Published online Dec 20, 2023. doi: 10.5662/wjm.v13.i5.456
Peer-review started: September 27, 2023
First decision: November 2, 2023
Revised: November 6, 2023
Accepted: December 7, 2023
Article in press: December 7, 2023
Published online: December 20, 2023
Processing time: 84 Days and 2.2 Hours
The coronavirus disease 2019 (COVID-19) pandemic is continuing. The disease most commonly affects the lungs. Since the beginning of the pandemic thorax computed tomography (CT) has been an indispensable imaging method for diagnosis and follow-up. The disease is tried to be controlled with vaccines. Vaccination reduces the possibility of a severe course of the disease.
The aim of this study is to investigate whether the vaccination status of patients hospitalized due to COVID-19 has an effect on the CT severity score (CT-SS) and CORADS score obtained during hospitalization.
The files of patients hospitalized between April 1, 2021 and April 1, 2022 due to COVID-19 were retrospectively reviewed. A total of 224 patients who were older than 18 years of age, whose vaccination status was accessible, whose severe acute respiratory syndrome coronavirus 2 polymerase chain reaction result was positive, and who had a Thorax CT scan during hospitalization were included in the study.
Among the patients included in the study, 52.2% were female and the mean age was 61.85 years. The patients applied to the hospital on the average 7th day of their complaints. While 63 patients were unvaccinated (Group 1), 20 were vaccinated with a single dose of CoronaVac (Group 2), 24 with a single dose of BioNTech (Group 3), 38 with 2 doses of CoronaVac (Group 4), 40 with 2 doses of BioNTech (Group 5), and 39 with 3 doses of vaccine (2 doses of CoronaVac followed by a single dose of BioNTech, Group 6). CT-SS ranged from 5 to 23, with a mean of 12.17.
CT-SS mean of the groups were determined as 14.17, 13.35, 11.58, 10.87, 11.28, 10.85, respectively. Accordingly, as a result of the comparisons between the groups, the CT-SS levels of the unvaccinated patients found to be significantly higher than the other groups. As the vaccination rates increased, the rate of typical COVID-19 findings on CT was found to be significantly lower.
Increased vaccination rates in COVID-19 patients reduce the probability of typical COVID-19 symptoms in the lungs. It also reduces the risk of severe disease and decreases CT Severity Scores. This may lead to a loss of importance of Thorax CT in the diagnosis of COVID-19 pneumonia as the end of the pandemic approaches.
Core Tip: This is a retrospective study to evaluate the effect of vaccination status on CORADS and computed tomography (CT) severity score in hospitalized coronavirus disease 2019 (COVID-19) patients. Accordingly, as a result of the comparisons between the groups, the CT severity score levels of the unvaccinated patients were significantly higher than the other groups. As the vaccination rates increased, the rate of typical COVID-19 findings on CT was found to be significantly lower.
- Citation: Binay UD, Karavaş E, Karakeçili F, Barkay O, Aydin S, Şenbil DC. Effect of vaccination status on CORADS and computed tomography severity score in hospitalized COVID-19 patients: A retrospective study. World J Methodol 2023; 13(5): 456-465
- URL: https://www.wjgnet.com/2222-0682/full/v13/i5/456.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i5.456
The coronavirus disease 2019 (COVID-19) pandemic still continues, although the number of cases has decreased[1]. The disease most commonly affects the lungs, and since the beginning of the pandemic, thorax computed tomography (CT) has been an indispensable imaging method for diagnosis and follow-up[2]. The disease is tried to be controlled with the introduction of vaccines and the increase in vaccination rates. Vaccination reduces the probability of severe course of the disease and the CORADS score, which is an indicator of lung involvement[3-6].
CoronaVac vaccine (Sinovac Life Sciences, Beijing, China), which is an inactive vaccine, has been started to use from the elderly population and healthcare workers in our country as of January 2021[7]. As of April 2021, the BNT-162b2 (BioNTech/Pfizer) vaccine, which is an mRNA vaccine, has begun to be used[8]. Reminder doses are also applied as the pandemic continues.
The aim of this study was to investigate whether the vaccination status of patients hospitalized due to COVID-19 had any effect on the CT severity score (CT-SS) and CORADS scoring assessed during hospitalization.
This study was planned as a retrospective, cross-sectional study and was conducted with the approval of Erzincan Binali Yildirim University Clinical Research Ethics Committee (Date: 27.10.2022 / Decision No: 04/16).
The files of patients admitted to the COVID-19 inpatient clinic between April 1, 2021 and April 1, 2022 were retrospectively reviewed.
Inclusion criteria: (1) Being over 18 years of age; (2) Having a positive polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and receiving inpatient treatment in one of the COVID-19 inpatient clinics; (3) Having a Thorax CT scan on the seventh day of complaints during hospitalization; (4) Having access to COVID-19 vaccination information during hospitalization; (5) Among the patients for whom vaccination information was available, it was defined as 14 days or more from the last vaccine dose for patients who received 2 or more vaccines, and 14 days or more from the date of vaccination for patients who received a single dose of vaccine.
Accordingly, 224 patients out of a total of 2000 patients were included in the study. In our study, patients who met the inclusion criteria were divided into 6 groups: Unvaccinated (Group 1), single dose CoronaVac vaccine (Group 2), single dose BioNTech vaccine (Group 3), 2 doses CoronaVac vaccine (Group 4), 2 doses BioNTech vaccine (Group 5) and 2 doses CoronaVac + 1 dose BioNTech vaccine (Group 6). Patients were evaluated according to the severity of involvement in thorax CT and whether there is typical involvement or not. At the same time, it was investigated whether the acute phase parameters of the patients were affected by the vaccination status.
The SARS-CoV-2 PCR test was studied with DS CORONEX COVID-19 Multiplex Real Time qPCR (DS Nano and Biotechnology). The hemogram test of the patients was performed using the Sysmex XN-1000 Hematology System (Sysmex Corporation, Kobe, Japan); and biochemical tests were studied with AU 5800 (Beckman Coulter, California, United States) and coagulation tests with Ceveron® alpha (Diapharma Group, Ohio, United States).
All patients had a CT scan without intravenous contrast material on the day they were admitted to the hospital (Siemens SOMATOM Sensation 16, Forchheim, Germany). All patients were scanned in the supine position using an adult CT protocol; reconstruction images of the 1.5 mm lung window were obtained using tube voltage = 130kV, effective mAs = 70, slice thickness = 5 mm, collimation = 16 × 1.2, pitch = 0.8. In children, reconstruction images of the lung window of 1.5 mm were obtained with protocol tube voltage = 110kV, effective mAs = 60, slice thickness = 8 mm, collimation = 16 × 1.2, pitch = 0.8 (14 years and younger). CT-SS and CORADS score were evaluated by 3 independent experienced radiologists.
The result of the CT examination on admission to hospital was used to define the CT severity value. CT severity of the patients was defined for each lung segment, and the sum of the severity value of each lobe was used to arrive at a final severity score. CT severity scores were calculated using the method described by Pan et al[9] (Table 1). The mean value of two measurements was recorded as the final value.
| CT severity score | Extent of lesions for each lung lobe, % |
| 0 | 0 |
| 1 | < 5 |
| 2 | 5-25 |
| 3 | 26-50 |
| 4 | 51-75 |
| 5 | > 75 |
The CORADS system was used to determine the probability of disease based on the severity of lung involvement in CT examinations performed during hospitalization. CORADS 0 indicates that the examination is not of sufficient quality to be evaluated. CORADS 1 indicates either a normal thorax scan or the presence of a non-infectious disease. CORADS 2 identifies findings that are unusual for COVID-19 but are common in other infectious diseases such as bronchitis and bronchopneumonia. CORADS 3 describes findings that could be related to COVID 19 as well as other viral pneumonias or non-infectious diseases. CORADS 4 identifies findings that are typical for COVID 19 but may also be relevant for other viral pneumonias. Its difference from CORADS 5 is its atypical involvement. CORADS 5 category implies a very high level of suspicion for pulmonary involvement by COVID-19. Patients who are positive according to the reverse transcription-PCR test result are defined as CORADS 6[10].
NCSS (Number Cruncher Statistical System) 2020 (Kaysville, Utah, United States) program was used for statistical analysis. Descriptive statistical methods (mean, standard deviation, median, frequency, percentage, minimum, maximum) were used while evaluating the study data. The suitability of the quantitative data to the normal distribution was tested by the Shapiro-Wilk test and graphical analyses.
Oneway ANOVA test was used for the comparison of normally distributed quantitative variables between groups, and Bonferroni test was used for post hoc evaluations. The Kruskal-Wallis test was used for the comparison of quantitative variables that did not show normal distribution, and the Dunn-Bonferroni test was used for post hoc evaluations.
Pearson Chi-Square test was used to compare qualitative data. Statistical significance was accepted as P < 0.05.
The study was conducted in a university hospital from the eastern of Turkey between April 1, 2021 and April 1, 2022, with a total of 224 cases, of which 52.2% (n = 117) were female and 47.8% (n = 107) were male. The ages of the cases ranged from 22 to 97, and the mean age was 61.85±15.36 years.
A statistically significant difference was found between the age distributions of the groups (P < 0.001); when the significances are analyzed; the mean age of those who were not vaccinated was found to be significantly lower than the single dose CoronaVac, two doses CoronaVac, two doses BioNTech and three doses vaccine groups (P = 0.021; P = 0.001; P = 0.011; P = 0.001; P < 0.05, respectively).
The mean age of those who received a single dose of CoronaVac was higher than those who received a single dose of BioNTech (P = 0.049), and significantly lower than the two-dose CoronaVac and three-dose vaccine groups (P = 0.002; P = 0.001; P < 0.01, respectively).
The mean age of those who received a single dose of BioNTech was also found to be significantly lower than the two-dose CoronaVac and three-dose vaccine groups (P = 0.001; P = 0.001; P < 0.01, respectively).
The ages of those who received two doses of CoronaVac were also significantly higher than those who received two doses of BioNTech (P = 0.001; P < 0.01). The age of those who received two doses of BioNTech was also significantly lower than the group that received three doses of vaccine (P = 0.001; P < 0.01).
No statistically significant difference was found between the distribution of the groups according to gender (P > 0.05) (Table 2).
| n | Age | Gender | ||
| mean ± SD | Male | Female | ||
| 1Unvaccinated | 63 | 50.95 ± 13.54 | 27 (42.9) | 36 (57.1) |
| 2Single dose CoronaVac | 20 | 60.75 ± 8.82 | 9 (45.0) | 11 (55.0) |
| 3Single dose BioNTech | 24 | 50.1 ± 11.015 | 7 (29.2) | 17 (70.8) |
| 4Two doses CoronaVac | 38 | 73.55 ± 9.34 | 21 (55.3) | 17 (44.4) |
| 5Two doses BioNTech | 40 | 59.13 ± 13.88 | 24 (60) | 16 (40) |
| 6Three doses | 39 | 74.23 ± 10.29 | 19 (48.7) | 20 (51.3) |
| Total | 224 | 61.09 ± 15.36 | 107 (47.8) | 117 (52.0) |
| P value | 0.001a,c | 0.204b | ||
| Post hoc | 1 < 2, 4, 5, 6; 2 > 3; 2 < 4, 6; 3 < 4, 6; 4 > 5; 5 < 6 | - | ||
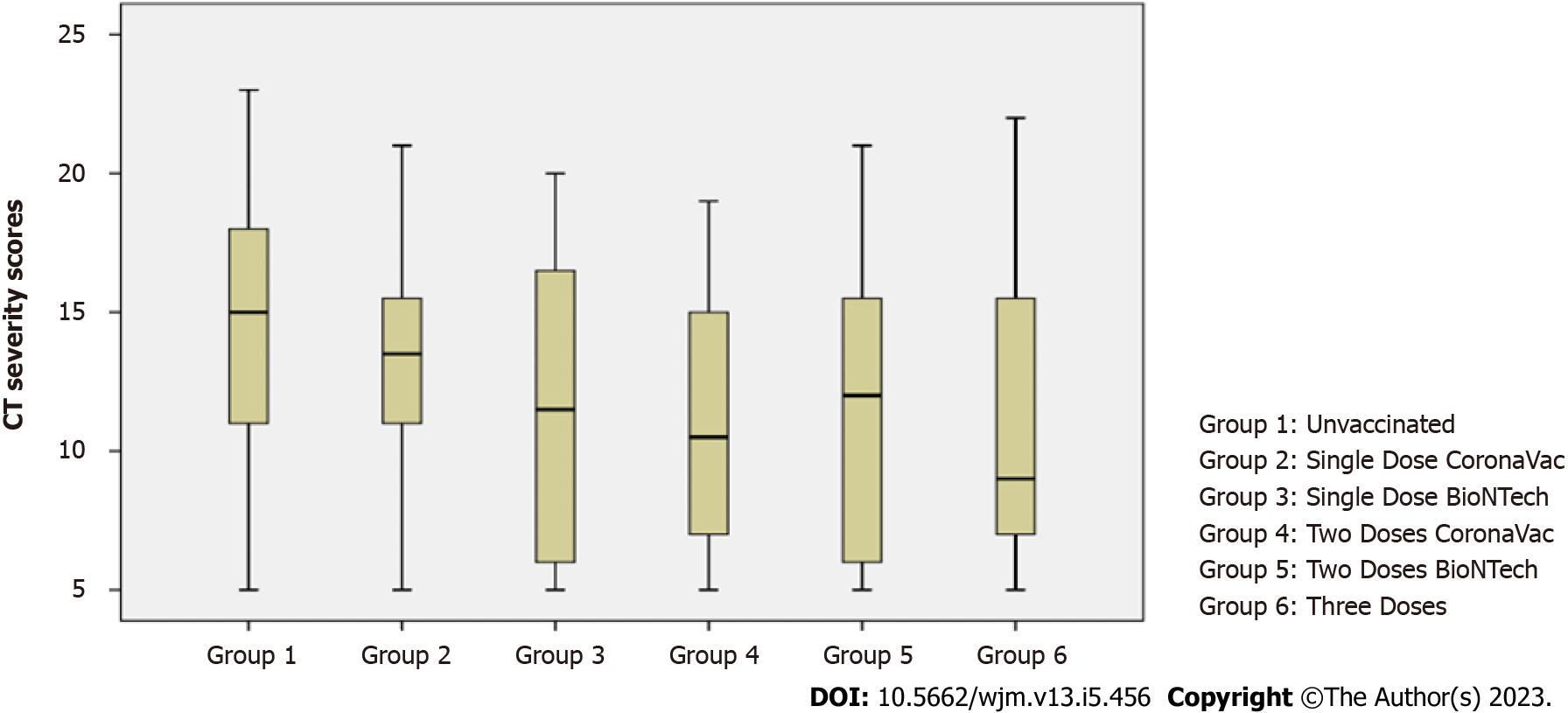
A statistically significant difference was found between the CT severity scores according to the groups (P < 0.01). When it was analyzed from which groups the significance originated, no significant difference was found between the severity scores of those who were not vaccinated and those who received a single dose of CoronaVac and a single dose of BioNTech (P > 0.05). The severity score was significantly higher than those who received two doses of CoronaVac, two doses of BioNTech, and three doses of vaccination (P < 0.05) (Table 3, Figure 1).
| Group | n | CT severity scores | P value | |||
| Mean | SD | Median | Min-Max | |||
| 1Unvaccinated | 63 | 14.17 | 4.56 | 15 | 5-23 | 0.002b |
| 2Single dose CoronaVac | 20 | 13.35 | 4.12 | 13.5 | 5-21 | |
| 3Single dose BioNTech | 24 | 11.58 | 5.29 | 11.5 | 5-20 | 1 > 4; P = 0.015a |
| 4Two doses CoronaVac | 38 | 10.87 | 4.42 | 10.5 | 5-19 | 1 > 5; P = 0.021a |
| 5Two doses BioNTech | 40 | 11.28 | 5.25 | 12.0 | 5-21 | 1 > 6; P = 0.045a |
| 6Three doses | 39 | 10.85 | 5.01 | 9 | 5-22 | |
| Total | 224 | 12.17 | 4.950 | 12 | 5-23 | |
A statistically significant difference was found between the distribution of the groups according to the CORADS classification according to their typicality (P = 0.001; P < 0.01). When the significances were analyzed, the typical incidence rate in those who were not vaccinated and those who received a single dose of CoronaVac vaccine was significantly higher than in all other groups. Typical rates of those who received a single dose of BioNTech and those who received two doses of CoronaVac vaccine were also found to be higher than those who received two doses of BioNTech and three doses of vaccine (Table 4).
| Group | n | CORADS | P value | |
| Typical | Not typical | |||
| 1Unvaccinated | 63 | 57 (90.5) | 6 (9.5) | 0.001a |
| 2Single dose CoronaVac | 20 | 18 (90) | 2 (10.3) | |
| 3Single dose BioNTech | 24 | 14 (58.3) | 10 (41.7) | |
| 4Two doses CoronaVac | 38 | 26 (68.4) | 12 (31.6) | |
| 5Two doses BioNTech | 40 | 15 (37.5) | 25 (62.5) | |
| 6Three doses | 39 | 17 (43.6) | 22 (56.4) | |
| Total | 224 | 147 (65.6) | 77 (34.4) | |
A statistically significant difference was found between the C-reactive protein (CRP) levels of the groups (P < 0.01). When it was analyzed from which groups the significance originated, CRP levels of those who received a single dose of BioNTech vaccine were found to be statistically significantly lower than those who received two doses of CoronaVac, two doses of BioNTech, and three doses of vaccine (P = 0.012; P = 0.014; P = 0.008; P < 0.05). No significant difference was found between the CRP levels of the other groups (P > 0.05) (Table 5, Figure 2).
| 1Unvaccinated | 2Single dose CoronoVac | 3Single dose BioNTech | 4Two doses CoronaVac | 5Two doses BioNTech | 6Three doses | P valuec | Post Hoc | ||
| CRP | mean ± SD | 52.95 ± 40.49 | 49.75 ± 31.26 | 34.0 ± 32.3 | 66.58 ± 52.75 | 84.35 ± 72.99 | 81 ± 62.88 | 0.006a | 3 < 4, 5, 6 |
| Median (Min-Max) | 46 (3-173) | 39.5 (12-124) | 22 (2-106) | 59 (4-204) | 66 (2-341) | 54 (2-286) | |||
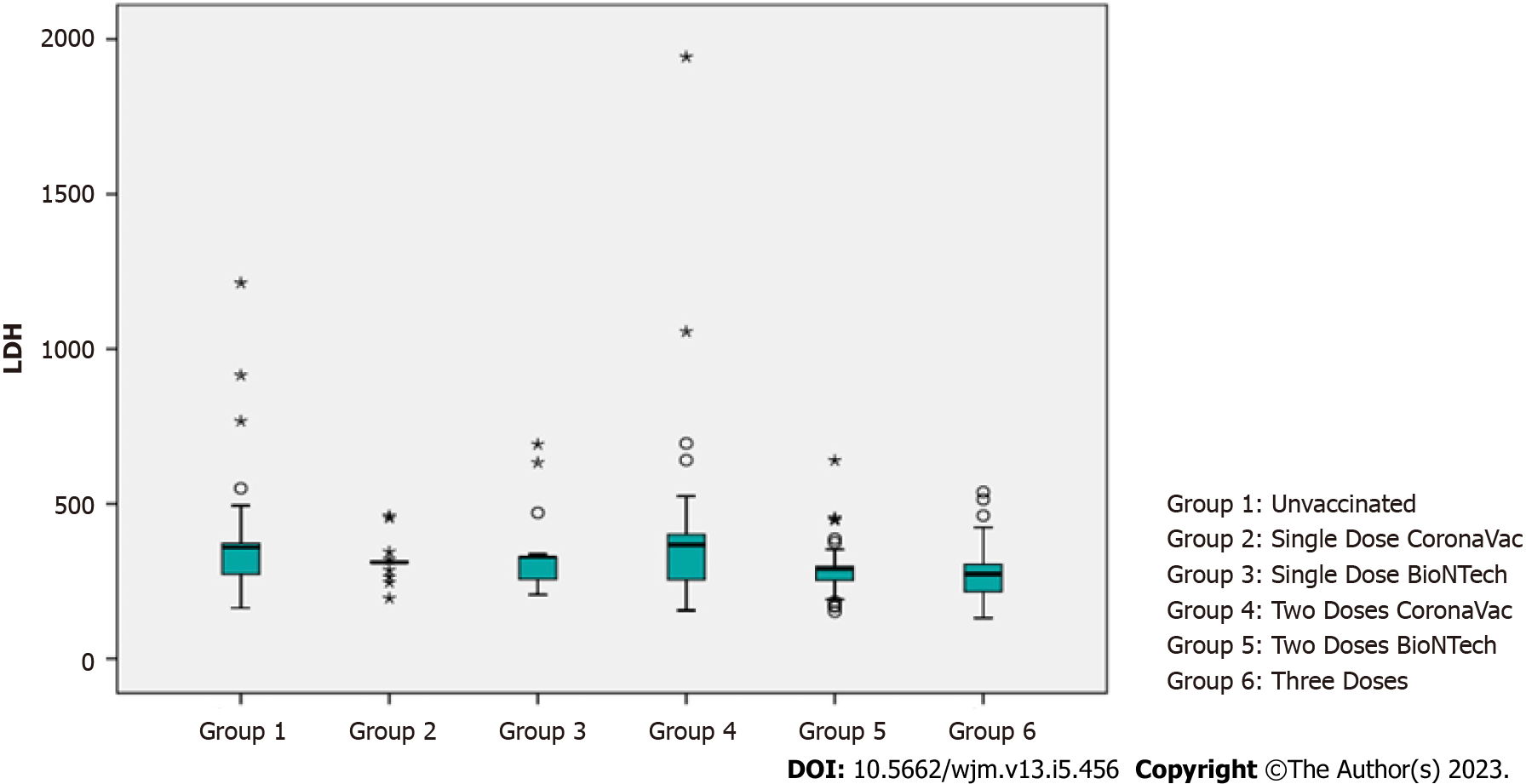
| LDH | mean ± SD | 359.17 ± 164.41 | 315.2 ± 58.2 | 331.83 ± 116.61 | 401.5 ± 305.74 | 294.05 ± 89.57 | 276.36 ± 94.2 | 0.001a | 6 < 5, 3, 1; 5 < 1 |
| Median (Min-Max) | 359 (164-1213) | 311 (195-461) | 328 (207-692) | 368 (156-1943) | 290 (152-640) | 273 (131-538) | |||
| Lymphocyte count | mean ± SD | 1335.4 ± 512.04 | 1396.5 ± 470.93 | 1502.92 ± 724.42 | 1637.11 ± 1896.86 | 1628.75 ± 86.02 | 1477.95 ± 867.82 | 0.660 | |
| Median (Min-Max) | 1290 (390-2790) | 1375 (510-2050) | 1340 (420-3450) | 1200 (340-12360) | 1575 (550-5200) | 1320 (260-4640) | |||
| Procalcitonin | mean ± SD | 0.52 ± 0.94 | 0.28 ± 0.29 | 0.13 ± 0.13 | 0.77 ± 2.39 | 0.25 ± 0.2 | 0.27 ± 0.31 | 0.001a | 3 < 1, 2, 4, 5 |
| Median (Min-Max) | 0.3 (0.1-6) | 0.3 (0.1-1.3) | 0.1 (0.1-0.7) | 0.2 (0.1-15) | 0.1 (0.1-0.7) | 0.1 (0.1-1.2) | |||
| D-DIMER | mean ± SD | 1041.08 ± 1559.79 | 955.95 ± 1156.16 | 1396.71 ± 1608.97 | 1561.63 ± 2118.83 | 1462.2 ± 2934.47 | 1388.41 ± 1922.45 | 0.106 | |
| Median (Min-Max) | 596 (24-11805) | 549.5 (133-4661) | 714.5 (140-6193) | 942.5 (251-13077) | 622 (166-17459) | 820 (194-9440) |
A statistically significant difference was found between the lactate dehydrogenase (LDH) levels of the groups (P < 0.01). When it was analyzed from which groups the significance originated, LDH levels of those who received three doses of vaccine, two doses of CoronaVac vaccine, one dose of BioNTech, and those who were not vaccinated were statistically significantly lower (P = 0.010; P = 0.038; P = 0.001; P < 0.05). The LDH levels of those who received two doses of BioNTech were significantly lower than those who were not vaccinated (P = 0.040; P < 0.05). No significant difference was found between the LDH levels of the other groups (P > 0.05) (Table 5, Figure 3).
There was no statistically significant difference between the lymphocyte levels of the groups (P > 0.05) (Table 5).
A statistically significant difference was found between the procalcitonin levels of the groups (P < 0.01). It was analyzed from which groups the significance originated, the procalcitonin levels of those who received a single dose of BioNTech were found to be statistically significantly lower than those who received a single dose of CoronaVac vaccine, two doses of CoronaVac and two doses of BioNTech (P = 0.001; P = 0.001; P = 0.042; P = 0.037; P <0.05). No significant difference was found between the Procalcitonin levels of the other groups (P > 0.05) (Table 5, Figure 4).
There was no statistically significant difference between D-Dimer levels according to the groups (P > 0.05) (Table 5).
In our country, COVID-19 vaccination was started with the CoronaVac vaccine, which is an inactive vaccine. The vaccine was first started to be administered to healthcare workers and elderly individuals, and vaccination continued from older individuals to younger individuals gradually. While the pandemic was continuing, the BNT-162b2 (BioNTech) vaccine, which is an mRNA vaccine, has also been started to be used in our country[7,8]. This explains why the average age of those who received two doses of CoronaVac and three doses of vaccine in our study was higher.
Thorax CT has been used since the beginning of the pandemic to diagnose COVID-19 suspected patients, especially those with negative SARS-CoV-2 PCR tests. With the CORADS scoring system, patients are evaluated for COVID-19 with Thorax CT. At the same time, Thorax CT is used for disease severity scoring[11,12]. The pandemic is tried to be controlled with the introduction of COVID-19 vaccines. In the study of Haas et al[13], it was shown that 2 doses of BioNTech vaccine reduced the risk of severe disease and death. Hu et al[14] also found that 2 doses of inactive vaccine were highly effective against the Delta variant and reduced the risk of severe disease. In the study of Sagiraju et al., it was shown that full dose vaccination reduces the risk of severe disease and mortality, regardless of the vaccine type[15]. In the study of Mahajan et al[16], where they evaluated vaccinated and unvaccinated patients with a positive SARS-CoV-2 PCR test, they found that the CT-SS of vaccinated people was mild-moderate, while unvaccinated individuals were moderate-severe. Again, in the studies conducted by Russo et al[4], Ravindra et al[5], Fatima et al[6], and Yavuz et al[17], when the CT SS of vaccinated and unvaccinated patients were compared, it was shown that vaccinated patients had milder pulmonary involvement. In our study, it was shown that unvaccinated individuals had moderate-severe lung involvement, and patients who received two doses of CoronaVac or two doses of BioNTech or three doses of vaccine had moderate lung involvement. Also, it was shown that incomplete vaccination was not effective in reducing CT SS. This finding supports the literature. At the same time, it is an indication that incomplete vaccination will not have any effect on severe disease.
In our study, unlike other studies, the group that had a reminder dose with mRNA vaccine after 2 inactivated vaccines was also evaluated. Accordingly, the CT SS of the patients who received 3 doses of vaccine was found to be lower than those who received 2 doses of vaccine, although it was not statistically significant. This is an evidence of the necessity of reminder doses.
Since the beginning of the pandemic, Thorax CT has had an important place in the diagnosis of suspected COVID-19 patients, especially those with negative PCR tests. Patients with negative SARS-CoV-2 PCR test were not included in our study, and patients were admitted to the hospital on the 7th day of their symptoms on average. When the CORADS scores of the diagnosed cases were evaluated, it was found that the typical appearance was significantly less as the number of vaccinations increased. This suggests that while the pandemic continues, the role of CT in the diagnosis of COVID-19 may remain in the background.
In a study evaluating the effectiveness of the CoronaVac vaccine and hospitalizing 292 patients diagnosed with COVID-19, no significant difference was found between the acute phase reactants of vaccinated and unvaccinated patients[17]. In another study conducted in Poland, no significant difference was found between acute phase reactants[18]. In our study, different acute phase reactants (CRP and procalcitonin) responses were observed depending on the number and type of vaccination, and it was thought that it could not be interpreted clearly since only the acute phase reactants of the patients at the time of admission to the hospital were examined. Otherwise, LDH level is associated with the prognosis of the disease[19]. In our study, the higher LDH levels of unvaccinated or incompletely vaccinated patients showed that vaccination would reduce the probability of a severe course of the disease but, only the measurements of the patients at the time of admission to the hospital were evaluated in our study.
The strength of our study is that, unlike other studies, we evaluated the group that received a booster dose with mRNA vaccine after 2 doses of inactive vaccine.
The acute phase reactants in the follow-up of the patients were not evaluated. This is one of the limitations of the study. Again, not evaluating the comorbid diseases and mortality rates of the patients are other limitations of our study.
In conclusion, our study showed that being vaccinated, regardless of the type of vaccine, leads to a decrease in CT severity scores. Again, our study suggests that with the effect of vaccination, we may no longer see the CT images that we are accustomed to seeing in COVID-19 patients, and CT may remain in the background in diagnosis.
The coronavirus disease 2019 (COVID-19) pandemic is still continuing. Vaccination has an important place in preventing the disease and vaccination reduces the possibility of a severe course of the disease.
It is important to investigate whether vaccination has any effect on the computed tomography (CT) severity score (CT-SS) and CORADS score of COVID-19 patients.
We aim to investigate whether the vaccination status of inpatient treatment for COVID-19 has any effect on the CT-SS and CORADS score taken during hospitalization.
This single-center retrospective study was conducted between April 1, 2021 and April 1, 2022 with a total of 224 patients older than 18 years of age, whose vaccination status was accessible, who had positive severe acute respiratory syndrome coronavirus 2 polymerase chain reaction results, and who had a thorax CT taken during hospitalization.
Among the patients included in the study, 52.2% were female and the mean age was 61.85 years. The patients applied to the hospital on the average 7th day of their complaints. While 63 patients were unvaccinated (Group 1), 20 were vaccinated with a single dose of CoronaVac (Group 2), 24 with a single dose of BioNTech (Group 3), 38 with 2 doses of CoronaVac (Group 4), 40 with 2 doses of BioNTech (Group 5), and 39 with 3 doses of vaccine (2 doses of CoronaVac followed by a single dose of BioNTech, Group 6). CT-SS ranged from 5 to 23, with a mean of 12.17. CT-SS mean of the groups were determined as 14.17, 13.35, 11.58, 10.87, 11.28, 10.85, respectively. Accordingly, as a result of the comparisons between the groups, the CT-SS levels of the unvaccinated patients found to be significantly higher than the other groups. As the vaccination rates increased, the rate of typical COVID-19 findings on CT was found to be significantly lower.
The increase in vaccination rates in COVID-19 patients reduces the CT Severity Score and the CORADS score.
It is important that COVID-19 vaccinations continue. With the effect of vaccination, the possibility of severe course of the disease will decrease. There will be a need for studies in which more patient data are analyzed and data obtained from patients with different vaccines are evaluated.
Provenance and peer review: Invited article; External peer review.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Seledtsov V, United States; Nasa P, United Arab Emirates S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | WHO COVID-19 Weekly Epidemiological Update. (Accessed on 30 July 2023). Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-july-2023. |
| 2. | Saeed GA, Gaba W, Shah A, Al Helali AA, Raidullah E, Al Ali AB, Elghazali M, Ahmed DY, Al Kaabi SG, Almazrouei S. Correlation between Chest CT Severity Scores and the Clinical Parameters of Adult Patients with COVID-19 Pneumonia. Radiol Res Pract. 2021;2021:6697677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Vishwanath T, Rajalakshmı B, Sadananda K, Manjunath C. Association of Chest CT Severity Scores and Vaccination Status in COVID-19 Disease: A Cross-sectional Study. JCDR. 2022;16. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Russo GM, Mangoni di Santo Stefano ML, Belfiore MP, Annunziata G, Zoi E, Gallo L, Ciani G, Urraro F, Nardone V, Reginelli A, Cappabianca S. Total Severity Score (TSS) comparison in vaccinated and unvaccinated patients during the fourth wave (December 2021 - January 2022) of COVID-19 in Italy. Eur Rev Med Pharmacol Sci. 2022;26:5971-5977. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Ravindra Naik B, Anil Kumar S, Rachegowda N, Yashas Ullas L, Revanth RB, Venkata Sai Aluru NR. Severity of COVID-19 Infection Using Chest Computed Tomography Severity Score Index Among Vaccinated and Unvaccinated COVID-19-Positive Healthcare Workers: An Analytical Cross-Sectional Study. Cureus. 2022;14:e22087. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Fatima S, Zafar A, Afzal H, Ejaz T, Shamim S, Saleemi S, Subhan Butt A. COVID-19 infection among vaccinated and unvaccinated: Does it make any difference? PLoS One. 2022;17:e0270485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Binay UD, Karakecili F, Barkay O, Gul O, Mertoglu C. Level of SARS-CoV-2 IgG Antibodies after Two Doses CoronaVac Vaccine: Primarily Report. J Antivir Antiretrovir S. 2021;18. [DOI] [Full Text] |
| 8. | "Bugün ilk kez uygulanmaya başladı! İşte BioNTech aşısı hakkında detaylar". hurriyet.com.tr. 2 Nisan 2021 (Accessed on 1.11.2022) . [DOI] [Full Text] |
| 9. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1761] [Article Influence: 352.2] [Reference Citation Analysis (0)] |
| 10. | Penha D, Pinto EG, Matos F, Hochhegger B, Monaghan C, Taborda-Barata L, Irion K, Marchiori E. CO-RADS: Coronavirus Classification Review. J Clin Imaging Sci. 2021;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Salaffi F, Carotti M, Tardella M, Borgheresi A, Agostini A, Minorati D, Marotto D, Di Carlo M, Galli M, Giovagnoni A, Sarzi-Puttini P. The role of a chest computed tomography severity score in coronavirus disease 2019 pneumonia. Medicine (Baltimore). 2020;99:e22433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115-E117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2088] [Cited by in RCA: 1873] [Article Influence: 374.6] [Reference Citation Analysis (2)] |
| 13. | Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, Southern J, Swerdlow DL, Jodar L, Levy Y, Alroy-Preis S. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819-1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1377] [Cited by in RCA: 1143] [Article Influence: 285.8] [Reference Citation Analysis (0)] |
| 14. | Hu Z, Tao B, Li Z, Song Y, Yi C, Li J, Zhu M, Yi Y, Huang P, Wang J. Effectiveness of inactivated COVID-19 vaccines against severe illness in B.1.617.2 (Delta) variant-infected patients in Jiangsu, China. Int J Infect Dis. 2022;116:204-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Sagiraju HKR, Elavarasi A, Gupta N, Garg RK, Paul SS, Vig S, Sirohiya P, Ratre B, Garg R, Pandit A, Nalwa R, Kumar B, Meena VP, Wig N, Mittal S, Pahuja S, Madan K, Das N, Dwivedi T, Gupta R, Wundawalli L, Singh AR, Singh S, Mishra A, Pandey M, Matharoo KS, Kumar S, Mohan A, Guleria R, Bhatnagar S. The effectiveness of SARS-CoV-2 vaccination in preventing severe illness and death—real-world data from a cohort of patients hospitalized with COVID-19. medRxiv. 2021;. [DOI] [Full Text] |
| 16. | Mahajan M, Gupta V, Ilyas M, Gupta K, Singh P. Comparative evaluation of severity of COVID-19 pneumonia on computed tomography of the chest in vaccinated and non-vaccinated individuals: an observational study. Pol J Radiol. 2022;87:e257-e262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Yavuz SŞ, Tunçer G, Altuntaş-Aydın Ö, Aydın M, Pehlivanoğlu F, Tok Y, Mese S, Gündüz A, Güçlü CG, Özdoğan İ, Hemiş-Aydın B, Soğuksu P, Benli A, Başaran S, Midilli K, Eraksoy H. Comparison of the Clinical and Laboratory Findings and Outcomes of Hospitalized COVID-19 Patients Who Were Either Fully Vaccinated with Coronavac or Not: An Analytical, Cross Sectional Study. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Rzymski P, Pazgan-Simon M, Simon K, Łapiński T, Zarębska-Michaluk D, Szczepańska B, Chojnicki M, Mozer-Lisewska I, Flisiak R. Clinical Characteristics of Hospitalized COVID-19 Patients Who Received at Least One Dose of COVID-19 Vaccine. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad Med J. 2022;98:422-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |