large-scale, comparative studies are needed to evaluate the yield and functionality of MSCs from different sites. Integrating new technologies like three-dimensional (3D) bioprinting, bioengineered scaffolds, and gene editing holds promise for improving the efficacy and safety of MSC-based therapies, particularly in regenerative applications like knee OA. The summary of challenges and limitations in isolation and characterization of bone marrow is summarized in Table 4.
| Challenges/limitations | Proposed solutions | Research gaps | Future directions |
| Lower MSC yield from alternative sites (distal femur, proximal tibia) | Optimize harvesting techniques at alternative sites to enhance MSC yield and viability | Lack of comprehensive comparative studies of MSC yield from different anatomical sites | Prioritize large-scale, randomized controlled trials across multiple anatomical sites |
| Necessity of larger volumes or in vitro expansion due to low yield | Refinements in aspiration technique and improvements in instruments | Limited data on long-term efficacy and safety of MSC-based therapies | Focus on personalized harvesting strategies based on biomarkers and patient characteristics |
| Influence of patient-specific factors (age and bone quality) | Develop protocols that combine cells from multiple sites for therapeutic dose | The absence of standardized protocols leads to variability in outcomes | Explore the integration of bone marrow harvesting techniques with emerging technologies (three-dimensional bioprinting, gene editing) |
| Variability in MSC yield and success rates across patients | Conduct large-scale comparative studies evaluating MSC yield, viability, and regenerative potential | Insufficient exploration of alternative harvesting sites for applications beyond knee osteoarthritis | Develop bioengineered scaffolds to enhance MSC survival and differentiation |
| Absence of standardized aspiration protocols for different sites | Establish standardized bone marrow aspiration protocols | Limited understanding of MSC functional heterogeneity from different sites | Investigate pre-operative and post-operative strategies to minimize complications |
| Complications at alternative sites | Explore less invasive harvesting techniques to reduce morbidity | Lack of personalized strategies considering genetic background, age, and disease state | Use advanced techniques (single-cell RNA sequencing, proteomics) to assess MSC characteristics |
| Donor-site morbidity from iliac crest harvesting | Innovate with rotational aspiration devices and powered biopsy systems | ||
| Age and health-related limitations (osteoporosis, lower MSC density) | Investigate personalized approaches based on patient-specific factors | ||
| Long-term efficacy and safety of MSC therapies not fully studied | Include extended follow-up in studies to assess long-term efficacy and safety | ||
Despite the potential of alternative harvesting sites, several limitations hinder the widespread adoption of these techniques. Many existing studies have small sample sizes and lack statistical power, which limits the generalizability of their findings. The heterogeneity in study designs, including variations in aspiration volumes, needle types, and processing methods, complicates comparisons and meta-analyses. The inconsistency in MSC yields from alternative sites is also a significant concern. Factors such as patient age, bone quality, and comorbidities contribute to this variability[35]. Without standardized protocols, it is challenging to determine whether differences in outcomes are due to the harvesting site or procedural inconsistencies. Most studies focus on short-term outcomes, with limited data on the long-term efficacy and safety of MSC therapies derived from alternative sites. This gap makes it difficult to assess the sustained benefits and potential risks associated with these approaches. To address these limitations, future research should conduct large-scale, multicenter trials to increase sample sizes and include diverse populations, thereby enhancing the robustness of findings and their applicability. Standardizing protocols by developing consensus guidelines on harvesting techniques, processing methods, and outcome measures will facilitate comparability across studies. Stratifying patients based on age, bone density, and comorbidities can help isolate the effects of these variables on MSC yield and therapeutic outcomes. Longitudinal follow-up studies, incorporating extended periods of observation, are needed to provide insights into the durability of treatment effects and potential late-onset complications. By critically analyzing current limitations and outlining strategies to overcome them, the field can advance towards more effective and reliable MSC-based therapies for knee OA.
The incorporation of emerging technologies has the potential to significantly enhance BMH techniques and MSC therapies for knee OA.
Single-cell sequencing: Single-cell RNA sequencing enables the detailed analysis of individual MSCs, revealing heterogeneity within cell populations[47]. By identifying subpopulations with superior regenerative potential or specific differentiation capabilities, clinicians can select or enrich for MSCs most likely to contribute to cartilage repair. This precision can improve therapeutic efficacy and allow for the development of customized MSC products tailored to patient needs.
Advanced imaging techniques: Utilizing advanced imaging modalities such as high-resolution MRI, micro-CT, and ultrasound elastography can assist in assessing bone quality and marrow composition before harvesting. These techniques can identify areas with higher MSC concentrations or better bone integrity, guiding needle placement to optimize cell yield. Intraoperative imaging can also enhance the accuracy of needle insertion, reducing procedural complications[48].
Artificial intelligence and machine learning: Artificial intelligence (AI) and machine learning algorithms can analyze large datasets to predict MSC yield, viability, and differentiation potential based on patient-specific variables. By integrating demographic data, imaging findings, and procedural parameters, AI can assist in personalizing harvesting protocols and predicting therapeutic outcomes. Machine learning models can also optimize processing techniques by identifying patterns that correlate with higher MSC viability and potency[49].
Intraoperative guidance systems: The development of AI-driven intraoperative guidance systems can improve the precision of bone marrow aspiration. Real-time feedback on needle positioning and aspiration parameters can enhance MSC yield while minimizing patient discomfort and procedural risks. Such systems can be particularly beneficial in patients with anatomical variations or compromised bone quality[50].
3D bioprinting and tissue engineering: Advancements in 3D bioprinting allow for the creation of custom scaffolds that can be seeded with MSCs to generate tissue constructs mimicking native cartilage. By integrating patient-specific imaging data, personalized implants can be designed to fit precisely within cartilage defects, enhancing integration and functional recovery[50].
Integrating these emerging technologies into clinical practice requires interdisciplinary collaboration and adherence to regulatory standards. Continued research and development in these areas hold promise for optimizing MSC harvesting and therapies, ultimately improving outcomes for patients with knee OA.
Advancing MSC-based therapies for knee OA necessitates exploring innovative research directions and developing theoretical models that can shape future studies. One promising avenue is the utilization of computational modeling to predict MSC yield and viability based on patient-specific factors such as age, bone density, and comorbidities[51,52]. By integrating patient data into predictive algorithms, clinicians can tailor harvesting strategies to optimize both the quantity and quality of MSCs obtained from individual patients. Systems biology approaches can be employed to unravel the complex interactions between MSCs and the osteoarthritic joint environment. By constructing computational models of signaling pathways and gene networks, researchers can identify key regulatory nodes that influence MSC differentiation and cartilage regeneration. This holistic understanding can inform the development of targeted therapies that modulate specific molecular pathways to enhance therapeutic outcomes.
Investigating the MSC secretome, particularly extracellular vesicles (EVs) and exosomes, represents another innovative direction. These vesicles carry bioactive molecules that can modulate inflammation and promote tissue repair. Exploring the therapeutic potential of MSC-derived EVs may lead to cell-free therapies that mitigate the risks associated with cell transplantation while harnessing the regenerative capabilities of MSCs[53,54]. Combining MSC therapies with biomaterials and scaffold technologies also holds significant promise. Developing bioengineered scaffolds that mimic the native extracellular matrix can provide structural support and enhance MSC survival, proliferation, and differentiation within the joint. Such scaffolds can be designed to deliver MSCs in a controlled manner, improving their integration and functional contribution to cartilage repair[55]. Utilising gene-editing technologies like CRISPR/Cas9 allows for the genetic modification of MSCs to enhance their regenerative properties[56–58]. By upregulating genes associated with chondrogenesis or downregulating inhibitory pathways, engineered MSCs can exhibit improved efficacy in cartilage repair. These innovative research directions and theoretical models offer valuable pathways for optimizing MSC-based therapies and warrant further exploration in future studies.
The exploration of alternative BMH sites, such as the proximal tibia, distal femur, and proximal humerus, offers promising avenues for optimizing MSC yield and minimizing donor-site morbidity, particularly in the context of knee OA treatment. While the iliac crest remains the gold standard due to its high MSC concentration, the emerging evidence suggests that alternative sites may provide viable MSCs with significant therapeutic potential, albeit with some limitations in yield and consistency. To fully harness the regenerative capabilities of MSCs, further research is essential to standardize harvesting techniques, improve cell viability, and refine clinical applications. As the field advances, personalized approaches, tailored to patient-specific factors and optimized harvesting strategies, will be crucial in enhancing the efficacy of MSC-based therapies for OA and other degenerative joint conditions, ultimately improving patient outcomes and quality of life.
| 1. | Li E, Tan J, Xu K, Pan Y, Xu P. Global burden and socioeconomic impact of knee osteoarthritis: a comprehensive analysis. Front Med (Lausanne). 2024;11:1323091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e508-e522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 570] [Article Influence: 285.0] [Reference Citation Analysis (0)] |
| 3. | Ondrésik M, Azevedo Maia FR, da Silva Morais A, Gertrudes AC, Dias Bacelar AH, Correia C, Gonçalves C, Radhouani H, Amandi Sousa R, Oliveira JM, Reis RL. Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng. 2017;114:717-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Uivaraseanu B, Vesa CM, Tit DM, Abid A, Maghiar O, Maghiar TA, Hozan C, Nechifor AC, Behl T, Patrascu JM, Bungau S. Therapeutic approaches in the management of knee osteoarthritis (Review). Exp Ther Med. 2022;23:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Jeyaraman M, Jeyaraman N, Ramasubramanian S, Ranjan R, Jha SK, Gupta A. Bone Marrow Aspirate Concentrate for Treatment of Primary Knee Osteoarthritis: A Prospective, Single-Center, Non-randomized Study with 2-Year Follow-Up. Indian J Orthop. 2024;58:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Allahabadi S, Haneberg EC, Elias TJ, Cole BJ, Yanke AB. Lateral Opening-Wedge Distal Femoral Osteotomy Made Easy: Tips and Tricks. Arthrosc Tech. 2024;13:102816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Jeyaraman M, Bingi SK, Muthu S, Jeyaraman N, Packkyarathinam RP, Ranjan R, Sharma S, Jha SK, Khanna M, Rajendran SNS, Rajendran RL, Gangadaran P. Impact of the Process Variables on the Yield of Mesenchymal Stromal Cells from Bone Marrow Aspirate Concentrate. Bioengineering (Basel). 2022;9:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Vasiliadis AV, Galanis N. Human bone marrow-derived mesenchymal stem cells from different bone sources: a panorama. Stem Cell Investig. 2020;7:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Narbona-Carceles J, Vaquero J, Suárez-Sancho S, Forriol F, Fernández-Santos ME. Bone marrow mesenchymal stem cell aspirates from alternative sources: is the knee as good as the iliac crest? Injury. 2014;45 Suppl 4:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Carneiro DC, Araújo LT, Santos GC, Damasceno PKF, Vieira JL, Santos RRD, Barbosa JDV, Soares MBP. Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage. Int J Mol Sci. 2023;24:9939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 11. | Rindy LJ, Chambers AR. Bone Marrow Aspiration and Biopsy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. |
| 12. | Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc Tech. 2017;6:e441-e445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Abla O, Friedman J, Doyle J. Performing bone marrow aspiration and biopsy in children: Recommended guidelines. Paediatr Child Health. 2008;13:499-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Konda B, Pathak S, Edwin I, Mishall P, Downie SA, Olson TR, Reed LJ, Friedman EW. Safe and successful bone marrow biopsy: an anatomical and CT-based cadaver study. Am J Hematol. 2014;89:943-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Shapiro SA, Arthurs JR. Ultrasound-guided needle placement for bone marrow aspiration of the anterior iliac crest. JCJP. 2022;2:100057. [DOI] [Full Text] |
| 16. | Holdsworth MT, Raisch DW, Winter SS, Frost JD, Moro MA, Doran NH, Phillips J, Pankey JM, Mathew P. Pain and distress from bone marrow aspirations and lumbar punctures. Ann Pharmacother. 2003;37:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Oliver K, Awan T, Bayes M. Single- Versus Multiple-Site Harvesting Techniques for Bone Marrow Concentrate: Evaluation of Aspirate Quality and Pain. Orthop J Sports Med. 2017;5:2325967117724398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Fennema EM, Renard AJ, Leusink A, van Blitterswijk CA, de Boer J. The effect of bone marrow aspiration strategy on the yield and quality of human mesenchymal stem cells. Acta Orthop. 2009;80:618-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Muench LN, Kia C, Otto A, Mehl J, Baldino JB, Cote MP, McCarthy MB, Beitzel K, Mazzocca AD. The effect of a single consecutive volume aspiration on concentrated bone marrow from the proximal humerus for clinical application. BMC Musculoskelet Disord. 2019;20:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Hernigou P, Homma Y, Flouzat Lachaniette CH, Poignard A, Allain J, Chevallier N, Rouard H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop. 2013;37:2279-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Kuebler D, Schnee A, Moore L, Kouri J, McLaughlin A, Hanson R, Kuebler P, Dallo I, Gobbi A. Short-Term Efficacy of Using a Novel Low-Volume Bone Marrow Aspiration Technique to Treat Knee Osteoarthritis: A Retrospective Cohort Study. Stem Cells Int. 2022;2022:5394441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Pabinger C, Dammerer D, Lothaller H, Kobinia GS. Reorientation technique has benefits in bone marrow aspiration of stem cells. Sci Rep. 2022;12:11637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Islam A. Bone marrow aspiration before bone marrow core biopsy using the same bone marrow biopsy needle: a good or bad practice? J Clin Pathol. 2007;60:212-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Feddahi N, Herten M, Tassemeier T, Rekasi H, Hackel A, Haversath M, Jäger M. Does Needle Design Affect the Regenerative Potential of Bone Marrow Aspirate? An In Vitro Study. Life (Basel). 2021;11:748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Malempati S, Joshi S, Lai S, Braner DA, Tegtmeyer K. Videos in clinical medicine. Bone marrow aspiration and biopsy. N Engl J Med. 2009;361:e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | D'Souza RS, Li L, Leng S, Hunt C, Law L, Muir C, Eldrige J, Bydon M, Chi M, Shapiro S, Mauck WD, Qu W. A three-dimensional computed tomography study to determine the ideal method for fluoroscopically-guided bone marrow aspiration from the iliac crest. Bosn J Basic Med Sci. 2021;21:370-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 27. | Grønkjær M, Hasselgren CF, Østergaard AS, Johansen P, Korup J, Bøgsted M, Bilgrau AE, Jensen P. Bone Marrow Aspiration: A Randomized Controlled Trial Assessing the Quality of Bone Marrow Specimens Using Slow and Rapid Aspiration Techniques and Evaluating Pain Intensity. Acta Haematol. 2016;135:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Glennon CA, Woodroof JM, Kambhampati S, Battershell AC, O'Connor SR, Roberts KB. Comparison of Bone Marrow Biopsy Specimens Obtained Using a Motorized Device and Manual Biopsy Systems. Asia Pac J Oncol Nurs. 2018;5:394-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Berenson JR, Yellin O, Blumenstein B, Bojanower D, Croopnick J, Aboulafia D, Upadhyaya G, Spadaccini C. Using a powered bone marrow biopsy system results in shorter procedures, causes less residual pain to adult patients, and yields larger specimens. Diagn Pathol. 2011;6:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Reed LJ, Raghupathy R, Strakhan M, Philbeck TE, Kim MY, Battini R, Hussain Z, Abdullah S, Schweber S, Bala K, Pacello T. The OnControl bone marrow biopsy technique is superior to the standard manual technique for hematologists-in-training: a prospective, randomized comparison. Hematol Rep. 2011;3:e21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Bixel MG, Kusumbe AP, Ramasamy SK, Sivaraj KK, Butz S, Vestweber D, Adams RH. Flow Dynamics and HSPC Homing in Bone Marrow Microvessels. Cell Rep. 2017;18:1804-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Brozovich A, Sinicrope BJ, Bauza G, Niclot FB, Lintner D, Taraballi F, McCulloch PC. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared With Traditional Bone Marrow Aspiration Technique. Orthop J Sports Med. 2021;9:23259671211058459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Selle M, Koch JD, Ongsiek A, Ulbrich L, Ye W, Jiang Z, Krettek C, Neunaber C, Noack S. Influence of age on stem cells depends on the sex of the bone marrow donor. J Cell Mol Med. 2022;26:1594-1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 34. | Massaro F, Corrillon F, Stamatopoulos B, Dubois N, Ruer A, Meuleman N, Bron D, Lagneaux L. Age-related changes in human bone marrow mesenchymal stromal cells: morphology, gene expression profile, immunomodulatory activity and miRNA expression. Front Immunol. 2023;14:1267550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Muthu S, Jeyaraman M, Narula A, Ravi VR, Gandi A, Khanna M, Maffulli N, Gupta A. Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate-A Retrospective Analysis of 58 Patients. Biomedicines. 2023;11:738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Li J, Wong WH, Chan S, Chim JC, Cheung KM, Lee TL, Au WY, Ha SY, Lie AK, Lau YL, Liang RH, Chan GC. Factors affecting mesenchymal stromal cells yield from bone marrow aspiration. Chin J Cancer Res. 2011;23:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Calvet L, Pereira B, Sapin AF, Mareynat G, Lautrette A, Souweine B. Contribution to diagnosis and treatment of bone marrow aspirate results in critically ill patients undergoing bone marrow aspiration: a retrospective study of 193 consecutive patients. J Intensive Care. 2017;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Moore C, Kotchetkov R. Anticoagulation and bone marrow biopsy: is it safe to proceed? Hematology. 2021;26:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Ahmad A, Crawford CH 3rd, Glassman SD, Dimar JR 2nd, Gum JL, Carreon LY. Correlation between bone density measurements on CT or MRI versus DEXA scan: A systematic review. N Am Spine Soc J. 2023;14:100204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Bhaskar N. Bone Marrow Aspiration and Biopsy in Critical Pediatric Patients: A Pathologist's Perspective. Cureus. 2021;13:e17423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 41. | Tomasian A, Long J, W Jennings J. Fluoroscopy-guided bone marrow aspiration and biopsy: technical note. Diagn Interv Radiol. 2021;27:283-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Kaufman CS, Kuo KT, Anand K. Image Guided Bone Marrow Biopsy. Tech Vasc Interv Radiol. 2021;24:100771. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Ngo ATL, Le HM, Trinh NTH, Jun APG, Bach TQ, Bui HTH, Hoang VT, Bui AV, Nguyen LT, Hoang DM. Clinically relevant preservation conditions for mesenchymal stem/stromal cells derived from perinatal and adult tissue sources. J Cell Mol Med. 2021;25:10747-10760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Gorin N, Herzig G, Bull M, Graw RJ. Long-term preservation of bone marrow and stem cell pool in dogs. Blood. 1978;51:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Schäfer R, DeBaun MR, Fleck E, Centeno CJ, Kraft D, Leibacher J, Bieback K, Seifried E, Dragoo JL. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J Transl Med. 2019;17:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Cavallo C, Boffa A, de Girolamo L, Merli G, Kon E, Cattini L, Santo E, Grigolo B, Filardo G. Bone marrow aspirate concentrate quality is affected by age and harvest site. Knee Surg Sports Traumatol Arthrosc. 2023;31:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 47. | Potter SS. Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol. 2018;14:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 390] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 48. | Krug R, Burghardt AJ, Majumdar S, Link TM. High-resolution imaging techniques for the assessment of osteoporosis. Radiol Clin North Am. 2010;48:601-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Nosrati H, Nosrati M. Artificial Intelligence in Regenerative Medicine: Applications and Implications. Biomimetics (Basel). 2023;8:442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 50. | Kolomenskaya E, Butova V, Poltavskiy A, Soldatov A, Butakova M. Application of Artificial Intelligence at All Stages of Bone Tissue Engineering. Biomedicines. 2023;12:76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 51. | Liu YYF, Lu Y, Oh S, Conduit GJ. Machine learning to predict mesenchymal stem cell efficacy for cartilage repair. PLoS Comput Biol. 2020;16:e1008275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Van Grouw A, Colonna MB, Maughon TS, Shen X, Larey AM, Moore SG, Yeago C, Fernández FM, Edison AS, Stice SL, Bowles-Welch AC, Marklein RA. Development of a Robust Consensus Modeling Approach for Identifying Cellular and Media Metabolites Predictive of Mesenchymal Stromal Cell Potency. Stem Cells. 2023;41:792-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Luo D, Zhu H, Li S, Wang Z, Xiao J. Mesenchymal stem cell-derived exosomes as a promising cell-free therapy for knee osteoarthritis. Front Bioeng Biotechnol. 2024;12:1309946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 54. | Jeyaraman M, Muthu S, Shehabaz S, Jeyaraman N, Rajendran RL, Hong CM, Nallakumarasamy A, Packkyarathinam RP, Sharma S, Ranjan R, Khanna M, Ahn BC, Gangadaran P. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp Cell Res. 2022;418:113274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Zhao X, Hu DA, Wu D, He F, Wang H, Huang L, Shi D, Liu Q, Ni N, Pakvasa M, Zhang Y, Fu K, Qin KH, Li AJ, Hagag O, Wang EJ, Sabharwal M, Wagstaff W, Reid RR, Lee MJ, Wolf JM, El Dafrawy M, Hynes K, Strelzow J, Ho SH, He TC, Athiviraham A. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front Bioeng Biotechnol. 2021;9:603444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 56. | Golchin A, Shams F, Karami F. Advancing Mesenchymal Stem Cell Therapy with CRISPR/Cas9 for Clinical Trial Studies. Adv Exp Med Biol. 2020;1247:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Li T, Yang Y, Qi H, Cui W, Zhang L, Fu X, He X, Liu M, Li PF, Yu T. CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduct Target Ther. 2023;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 263] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 58. | Varkouhi AK, Monteiro APT, Tsoporis JN, Mei SHJ, Stewart DJ, Dos Santos CC. Genetically Modified Mesenchymal Stromal/Stem Cells: Application in Critical Illness. Stem Cell Rev Rep. 2020;16:812-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |