Published online Sep 20, 2023. doi: 10.5662/wjm.v13.i4.296
Peer-review started: April 15, 2023
First decision: May 12, 2023
Revised: June 7, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 20, 2023
Processing time: 157 Days and 12.8 Hours
The coronavirus disease 2019 (COVID-19) pandemic has had a profound impact on global health, primarily characterized by severe respiratory illness. However, emerging evidence suggests that COVID-19 can also lead to secondary sclerosing cholangitis (SC), referred to as post-COVID-19 cholangiopathy.
To synthesize currently reported cases to assess the current state of knowledge on post-COVID-19 cholangiopathy.
Medical Subject Headings and Health Sciences Descriptors were used to retrieve relevant studies, which were combined using Boolean operators. Searches were conducted on electronic databases including Scopus, Web of Science, and MEDLINE (PubMed). Studies published in English, Spanish, or Portuguese were included, with no restrictions on the publication date. Additionally, the reference lists of retrieved studies were manually searched. Simple descriptive analyses were used to summarize the results. Then the data were extracted and assessed based on Reference Citation Analysis (https://www.referencecitationanalysis.com/).
The initial search yielded a total of 192 articles. After screening, 85 articles were excluded due to duplication, leaving 107 articles for further review. Of these, 63 full-length articles met the inclusion criteria and were included in the analyses. Most of the patients were male and exhibited elevated liver function tests (93.8%). Magnetic resonance imaging revealed duct thickening with contrast enhancement (47.7%), as well as beading of the intrahepatic ducts (45.7%) with peribiliary contrast enhancement on diffusion (28.7%). Liver biopsy results confirmed SC in most cases (74.4%). Sixteen patients underwent liver transplantation, with three experiencing successful outcomes.
Post-COVID-19 cholangiopathy is a serious condition that is expected to become increasingly concerning in the coming years, particularly considering long COVID syndromes. Although liver transplantation has been proposed as a potential treatment option, more research is necessary to establish its efficacy and explore other potential treatments.
Core Tip: Post-coronavirus disease 2019 (COVID-19) cholangiopathy is a rare but serious complication that can occur after contracting COVID-19. It is characterized by inflammation and damage to the bile ducts. To better understand this condition and its treatment, we conducted a systematic review of post-COVID-19 cholangiopathy cases. Sixty-three articles met the inclusion criteria, representing 540 patients. Males over 50-years-old were more prone to this condition, which is often accompanied by elevated liver function, bile duct thickening, and kidney failure after prolonged use of mechanical ventilation. Further research is needed to confirm the effectiveness of liver transplantation in treating post-COVID-19 cholangiopathy.
- Citation: Rasheed MA, Ballotin VR, Bigarella LG, Soldera J. Post-COVID-19 cholangiopathy: Systematic review. World J Methodol 2023; 13(4): 296-322
- URL: https://www.wjgnet.com/2222-0682/full/v13/i4/296.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i4.296
On March 2020, the World Health Organization declared a global health pandemic after the first case was recognized on December 2019 in Wuhan City, China, of what was called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. This led to catastrophic events in the world resulting in more than 6 million deaths globally. The pandemic has led to a great financial and humanitarian loss due to prolonged lockdowns, which have had a tragic effect on the global economy[2].
Also, coronavirus disease 2019 (COVID-19) keeps enduring second and third waves of outbreaks in many countries, probably caused by mutant new variants of the virus[2]. Despite the accelerated speed of vaccine development for the prevention of COVID-19 to control the disease and robust mass vaccination worldwide including booster doses, these new SARS-CoV-2 variants threaten the progress made so far with the purpose of controlling the spread of the disease[2,3].
Respiratory symptoms are the most common manifestation of the disease, which range from mild to severe and may include fever, dry cough, shortness of breath, anosmia, ageusia, and fatigue[4]. It may lead to viral pneumonia with severe complications such as acute respiratory failure, acute respiratory distress syndrome requiring intubation, mechanical ventilation (MV), and intensive care management[5,6].
In addition to respiratory symptoms, COVID-19 might also cause a range of extrapulmonary manifestations including cardiovascular, neurological, and renal complications[7]. Gastrointestinal symptoms, including diarrhea, nausea, and vomiting, are also commonly reported[8]. Post-COVID-19, derangement of liver enzymes is a potential complication observed in admitted COVID-19 patients, with a prevalence ranging from 14% to 83%[9]. Other liver-related conditions such as autoimmune hepatitis, vascular thrombosis, and hemophagocytic lymphohistiocytosis have also been associated with the post-COVID-19 period[9,10].
However, one emerging complication of COVID-19 is post-COVID-19 cholangiopathy (PCC), a novel clinical entity characterized by inflammation and damage to the bile ducts in individuals who have recovered from COVID-19 infection[11]. The clinical presentation of PCC can vary, but common symptoms may include abdominal pain, fever, and jaundice[12]. PCC has been observed in patients without a history of prior liver disease. This condition can manifest in various clinical settings, such as in individuals with severe COVID-19 infection requiring MV, as well as in those experiencing milder forms of the disease[5,13]. The prevalence of PCC is not well understood, and it is not clear if it is more common in certain patient populations. Some researchers have suggested a potential association between certain drugs, including immunomodulator agents, ketamine, and antiviral medications, and the development of PCC. However, the available evidence regarding these drugs causing cholangiopathy remains insufficient[9].
This systematic review comprehensively analyzes and synthesizes the existing evidence pertaining to PCC. The primary objective is to explore the clinical presentation and management approaches documented in the available cases reported in the literature. By conducting this review, we provide a comprehensive overview of the current understanding and knowledge gaps surrounding PCC, which can contribute to the development of effective strategies for diagnosis and treatment in clinical practice.
This study was conducted in accordance with the guidelines for preferred reporting items for systematic reviews and meta-analyses (PRISMA) protocol guidelines[14].
The studies included in this review were identified using the search strategy: in ("COVID-19" OR "SARS-COV-2") AND ("cholangiopathy" OR "cholangitis" OR "liver transplantation"). This search command was run on the electronic databases Scopus, Web of Science, and Medline (PubMed). Languages were restricted to English, Spanish, and Portuguese. There was no date of publication restrictions. The reference lists of the retrieved studies were also manually searched. The databases were searched in March 2023. Reference Citation Analysis (https://www.referencecitationanalysis.com/) was used to supplement the search.
Inclusion criteria were clinical case reports or case series of post-COVID cholangiopathy. Studies needed to include detailed information about the clinical presentation, diagnosis, management, and outcomes. Articles unrelated to the topic were excluded as were those that did not provide sufficient detail about the cases. If there was more than one study published using the same case, the variables were complemented with both articles. Studies published only as abstracts were included, as long as the available data made data collection possible.
A comprehensive search of various databases was conducted using the search terms listed in the COVID-19, cholangitis, and liver transplantation ("COVID-19" OR "SARS-COV-2") AND ("cholangiopathy" OR "cholangitis" OR "liver transplantation"). The initial screening process involved reviewing titles and abstracts to identify potentially relevant studies. These studies were then analyzed in full, and some were excluded due to a lack of clinical information. Two reviewers independently extracted data from eligible studies using a standardized form and assessed the characteristics of the subjects and outcomes measured. Any discrepancies in study selection or data extraction were resolved by a third party.
Variables included were age, sex, clinical presentation, liver function tests, renal function test, imaging findings, histopathology, whether or not the patient had undergone orthotopic liver transplantation (OLT), and outcome.
Data were analyzed and summarized using descriptive techniques such as frequency, means, and median. The analyses were performed using Microsoft Excel 2010.
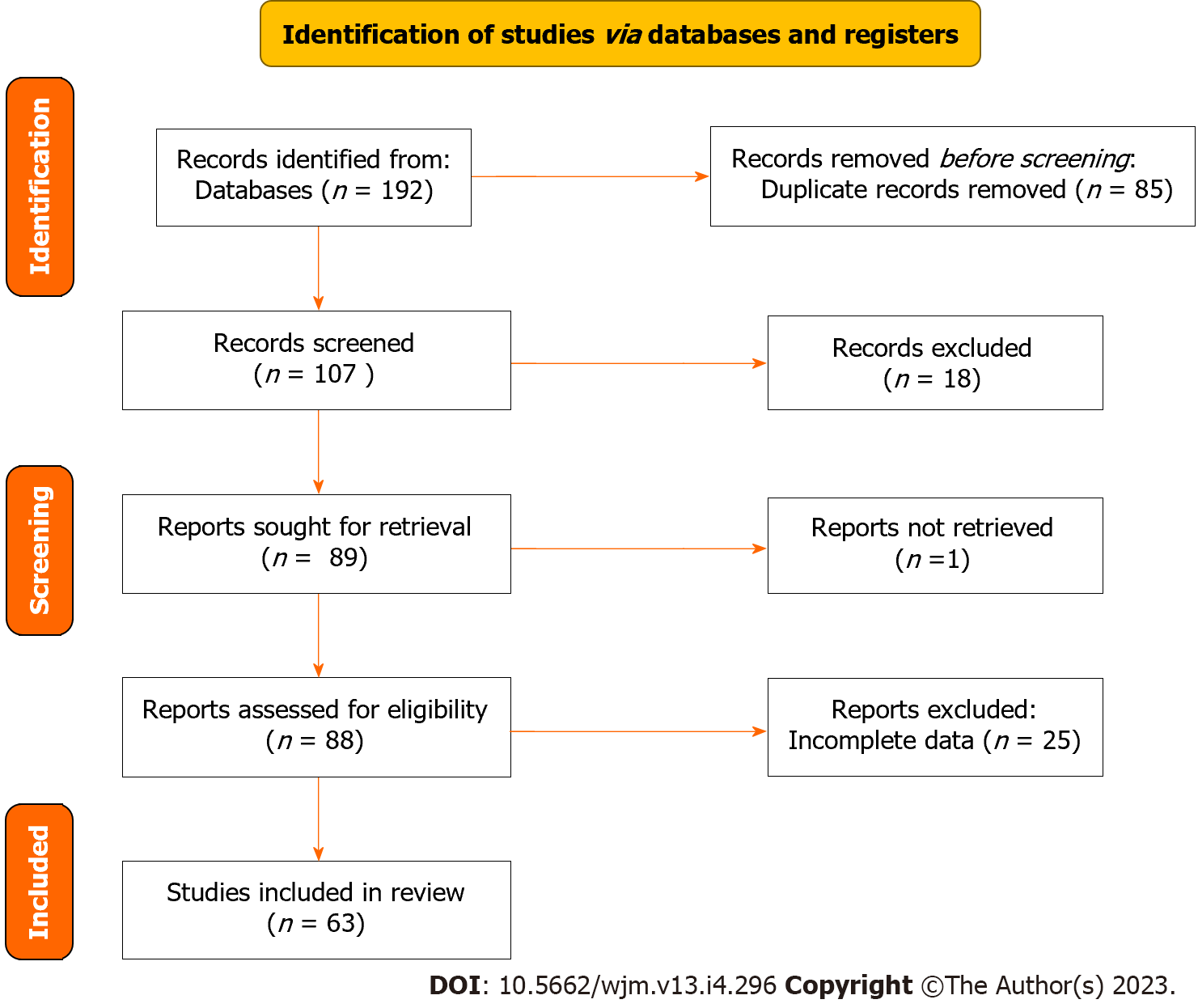
The search strategy retrieved 192 articles; 85 articles were excluded because they were duplicates and 107 articles were screened in the review. A total of 88 full-length articles were included and retrieved, of which 63 were included in the review. The PRISMA flowchart illustrating the search strategy is shown in Figure 1. Studies reviewed were either a case report or a case series.
This systematic review included a total of 540 patients, of whom 69 (12.7%) were male, 26 (4.8%) were female, and 445 (82.5%) did not note their sex. The majority of patients (66, 12.2%) were over 50-years-old. Almost all patients (93.8%) had elevated liver enzymes in the acute phase, with an increase of these levels in the chronic phase. Total bilirubin was elevated in 343 patients (63.5%), while only 80 (14.8%) had levels lower than 1.2 mg/dL. Data on bilirubin levels were not reported for 19 cases. Levels of alkaline phosphatase were high among 488 patients (90.3%) and gamma-glutamyl transferase were consistently elevated, often surpassing 1000 U/L.
In this study, based on imaging findings, 225 of 540 (41.6%) patients had biliary ductal dilatation with fibrosis on ultrasound, while 50 (9.2%) patients did not show any alteration. Furthermore, according to magnetic resonance imaging (MRI) results, 258 (47.7%) patients had bile duct thickening with contrast enhancement, 247 (45.7%) had beading of the intrahepatic ducts, and 155 (28.7%) had peribiliary enhancement on diffusion.
Moreover, 223 (41.3%) patients with PCC had respiratory failure type 2, which was characterized by acute respiratory distress syndrome (ARDS). Some of these patients underwent bilateral lung transplantation, but unfortunately 1 patient died. Additionally, 355 patients (65.7%) had acute renal injury that required either dialysis or renal transplantation after OLT. Data on renal function were not reported for 16 patients. According to liver biopsy results, 402 patients (74.4%) had sclerosing cholangitis (SC). Moreover, 16 patients (2.96%) with post-COVID-19 cholangitis underwent OLT. Of these, 15 patients experienced successful outcomes, with an improvement in liver enzyme levels post-transplantation.
After the first of case of SARS-CoV-2 disease in 2019[1], a novel clinical entity emerged. This condition has been reported in a small number of patients who have recovered from the virus and is characterized by elevated liver enzymes, biliary ductal dilatation on imaging, and histopathological findings of secondary SC (SSC)[11]. This systematic review examined the clinical presentations and outcomes of 540 patients with PCC, a rare complication of COVID-19 that affects the biliary system.
It is important to consider the differential diagnosis, as other diseases may present with a similar presentation[15]. Ketamine-induced cholangiopathy can lead to fusiform dilatation of the common bile ducts, without evidence of extrinsic or intrinsic obstruction[16]. The severity depends on the duration of using ketamine, and it is reversible in abstinent patients. Another difference is ischemic cholangitis, which occurs as a consequence of deficient blood flow to the bile duct wall[17]. This can affect the bile ducts leading to segmental strictures and cholangiectasis, resulting in mechanical restriction of bile acid flow.
SC is a medical condition characterized by the destruction of bile ducts due to inflammation and fibrosis and severe progressive stenosis of the bile tracts including three types: primary SC (PSC); immunoglobulin G-related SC (IgG-SC); and secondary cholangitis such as bacterial cholangitis, viral cholangitis (cytomegalovirus), postoperative biliary stenosis, and choledocholithiasis. Usually the patients present with similar cholestatic features such as itching and jaundice, and blood tests reveal high cholestatic enzymes[18,19]. Although the clinical presentation of PSC and IgG4-SC are nearly the same, they differ in treatment response, outcomes and comorbidities, and how to differentiate it from cholangiocarcinoma[18,19]. The difference between them is that IgG4-SC patients respond actively to prednisolone and steroid therapy, whereas PSC has no standard treatment approved; only ursodeoxycholic acid can be used in some patients, but it does not improve the overall prognosis[18-21].
Distinguishing and differentiating between PSC-high IgG and IgG-SC is challenging. A promising study that calculated serum IgG4:IgG1 ratios showed excellent specificity in distinguishing IgG4-SC from PSC-high IgG4[18,19]. The most common diagnostic test for PSC is cholangiography, which shows a pruned tree appearance, beaded ducts, and band-like stricture; thus, endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography is highly recommended. PSC is also highly associated with inflammatory bowel disease (ulcerative colitis more than Crohn’s disease); thus, a colonoscopy is recommended for the diagnosis, which increases the risk of cholangiocarcinoma and gallbladder carcinoma[18,19]. Therefore, more studies are required on the diagnostic procedures of PSC, IgG4-SC, and cholangiocarcinoma and their treatment and management[18,19].
The present results on PCC showed that most patients are male (12.7%) older than 50-years-old, consistent with the previous literature[9]. Every patient had elevated liver enzymes in the acute phase, and their levels increased in chronic phase if left untreated.
Also, ultrasound findings showed that 225 patients (41.6%) presented with biliary ductal dilatation. The MRI findings in this systematic review showed that only a small number of patients (28.7%) had peribiliary enhancement on diffusion, while a larger number of patients (47.7%) had bile duct thickening and enhancement, and 247 patients (45.7%) had beading of the intrahepatic ducts. By contrast, a previous retrospective study by Faruqui et al[13] showed that a higher proportion of patients (11/12, 92%) had beading of the intrahepatic ducts, 7/12 (58%) had bile duct wall thickening with enhancement, and 10/12 (83%) had peribiliary diffusion high signal[11]. Details can be found in Table 1 and Table 2.
| Variable | Patients, n = 540 (100) |
| Sex | |
| Male | 69 (12.7) |
| Female | 26 (4.8) |
| Age > 50 year | 66 (12.2) |
| Liver enzymes | |
| High (> 45) | 507 (93.8) |
| Total bilirubin | |
| High (> 1.2 mg/dL) | 343 (63.5) |
| Alkaline phosphatase | |
| High (> 147 IU/L) | 488 (90.3) |
| Ultrasound findings | |
| Biliary ductal dilatation with fibrosis | 225 (41.6) |
| MRI Findings | |
| Bile duct thickening and enhancement | 258 (47.7) |
| Beading of intrahepatic ducts | 247 (45.7) |
| Peribiliary diffusion | 155 (28.7) |
| Histopathology with secondary sclerosing cholangitis | 402 (74.4) |
| Orthotopic liver transplantation | 16 (2.96) |
| Ref. | Age, yr | Sex | Clinical presentation | Elevated liver enzyme | U/S findings | MRI findings | Respiratory failure | Renal failure | Histopathology | OLT | Outcome |
| Roth et al[28], 2021 | 38 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile ducts beading, with sub-segmental strictures and dilatation | Beading of intrahepatic ducts | Yes, required MV; On supplemental oxygen, then off on day 63 and decannulated | Yes, recovered | Portal tract findings; Mild duct paucity, moderate bile duct swelling & reaction; Mild portal tract inflammation; Endothelial hepatic artery swelling; Portal veins with focal endo phlebitis | Not done | Recovered |
| 25 | Male | Post-COVID-19 cholangiopathy | Yes | Hepatomegaly, extrahepatic bile duct dilatation, intrahepatic bile duct dilatation | Beading of intrahepatic ducts | Yes, required MV; On supplemental oxygen, then off on day 112 and decannulated | Yes, recovered | Portal tract findings; Moderate duct paucity, moderate bile duct swelling & reaction. Moderate portal tract inflammation; Endothelial hepatic artery swelling; Portal veins with focal endo phlebitis | Not done | Recovered | |
| 40 | Female | Post-COVID-19 cholangiopathy | Yes | Hepatomegaly, no dilatation | Peribiliary diffusion, moderate portal and periportal fibrosis | Yes, remains with tracheostomy & MV, and then off MV on day 63 | Yes, recovered | Portal tract findings; Moderate duct paucity, moderate bile duct swelling & reaction; Severe portal tract inflammation; Endothelial hepatic artery swelling; Portal veins with focal endo phlebitis | Not done | Death, cardiac arrest | |
| Faruqui et al[13], 2021 | Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | U/S showed; extrahepatic bile duct dilatation and intrahepatic bile duct dilatation and periportal diffusion | MRI showed, beading of intrahepatic ducts (11/12, 92%); Peribiliary diffusion (10/12, 83%); Bile duct wall thickening (7/12, 58%) | Patients required MV | Yes, recovered | Large duct obstruction without clear bile duct loss | Done OLT | Had t successful recovery and rapid clinical improvement |
| Mean age 58 | Female | Post-COVID-19 cholangiopathy | Yes | Experiencing persistent jaundice, hepatic insufficiency, and/or recurrent bacterial cholangitis | Beading of intrahepatic ducts; Peribiliary diffusion; Bile duct wall thickening | Patients required MV | Yes, recovered | Large duct obstruction without clear bile duct loss | Highly recommended for OLT. Patient on transplantation waiting list, still not done OLT at time of study | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Experiencing persistent jaundice, hepatic insufficiency, and/or recurrent bacterial cholangitis | Beading of intrahepatic ducts; Peribiliary diffusion; Bile duct wall thickening | Patients required MV | Yes, recovered | Large duct obstruction without clear bile duct loss | Highly recommended for OLT. Patient on transplantation waiting list, still not done OLT at time of study | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Experiencing persistent jaundice, hepatic insufficiency, and/or recurrent bacterial cholangitis | Beading of intrahepatic ducts; Peribiliary diffusion; Bile duct wall thickening | Patients required MV | Yes, recovered | Large duct obstruction without clear bile duct loss | Highly recommended for OLT. Patient on transplantation waiting list, still not done OLT at time of study | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Experiencing persistent jaundice, hepatic insufficiency, and/or recurrent bacterial cholangitis | Beading of intrahepatic ducts; Peribiliary diffusion; Bile duct wall thickening | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | Highly recommended for OLT, patient on transplantation waiting list, still not done OLT at time of study | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | Beading of intrahepatic ducts; Peribiliary diffusion; Bile duct wall thickening | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | Beading of intrahepatic ducts; Peribiliary diffusion; Bile duct wall thickening | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | Beading of intrahepatic ducts; Peribiliary diffusion | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | Beading of intrahepatic ducts; Peribiliary diffusion | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | Beading of intrahepatic ducts; Peribiliary diffusion | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | Beading of intrahepatic ducts | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Mean age 58 | Male | Post-COVID-19 cholangiopathy | Yes | Intrahepatic bile duct dilatation and periportal diffusion | MRI not available | Patients required MV | Yes, recovered | Secondary sclerosing cholangitis | OLT Not done | Recovery with long-term liability and comorbidity | |
| Li et al[29], 2022 | N/A | Two sample mendelian randomization | The autoimmune diseases showed not associated with COVID-19 infection | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hunyady et al[30], 2023 | N/A | 24 Patients | Post-COVID-19 cholangiopathy developed after a median of 91 d | Yes | N/A | N/A | Patients required MV, the median was 48 d among all patients | N/A | COVID-SSC and CIP-SSC share the same clinical phenotype | N/A | UDCA showed great improvement in patients without liver cirrhosis and reduced severity in patients with liver cirrhosis, while OLT showed significant improvement in patient with liver cirrhosis |
| Weaver et al[31], 2021 | 63 | Male | Post-COVID-19 cholangiopathy | Yes | Sludge in the gallbladder, no biliary ductal dilation, and patent vasculature | N/A | Patients required MV | N/A | Filling defects in the common bile duct as well as an irregular and beaded appearance of the intrahepatic ducts | Not done | Recovered, after ERCP sphincterotomy followed by balloon sweep of the biliary ducts and removal of thick stone |
| Hartl et al[32], 2022 | N/A | N/A | Post-COVID-19 cholangiopathy (65 patients with CLD of 496 patients included in the study, around 24.6% non-ACLD vs ACLD 10.6% associated with COVID | Yes. Alkaline phosphatase showed (pre: 91.0 vs T1: 121.0 vs last: 175.0 U/L) and gamma glutamyl transferase GGT (pre: 95.0 vs T1: 135.0 vs last: 202.0 U/L) | N/A | N/A | N/A | N/A | 20% of patients with CLD developed progressive cholestasis post-COVID-19 cholangiopathy, and patients with NASH/NAFLD also have a risk of developing cholestatic liver failure and secondary sclerosing cholangitis post-COVID-19 | N/A | N/A |
| Duengelhoef et al[33], 2022 | N/A | N/A | Post-COVID-19 cholangiopathy, associated more with Autoimmune hepatitis AIH as well as post COVID vaccine than PBC and PSC patients | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| John et al[34], 2023 | N/A | N/A | Post-COVID -19 cholangiopathy study included 1607 patients with liver cirrhosis used UDCA | Yes | N/A | N/A | N/A | N/A | N/A | N/A | UDCA showed great improvement in patients with liver cirrhosis, by decreased symptoms and decreased COVID-19 infection |
| Heucke et al[35], 2022 | N/A | 48 | Post-COVID-19 cholangiopathy 13% from 496 patients developed CLD; 23% of patients with CLD developed cholestasis/cholangiopathy | Yes (ALT & AST were elevated in 50 patients less than 5 times upper limit of normal. While in late-stage alkaline phosphatase and GGT were highly progressively elevated | N/A | N/A | Yes, require oxygen supply & some patients MV | Yes, some patients required dialysis for renal failure | The histopathology reported SARS-CoV-2 RNA and/or proteins in human liver tissue and bile samples, this SARS-CoV-2 RNA may lead to provoke a strong proinflammatory cytokine response (TNF, IL‐1, IL‐6) with hypercoagulation, endothelial damage, consecutive venous and arterial embolism, as well as secondary parenchymal damage | 9 patients listed for OLT and 6 patients done OLT with good recovery | 16 patients died, and 24 patients were treated with ketamine during the acute phase of COVID-19 and around 28 patients with SSC from 48 were reduced after using UDCA treatment |
| Bazerbachi et al[36], 2022 | 56 | Female | Post-COVID-19 cholangiopathy | Yes alkaline phosphatase 1574U/L, total bilirubin 11 mg/dL, ALT 88 U/L, AST 101 U/L | EUS showed a left hepatic duct stricture and heterogenous, non-shadowing cylindric objects in the main bile duct | N/A | Yes, require tracheostomy & MV | Yes, developed renal failure and required hemodialysis | LHD stricture with upstream dilation of the left ducts, and obliteration of right intrahepatic with secondary sclerosing changes | Not done | Improved, casts were swept and removed, and left lobe was stented with a 10 Fr 20 cm plastic stent improving bilirubin level to a baseline of 3 mg/dL |
| Cho et al[37], 2022 | 47 | Female | Post-COVID-19 cholangiopathy | Yes, highly elevated ALP-positive ANA, anti-mitochondrial highly positive | N/A | N/A | N/A | N/A | N/A | N/A | Post-COVID-19 cholangiopathy may be due to direct cytotoxicity from SARS-CoV-2 active replication, hypoxia induced respiratory failure, drug induced liver injury, vascular coagulopathy, immune mediated liver damage |
| 57 | Male | Post-COVID-19 cholangiopathy | Yes, hypogammaglobinemia, high GGT, elevated AST/ALT, positive anti-mitochondrial antibody, anti-smooth muscle antibodies, and anti-double stranded DNA antibodies | N/A | N/A | N/A | N/A | N/A | OLT Considered for some patients | ||
| N/A | N/A | Post-COVID-19 cholangiopathy | Yes, ALP > three times | N/A | MRCP showed dilatation of hepatic ducts with stenosis and beading of intrahepatic ducts | N/A | N/A | N/A | |||
| N/A | 24 Patients | Post COVID-19 Cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | |||
| Yu et al[38], 2022 | N/A | N/A | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | N/A | The patients are not only related to liver disease, but also cholangitis may be due to viral cholangitis, systemic inflammation response, and hypoxic liver injury |
| Sanders et al[39], 2021 | 57 | Male | Post-COVID-19 cholangiopathy | Yes | Dilated CBD with a distal CBD stone | N/A | Yes, required MV. (Tracheostomy & gastrostomy) | Renal impairment required fluid resuscitation | N/A | N/A | Improved, biliary cast removed by ERCP, and bile duct stent and patient referred for cholecystectomy |
| López Romero-Salazar et al[40], 2022 | 76 | Male | Post-COVID-19 cholangiopathy | Yes, elevated ALT & AST developed AIH and complicated to liver cirrhosis secondary to primary biliary cholangitis (PBC) igg positive, ANA | U/S showed hepatic fibrotic inflammation, dilated lobes, and biliary ducts | N/A | N/A | N/A | Biopsy showed lobular hepatitis, with intense interface, centrilobular necrosis with lymphoplasmacytic inflammation | N/A | The patient has poor prognosis due to liver cirrhosis, the study emphasizes the hypothesis that AIH induced due to or post COVID-19 vaccination. Patient given UDCA and obeticholic acid |
| Wall et al[41], 2022 | N/A | N/A | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | N/A | The study showed to avoid using SARS-CoV-2-positive donors for liver transplantation unless there is a justifying indicator such as recipient illness severity |
| Ghafoor et al[42], 2022 | Mean Age 60.5 | 15 Male patients | Post-COVID-19 cholangiopathy | Yes | N/A | All patients had intrahepatic bile duct strictures and 10 patients had associated upstream dilatation. Fourteen patients showed intrahepatic bile duct beading. One patient had extrahepatic bile duct structuring; 9 patients showed high signal on T2 and diffusion weighted images & 7 patients showed patchy arterial phase hyperenhancement; 2 patients showed biliary casts. Vascular complication, and periportal lymphadenopathy were not seen on MRI/MRCP | N/A | N/A | N/A | N/A | The post-COVID-19 cholangiopathy patients showed on MRI/MRCP multiple intrahepatic bile duct strictures with intrahepatic bile duct beading |
| Singh et al[43], 2021 | 57 | Male | Post-COVID-19 cholangiopathy | Yes, elevated ALT, AST, GGT, hypergammaglobulinemia and anti-mitochondrial antibody, anti-smooth muscle antibody and anti-double stranded DNA antibodies | N/A | N/A | N/A | N/A | N/A | N/A | The patient diagnosed with auto immune hepatitis with primary biliary cholangitis overlap syndrome triggered by COVID-19 |
| Seifert et al[44], 2023 | N/A | 7 patients (3 males & 4 females) | Post-COVID-19 cholangiopathy among 7 patients of 544 patients with cholangitis. 4 patients had SSC due to other reasons | Yes, elevated GGT, Alkaline phosphatase ALP among 7 patients more than 4 patients non COVID-19 | N/A | N/A | N/A | N/A | N/A | N/A | The 7 patients with post-COVID-19 cholangiopathy showed more hepatitis and cholangitis than other group non-COVID cholangitis most probably due to direct cytopathologic effect of COVID virus |
| Lee et al[45], 2021 | 64 | Male | Post-COVID-19 cholangiopathy | Yes | U/S intrahepatic bile ducts loss | MRI not available | Required MV | Yes, Recovered | Diffuse hepatic injury, onion skinning of the bile ducts and bile duct loss in scattered portal tracts | OLT not done; patient need to be stable for the operation | Not recovered |
| Cunha-Silva et al[46], 2023 | 45 | Male | Post-COVID-19 cholangiopathy | Yes, elevated in the first 2-wk AST, ALT, GGT, Alkaline phosphatase post SARS-CoV-2 infection: ANA and anti-smooth muscle-positive. Negative viral hepatitis & anti-mitochondrial antibodies | N/A | No dilatation of biliary ducts | N/A | AKI after recovering 2 wk from COVID-19 | Numerous foci of lobular necrosis but with no ductopenia or portal biliary reaction. After 2 mo: Biopsy showed: extensive areas of confluent necrosis, hepatocytes regenerating into pseudorosettes and numerous plasma cells, non-suppurative cholangitis all these features diagnosed by PARIS Criteria as AIH-PBC-OS | N/A | The patient is given prednisolone in the first phase, then after 2 mo added azathioprine and UDCA to management and showed great response and recovery |
| Hamid et al[47], 2021 | N/A | N/A | Post-COVID-19 cholangiopathy | Yes, elevated AST, ALT, low albumin, and low platelet | N/A | N/A | N/A | N/A | Endoscopy and ERCP are recommended by WGO | N/A | OLT is advised to be postposed till SARS-CoV-2 infection treated and patient recovered |
| Kroepfl et al[48] | N/A | 2 patients | Post-COVID-19; cholangiopathy | Yes | N/A | N/A | N/A | N/A | ERCP biopsy showed severely destructed biliary mucosa with ischemia and epithelial roughness | N/A | N/A, early cholangioscopy can confirm the diagnosis |
| Mayorquín-Aguilar et al[24] | 3 Cases | Post-COVID-19 cholangiopathy | Yes | Not available | Mild intrahepatic; Biliary ductal; Dilatation with; Multifocal strictures or; Beading without; Extrahepatic biliary; Dilatation | Yes, required MV | Yes, recovered | SSC-CIP beading of intrahepatic ducts, bile duct wall thickening with enhancement, and peribiliary diffusion high signal | 2 Done OLT, 1 Not done | 2 males death; 1 female recovered | |
| 45 | Male | ||||||||||
| 52 | Male | ||||||||||
| 46 | Female | ||||||||||
| Graciolli et al[49] | 63 | Male | Post-COVID-19 cholangiopathy | Yes | Not available | Dilations with intercalated stenotic segments in intra and extrahepatic bile ducts and edema of the bile ducts corresponding to inflammation of the adjacent parenchyma | Yes | Not available | Intrahepatocellular cholestasis | Not done | Death, infected ulcer, palliative care |
| Keta-Cov research group[50] | Median Age 59 (35-65) | Male | Post-COVID-19 cholangiopathy | Yes, elevated AST, ALTGGT, ALP, total bilirubin all elevated | N/A | Aspects of sclerosing cholangitis, with strictures and dilatations of intrahepatic bile ducts, peribiliary cysts and multiple biliary casts | All patients required M/V | All patients developed acute kidney injury required renal replacement therapy | ERCP showed filling defects in the common bile duct and rarefication of the intrahepatic biliary tract and biopsy showed biliary obstructions, including cholangiolar proliferation, biliary plugs, portal inflammation with neutrophil infiltrates, extensive biliary fibrosis and cirrhosis | N/A | Intravenous ketamine is dose dependant and used for maintenance sedation of patients required M/V for acute respiratory distress syndrome ARDS, and showed associated with biliary obstructions, cholestatic liver injury, biliary cirrhosis, and end-stage liver disease, that’s the reason the new guidelines is not recommend ketamine especially if prolonged or at higher dose |
| Male | |||||||||||
| Male | |||||||||||
| Female | |||||||||||
| Female | |||||||||||
| Zdanowicz et al[51], 2022 | Paediatric patient | Male | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | N/A | Patient developed autoimmune hepatobiliary diseases, autoimmune sclerosing cholangitis ASC which required long-term liver function monitoring |
| Schwarz et al[52], 2022 | N/A | 15 patients | Post-COVID-19 cholangiopathy | Yes, GGT is elevated in 15 patients with SSC-CIP after lung transplantation out of 40 patients in the study. ALP is elevated after lung transplant | N/A | N/A | 15 patients out of 40 developed SSC-CIP underwent lung transplant | N/A | N/A | N/A | GGT showed to be a sensitive parameter to predict severity in SSC-CIP |
| Keskin et al[53], 2022 | N/A | 32 patients | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | N/A | Technical problems with ECRP were more common in biliary patients with delay group than in those without delay, while 7 pancreatic patients showed no difference in ERCP with or without delay of intervention. Technical issues considered such as abundant stone sludge in bile duct, stent migration, etc |
| Bartoli et al[54], 2021 | 44 | Female | Post-COVID-19 cholangiopathy | Yes, AST, ALT elevated and GGT, ALP elevated more ANA positive, anti-mitochondrial-positive, anti-smooth muscle negative | U/S showed slightly enlarged liver with moderate steatosis and a mildly enlarged spleen | N/A | Yes, required intubation and MV | N/A | Florid ductal lesions, moderate peri-portal fibrosis, portal chronic inflammatory infiltrate | Not done | Patient treated with UDCA and discharged and breathing normally, also treated from Guillain barre syndrome GBS by intravenous immunoglobulin |
| Ferreira et al[55], 2022 | N/A | 4 cases | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | ERCP showed beaded appearance of intrahepatic bile ducts and bile casts | N/A | One patient undergone stone removal, and one patient complicate with liver cirrhosis, the other two progressed to advanced chronic liver disease |
| Bütikofer et al[56], 2021 | N/A | 20 Cases | Post-COVID-19 cholangiopathy | Yes 9 patients with severe cholestasis 11 patients with mild cholestasis | N/A | N/A | N/A | N/A | Ischemic changes to the perihilar bile ducts | N/A | SSC is more common and severe in critically COVID-19 patients, with prolonged ICU period |
| Zafar et al[57], 2022 | N/A | 2 Cases | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | N/A | Both patients developed SSC post-COVID-19 vaccination, which lead to hepatitis and eventually cholangitis |
| Otani et al[58], 2022 | N/A | N/A | Post-COVID-19 cholangiopathy in some cases of 166 cases | Yes | N/A | N/A | N/A | N/A | N/A | N/A | 166 cases for endoscopic procedures for causes; Cholangitis, GI bleeding, Obstructive jaundice, neoplasia, COVID-19 led to delay in endoscopic procedures which led to delayed diagnosis of cholangitis, cancers, etc. |
| Cesar Machado et al[59], 2022 | 66 | Male | Post-COVID-19 cholangiopathy | Yes | Ultrasound showed slight hepatomegaly and no bile duct dilatation | MRI showed biliary cast, also revealed. Diffuse irregularity of intra- and extrahepatic bile ducts, with multiple focal strictures alternating with mild focal dilations of the biliary tree, suggesting a sclerosing cholangiopathy | Yes, required MV | Yes, required haemodialysis | Biopsy showed a prominent bile ductular reaction, cholangiocyte injury, inflammatory infiltrate rich in neutrophils, biliary infarctions, marked cholestasis, and portal fibrosis | Not done OLT, due to poor clinical condition | Slight recovery, under observation & follow-up |
| Steiner et al[60], 2022 | 33 | Female | Post-COVID-19 cholangiopathy | Yes, elevated liver enzymes AST, ALT, marked elevated GGT, ALP | N/A | MRCP done showed cholangiopathy | Yes hypoxia required intubation and MV, patient developed respiratory distress syndrome in which she was given veno-venous extracorporeal membrane oxygenation | Yes renal failure, and went through haemodialysis frequently | ERCP done showed cholangiopathy | OLT not done | Patient passed away, her clinical condition deteriorated, with severe hypoxia, renal failure, and multi-organ failure |
| Gourjault et al[61], 2021 | 55 | Male | Post-COVID-19 cholangiopathy | Yes, elevated AST, ALT high GGT, ALP, elevated bilirubin, LDH | N/A | Periportal hypersignal without hepatic biliary dilatation | Yes, Required intubation& MV for 20 d with four sessions prone position | N/A | Interlobular biliary lesions with cholestasis | Waiting list for OLT | Discharged home, he had sphincterotomy and stone removal, planned for OLT |
| 45 | Male | Hepatic steatosis without hepatomegaly or biliary dilatation | Diffuse intra-hepatic dilatation and liver steatosis without any focal obstructing lesion | MV for 26 d and sedated with ketamine for 24 d then he was on ECMO for 18 d | Fifteen sessions of hemodialysis | Discharged home, improved, not done OLT | |||||
| 30 | Male | US normal | Progressive irregular intrahepatic ductal dilatation | MV for 12 d with ketamine sedation, then replaced by ECMO for 29 d with 6 sessions of prone position | 30 sessions of hemodialysis | Biopsy showed cholestatic hepatitis, bile ducts dystrophy | OLT done 11 mo after his admission | Developed liver failure with ascites, prolonged prothrombin, OLT done | |||
| Tafreshi et al[62], 2021 | 38 | Male | Post-COVID-19 cholangiopathy | Yes, mildly elevated AST, ALT and GGT mild bilirubin level | Intrahepatic biliary ductal irregularity and a markedly thickened common bile duct | Diffuse mild intrahepatic biliary distention, marked beading and irregularity& mild irregularity of the extra hepatic common bile duct | Required intubation & MV | N/A | Biopsy showed cholestatic hepatitis with cholangiocyte injury, bile ductular proliferation, canalicular cholestasis | Waiting list for OLT | Improved by treatment, waiting list for OLT |
| Leonhardt et al[63], 2023 | N/A | N/A | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | Yes. Intubated on MV | N/A | N/A | N/A | One patient developed SSC-CIP in every 43 invasive ventilated COVID-19 patients (total 1082 patients) |
| Zengarini et al[64], 2022 | 30 | Female | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | N/A | Patient developed subacute cutaneous lupus erythematosus post COVID-19 vaccination in patient with PBC |
| Wendel-Garcia et al[65], 2022 | N/A | N/A | Post-COVID-19 cholangiopathy | Yes. High total bilirubin | N/A | N/A | N/A | N/A | N/A | N/A | The study showed 243 patients; 170 Patients infused with ketamine developed post-COVID-19 cholangiopathy while other patients received propofol, fentanyl were not associated with cholestatic liver injury |
| Morão et al[66], 2022 | 46 | Female | Post-COVID-19 cholangiopathy | Yes | N/A | MRCP; liver abscesses, intrahepatic bile duct dilation with multiple strictures and some linear repletion defects at the bifurcation of the common hepatic duct | Intubation with MV 12 d | N/A | ERCP Showed; biliary casts | N/A | N/A |
| Lee et al[67], 2022 | 56 | Female | Post-COVID-19 cholangiopathy | Yes, hepatitis C, AST, 243, ALT 630, ALP 449, GGT 2765 | N/A | N/A | N/A | N/A | Granulomatous cholangitis, nonsuppurative with destruction and proliferation of bile ducts with PBC Also immune infiltrations of CD3 T-cells, CD8 T-cells | N/A | Patient improved and discharged after high dose UDCA treatment, liver enzymes become normal |
| Nikoupour et al[68], 2020 | 35 | Male | Post-COVID-19 cholangiopathy | Yes | N/A | N/A | N/A | N/A | N/A | OLT done before 3 yr from COVID-19 infection | Two identical twins had COVID-19 infection, both developed PSC, one of them who had OLT showed improvement with mild symptoms, while the other twin had more severe symptoms |
| 35 | Male | Did not have OLT | |||||||||
| Arnstadt et al[69], 2021 | 62 | N/A | Post-COVID-19 cholangiopathy | Yes | Echogenic intraductal longitudinal structures characteristic for intraductal casts and for SSC-CIP | MRCP showed irregular intrahepatic bile ducts | Yes, need long-term ventilation | N/A | Necrotic bile ducts | N/A | N/A |
| Meersseman et al[70], 2021 | Mean age 48-68 | Male | Post-COVID-19 cholangiopathy | Yes, elevated GGT, ALP, AST, ALT | N/A | MRCP showed focal strictures in intrahepatic bile ducts with intraluminal sludge and casts | Yes, intubated & MV then VV- ECMO | Yes, required renal support | ERCP: Patient 1 diffuse beading of the intrahepatic biliary system, patient 2 & 3 diffuse beading of the intrahepatic biliary ducts, patient 4 focal strictures on the right hepatic duct | OLT done for patient 1 and 2patient 3 & 4 did not undergo OLT | Patient 1 is doing well, patient 2 died due to septic shock and pneumonia, patient 3 have mild SSC-CIP, patient 4 died due to lethal liver hemorrhage |
| Durazo et al[5], 2021 | 47 | Male | Post-COVID cholangiopathy | Yes | Cholelithiasis without evidence of acute cholecystitis | Mild intrahepatic biliary ductal dilatation with multifocal strictures and beading with intra hepatic dilatation but without extrahepatic biliary dilatation | Yes, off MV on day 29 | Yes, recovered | Liver abscess; Bile collection associated with bile duct dilatation with vacuolization and neutrophilia. Endothelial hepatic arteries swelling. Severe portal tract inflammation with Obliterative venopathy | OLT done | Recovered |
| Raes et al[71], 2022 | 64 | Male | Post-COVID-19 cholangitis | Yes | N/A | N/A | Yes, MV then venovenous ECMO VV-ECMO | N/A | N/A | N/A | Passed away; patient having CAHA, progressive liver failure, secondary to ischemic cholangitis |
| Fajardo et al[72], 2021 | 24 | Female | Post-COVID-19 cholangitis | Yes, GGT, ALP, AST, ALT, bilirubin | US: thickening of the gallbladder without stones | MRI: showed normal biliary tree and wall oedema of the gallbladder | N/A | N/A | Cholangitis of the small bile ducts consisting of a mixed inflammatory infiltrate with lymphocytes, plasma cells and neutrophils, accompanied by eosinophils, localized around and within the bile ducts | Not done OLT | Improved, patient discharged after laparoscopic cholecystectomy and liver biopsy |
| Pizarro Vega et al[73], 2023 | 63 | Male | Post-COVID-19 cholangiopathy | Yes, GGT high in all patients especially in patient NO. 3 to 143 U/L then reached to 1130 U/L and patient 4 reached 3550 U/L. AST is high and higher in patient 4 to 82 U/L and patient 5 to 85 U/L then reached maximum 250 and 148, respectively. And patient 1 reached 1520 U/L. High tot. Bilirubin, ALT, ALP | N/A | MRI showed intrahepatic duct dilatations, stenosis without lithiasis, no extrahepatic duct alteration | Yes, required intubation, MV. Pronosupination | Yes, impaired renal function, required vasoactive drugs | No liver biopsy | One patient planned for OLT | All patients treated with UDCA and discharge. 3 patients re-admitted due to complication, patient 4 had pleural empyema. Patient 5 had cholecystectomy, patient 6 readmitted for acute cholangitis without lithiasis, no patients died during follow up |
| 66 | Female | ||||||||||
| 60 | Male | ||||||||||
| 65 | Male | ||||||||||
| 44 | Female | ||||||||||
| 68 | Male | ||||||||||
| Knooihuizen et al[74], 2021 | 54 | Female | Post-COVID-19 cholangiopathy KISC | Yes, ALP peaked 2239 U/L, GGT 773 U/L, AST 1260 U/L, ALT 1729 U/L | N/A | MRI showed intrahepatic biliary dilatation with a beaded appearance & dilated common bile duct with distal narrowing | Yes | Yes | Liver biopsy showed minimal infiltration of neutrophils in the portal tract and lobule without cholestasis, also showed portal tract with bile duct injury | Not done | Patient have KISC during intensive sedation, then ceased the KISC is transient, patient for repeat MRCP |
| Zhou et al[75], 2022 | 36 | Female | Post-COVID-19 vaccination leading autoimmune hepatitis | Yes, AST 581, ALT 588 elevated, GGT, ALP slight elevation, bilirubin 1.4 | N/A | N/A | N/A | N/A | Liver biopsy showed interface hepatitis with portal infiltration and discrete presence of rosette formation and apoptotic hepatocytes | Not done | Patients have Autoimmune hepatitis AIH post vaccination (Moderna mRNA), treatment given after 2nd dose vaccine with prednisolone PSC treated with UDCA and ERCP |
| Muehlenberg et al[76], 2021 | 80 | Female | Post-COVID-19 cholangiopathy | Yes, AST 100 U/L, ALT 113 U/L, bilirubin 12 mg/dL | US of liver and bile duct were normal | N/A | Yes, intubation and MV with antibiotics and catecholamine treatment | N/A | N/A | N/A | Patient done ERCP with papillotomy and foreign body extraction |
| Soldera and Salgado[77], 2021 | 62 | Male | Post-COVID-19 cholangiopathy | Yes | Not Available | Diffuse irregularity of the intrahepatic bile ducts associated with sacular dilations suspicious for cholangiolithic abscesses | Yes | Not available | Intense cytoplasmic vacuolization of cholangiocytes and microvascular alterations | OLT done | Recovery |
| Rojas et al[78], 2021 | 29 | Female | Post-COVID-19 cholangiopathy | Yes | Resemble SSC (secondary sclerosing cholangitis) but no portal inflammation, dilatation, or fibrosis) | MRI is negative | Yes, off MV on day 30 | Yes, recovered | Low periportal inflammatory infiltrate without necrosis but with a severe obstructive cholestatic pattern | Not done | Not recovered |
| Dhaliwal et al[23], 2022 | 42 | Female | Post-COVID-19 cholangiopathy | Yes | Not Available | Mild intrahepatic biliary ductal dilation. With subtle central biliary enhancement concerning for. Cholangitis along with hypodense material hin extrahepatic. Biliary system likely representing transiting gallbladder. Sludge | Not required MV | No | Filling defects secondary to multiple large biliary casts (Biliary case syndrome) | OLT Done | Recovery with long-term liability and comorbidity |
| Caballero-Alvarado et al[79], 2023 | 7 cases | 7 cases | Post-COVID-19 cholangiopathy | Yes | Not available | Not available | Not available | Yes, recovered | Secondary sclerosing cholangitis | 1 done OLT 6 send for consideration of OLT | One recovered, 6 no data available |
| Soldera et al l[26], 2023 | 50 | One male | Post-COVID-19 cholangiopathy | Yes | Not available | MRI showed intra-hepatic sclerosing cholangitis and a dilated chilidium, with no signs of lithiasis (11 mm) | Yes, required MV | Yes, required haemodialysis | ERCP which showed a cast in the format of the external biliary tract, which was removed | Not done OLT | Recovered post cast removal |
| Franzini et al[80], 2022 | 65 | Male | Post-COVID-19 cholangiopathy | Yes | U/S showed no abnormalities | MRI Not available | Yes, required MV; under Fentanyl, Midazolam, and Ketamine sedation | Yes, required haemodialysis | ERCP revealed rarefaction of intrahepatic bile ducts, and removal of biliary casts | OLT Not done | No improvement |
| Roda et al[81], 2022 | 63 | Male | Post-COVID-19 cholangiopathy | Yes | Ultrasound results was inconclusive | MRI not done | Yes, required MV, and veno-venous extracorporeal membrane oxygenation support (VV-ECMO). And eventually done bilateral lung transplant | Acute renal failure (AKI III); chronic illness neuropathy; several episodes of bacterial superinfections and lastly, PLS, characterized by severe haemolysis | Transjugular hepatic biopsy was performed with histopathological evidence of portal and periportal fibrosis, and intraparenchymal cholestasis with cholangiopathy and vasculopathy | OLT not done, patient did bilateral lung transplant | Not recovered, patient passed away due to Multiorgan failure MOF due to septic shock |
| Tebar et al[82], 2022 | 43 | Male | Post-COVID-19 cholangiopathy | Yes | Ultrasound Not available | MRI not done | Yes, required MV | Not available | ERCP, MRCP Showed: Bile cholestatic, toxic. Cause necrosis of cholangiocytes and stenosis, determining persistent and irreversible biliary obstruction, with rapid progression to liver cirrhosis | Not available | Not available |
| Santisteban Arenas et al[83], 2022 | 6 cases | Post-COVID-19 cholangiopathy | Yes | Ultrasound not available | Destruction and curling of the pathway, beading of the intrahepatic bile duct | Yes, all 6 cases required MV | 1 male not having renal failure or haemodialysis. All other 5 cases have renal failure; 2 females not required haemodialysis; 2 males required haemodialysis; 1 male have renal failure but not required haemodialysis | MRCP/ERCP showed destruction of biliary tract. In three of the six patients underwent liver biopsy, the most frequent findings were the presence of a reaction. Ductular, proliferation of cholangioles, infiltrate. Inflammatory associated with the biliary epithelium with component. Lymphoplasmocyte and polymorphonuclear neutrophils | Not available | 1 patient died, 5 other survived with severe comorbidities such as pneumonia, tracheal stenosis, pressure ulcers etc. | |
| 55 | Male | ||||||||||
| 54 | Male | ||||||||||
| 62 | Male | ||||||||||
| 56 | Female | ||||||||||
| 73 | Female | ||||||||||
| 34 | Male | ||||||||||
PCC appears to have different histologic characteristics compared to SSC in critically ill patients caused by other factors. Biopsy samples from patients with PCC show extensive degeneration and injury of cholangiocytes, as well as unique microvascular features such as swelling of hepatic artery endothelial cells, phlebitis in the portal vein, and sinusoidal obstruction syndrome[5]. Several studies have suggested that COVID-19 cholangiopathy is the result of progressive paucity of bile ducts; however, the exact pathophysiology is not well known[11]. Our histopathology biopsy results showed SSC in 402 patients (74.4%).
On the other hand, PCC presentation is difficult to treat, and sometimes requires OLT[5,6,21]. Almost all patients presented with respiratory failure type 2 as they had ARDS, and 1 patient had bilateral lung transplant and unfortunately died. Every patient presented with acute kidney injury, which required either dialysis or renal transplantation post OLT. As described in the literature, PCC is often accompanied by respiratory failure and acute renal injury[22-25]. Also, some cases of biliary casts have been described, removed via ERCP. The diagnosis and management of post-COVID 19 cholangiopathy requires an ERCP, especially in the presence of a dilated choledocus in imaging studies[9,26].
Also, 16 patients (2.96%) underwent OLT, which can be a viable treatment option for this condition[5,27]. One of these cases was reported by Durazo et al[5], which comprised SSC in a 47-year-old patient who was recovering from severe acute respiratory distress syndrome caused by COVID-19 infection. He was admitted to the intensive care unit for prolonged MV (29 d) and was listed for liver transplantation with a model for end-stage hepatic disease score of 37. On day 108 from his presentation, the patient underwent successful OLT with a whole liver allograft from a deceased donor.
In conclusion, this paper presents an extensive review of post-COVID-19 cholangiopathy published in medical journals. Our analysis indicates that post-COVID-19 cholangiopathy is a serious systemic illness that can affect the liver in addition to the lungs. Most cases were found in males over 50-years-old, and patients with cholangiopathy exhibited elevated liver enzymes particularly alkaline phosphatase and gamma-glutamyl transpeptidase, and signs of liver dysfunction. Radiology showed bile duct thickening and enhancement, beading of the intrahepatic ducts, and peribiliary enhancement on diffusion. Additionally, every patient had severe respiratory distress syndrome and kidney failure reported as complications. Liver transplantation has been suggested as a potential management option for PCC, although its efficacy as a curative treatment requires further validation. Not all PCC patients require liver transplantation, as some may recover without undergoing this procedure. Studies have demonstrated that liver enzymes, especially alkaline phosphatase, total bilirubin, and gamma-glutamyl transferase, decrease after medical treatment of PCC. While liver transplantation is not suitable for all PCC patients, it remains the most effective option for select cases. Further research, clinical studies, and international collaborations are needed to gain a better understanding of this novel disease and explore potential treatment avenues.
The coronavirus disease 2019 (COVID-19) pandemic, declared by the World Health Organization in March 2020, has had devastating global impacts, resulting in millions of deaths and significant economic and humanitarian losses. Despite vaccination efforts, new variants of the virus continue to pose a threat, hindering control measures. While respiratory symptoms are common in COVID-19, extrapulmonary manifestations and derangement of liver enzymes have been observed. One emerging complication is post-COVID-19 cholangiopathy (PCC), characterized by bile duct inflammation and damage in recovered individuals. PCC presents with symptoms such as abdominal pain, fever, and jaundice, affecting both severe and milder cases. The prevalence and potential drug associations with PCC remain uncertain.
Understanding post-COVID-19 cholangiopathy is crucial due to its novelty and potential impact on recovered patients. Exploring the clinical presentation and management of PCC can provide valuable insights into its diagnosis and treatment. By addressing the knowledge gaps surrounding this condition, future research can develop effective strategies for patient care and improve outcomes in clinical practice. The significance of solving these problems lies in advancing our understanding of this novel disease and facilitating evidence-based approaches to manage post-COVID-19 cholangiopathy.
The primary objectives of this systematic review were to comprehensively analyze and synthesize existing evidence on post-COVID-19 cholangiopathy, focusing on the clinical presentation and management approaches documented in reported cases. By realizing these objectives, we provide a comprehensive overview of the current understanding of post-COVID-19 cholangiopathy, identify knowledge gaps, and contribute to the development of effective diagnostic and therapeutic strategies for this condition. The findings from this study can guide future research endeavors, leading to improved patient care and outcomes in the field of post-COVID-19 cholangiopathy.
The research methods employed in this study adhered to the guidelines for preferred reporting items for systematic reviews and meta-analyses protocols. A comprehensive search was conducted in electronic databases (Scopus, Web of Science, and Medline/PubMed) using specified search terms. The search was limited to English, Spanish, and Portuguese language publications without any date restrictions. In addition to database searches, the reference lists of identified studies were manually searched. The inclusion criteria encompassed clinical case reports or case series focusing on post-COVID cholangiopathy, with detailed information on clinical presentation, diagnosis, management, and outcomes. Studies that lacked relevant clinical information or were unrelated to the topic were excluded. Two independent reviewers performed data extraction using a standardized form, and any discrepancies were resolved through discussion or consultation with a third reviewer. The extracted data included variables such as age, sex, clinical presentation, liver and renal function tests, imaging findings, histopathology, liver transplantation status, and outcomes. Data analysis involved descriptive techniques, including frequencies, means, and medians.
This systematic review identified a total of 540 patients with post-COVID-19 cholangiopathy, predominantly male (12.7%) and over 50-years-old (12.2%). Elevated liver enzymes were observed in nearly all patients during the acute phase (93.8), persisting in the chronic phase. Total bilirubin levels were elevated in 63.5% of cases, while alkaline phosphatase was 488 (90.3%) and gamma-glutamyl transferase levels consistently exceeded 1000 U/L. Imaging findings revealed biliary ductal dilatation with fibrosis on ultrasound in 41.6% of patients and bile duct thickening with contrast enhancement on MRI in 47.7% of patients. Respiratory failure type 2, associated with acute respiratory distress syndrome, was observed in 41.3% of patients, with 1 patient undergoing lung transplantation. Acute renal injury requiring dialysis or renal transplantation was present in 65.7% of cases. Liver biopsy showed sclerosing cholangitis in 74.4% of patients. Sixteen patients (2.96%) underwent orthotopic liver transplantation, with successful outcomes observed in 93.75% of these cases. These findings provide important insights into the clinical characteristics and complications of post-COVID-19 cholangiopathy, highlighting the need for further research to elucidate its pathogenesis and optimal management strategies.
This study proposes several new theories and methods in the field PCC. First, the study suggests that PCC is a serious systemic illness that affects not only the lungs but also the liver. It provides evidence that PCC is characterized by elevated liver enzymes, biliary ductal dilatation, and histopathological findings of secondary sclerosing cholangitis. The study highlights the importance of considering the differential diagnosis, as other diseases may present with similar symptoms, such as ketamine-induced cholangiopathy and ischemic cholangitis. The study emphasizes the diagnostic procedures for PCC. It recommends the use of cholangiography, endoscopic retrograde cholangiopancreatography, or magnetic resonance cholangiopancreatography to visualize the biliary system and identify characteristic features of PCC, such as pruned tree appearance, beaded ducts, and band-like strictures.
The future research in the field of PCC should focus on understanding its pathophysiology, including the mechanisms of bile duct paucity and unique microvascular features. Improving diagnostic procedures through novel imaging techniques and biomarkers is essential for early and accurate detection. Comparative studies with other cholangiopathies can enhance treatment approaches. Additionally, investigating the management and treatment of PCC, including the efficacy of liver transplantation, is crucial. Identifying predictive factors for transplantation and determining long-term prognosis are valuable areas of research. Overall, future studies should deepen our understanding, develop improved diagnostics, and explore effective treatments to enhance patient outcomes. Collaboration among researchers and international efforts will play a vital role in advancing knowledge and management of this disease.
We would like to extend our sincere appreciation to the Acute Medicine MSc program at the University of South Wales for their invaluable assistance in our work. We acknowledge and commend the University of South Wales for their commitment to providing advanced problem-solving skills and life-long learning opportunities for healthcare professionals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Federação Brasileira De Gastroenterologia; Sociedade Brasileira de Hepatologia.
Specialty type: Medical laboratory technology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Gaspar R, Portugal; Giacomelli L, Italy S-Editor: Li L L-Editor: Filipodia P-Editor: Chen YX
| 1. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2625] [Reference Citation Analysis (0)] |
| 2. | Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). 2023 Jan 9. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 3. | Sharma O, Sultan AA, Ding H, Triggle CR. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front Immunol. 2020;11:585354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 4. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 5. | Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, Loy V, Kim J, Zimmerman MA, Hong JC. Post-Covid-19 Cholangiopathy-A New Indication for Liver Transplantation: A Case Report. Transplant Proc. 2021;53:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 7. | Johnson KD, Harris C, Cain JK, Hummer C, Goyal H, Perisetti A. Pulmonary and Extra-Pulmonary Clinical Manifestations of COVID-19. Front Med (Lausanne). 2020;7:526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 8. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 9. | Veerankutty FH, Sengupta K, Vij M, Rammohan A, Jothimani D, Murali A, Rela M. Post-COVID-19 cholangiopathy: Current understanding and management options. World J Gastrointest Surg. 2023;15:788-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Soldera J, Bosi GR. Haemophagocytic lymphohistiocytosis following a COVID-19 infection: case report. J Infect Dev Ctries. 2023;17:302-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 11. | Yanny B, Alkhero M, Alani M, Stenberg D, Saharan A, Saab S. Post-COVID-19 Cholangiopathy: A Systematic Review. J Clin Exp Hepatol. 2023;13:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Bethineedi LD, Suvvari TK. Post COVID-19 cholangiopathy - A deep dive. Dig Liver Dis. 2021;53:1235-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 14. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40536] [Article Influence: 10134.0] [Reference Citation Analysis (2)] |
| 15. | Yu WL, Cho CC, Lung PF, Hung EH, Hui JW, Chau HH, Chan AW, Ahuja AT. Ketamine-related cholangiopathy: a retrospective study on clinical and imaging findings. Abdom Imaging. 2014;39:1241-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | de Tymowski C, Dépret F, Dudoignon E, Legrand M, Mallet V; Keta-Cov Research Group. Ketamine-induced cholangiopathy in ARDS patients. Intensive Care Med. 2021;47:1173-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Batts KP. Ischemic cholangitis. Mayo Clin Proc. 1998;73:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Tanaka A. IgG4-Related Sclerosing Cholangitis and Primary Sclerosing Cholangitis. Gut Liver. 2019;13:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Manganis CD, Chapman RW, Culver EL. Review of primary sclerosing cholangitis with increased IgG4 levels. World J Gastroenterol. 2020;26:3126-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Ballotin VR, Bigarella LG, Riva F, Onzi G, Balbinot RA, Balbinot SS, Soldera J. Primary sclerosing cholangitis and autoimmune hepatitis overlap syndrome associated with inflammatory bowel disease: A case report and systematic review. World J Clin Cases. 2020;8:4075-4093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Brambilla B, Barbosa AM, Scholze CDS, Riva F, Freitas L, Balbinot RA, Balbinot S, Soldera J. Hemophagocytic Lymphohistiocytosis and Inflammatory Bowel Disease: Case Report and Systematic Review. Inflamm Intest Dis. 2020;5:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv:931766. [DOI] [Full Text] |
| 23. | Dhaliwal A, Dhindsa BS, Esquivel RG. COVID Bile Duct: Biliary Cast Syndrome as a Complication of SARS-CoV-2 Infection. J Gastrointest Surg. 2022;26:1806-1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Mayorquín-Aguilar JM, Lara-Reyes A, Revuelta-Rodríguez LA, Flores-García NC, Ruiz-Margáin A, Jiménez-Ferreira MA, Macías-Rodríguez RU. Secondary sclerosing cholangitis after critical COVID-19: Three case reports. World J Hepatol. 2022;14:1678-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Graciolli AM, De Bortoli BR, Maslonek C, Gremelmier EMC, Henrich CF, Salgado K, Balbinot RA, Balbinot SS, Soldera J. Post-COVID-19 cholangiopathy. Dig Med Res. 2023. In press. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Soldera J, Balbinot RA, Balbinot SS. Billiary casts in post-COVID-19 cholangiopathy. Gastroenterol Hepatol. 2023;46:319-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 27. | Kulkarni AV, Khelgi A, Sekaran A, Reddy R, Sharma M, Tirumalle S, Gora BA, Somireddy A, Reddy J, Menon B, Reddy DN, Rao NP. Post-COVID-19 Cholestasis: A Case Series and Review of Literature. J Clin Exp Hepatol. 2022;12:1580-1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 29. | Li S, Yuan S, Schooling CM, Larsson SC. A Mendelian randomization study of genetic predisposition to autoimmune diseases and COVID-19. Sci Rep. 2022;12:17703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Hunyady P, Streller L, Rüther DF, Groba SR, Bettinger D, Fitting D, Hamesch K, Marquardt JU, Mücke VT, Finkelmeier F, Sekandarzad A, Wengenmayer T, Bounidane A, Weiss F, Peiffer KH, Schlevogt B, Zeuzem S, Waidmann O, Hollenbach M, Kirstein MM, Kluwe J, Kütting F, Mücke MM. Secondary Sclerosing Cholangitis Following Coronavirus Disease 2019 (COVID-19): A Multicenter Retrospective Study. Clin Infect Dis. 2023;76:e179-e187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Weaver M, McHenry S, Das KK. COVID-19 and Jaundice. Gastroenterology. 2021;160:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Hartl L, Haslinger K, Angerer M, Semmler G, Schneeweiss-Gleixner M, Jachs M, Simbrunner B, Bauer DJM, Eigenbauer E, Strassl R, Breuer M, Kimberger O, Laxar D, Lampichler K, Halilbasic E, Stättermayer AF, Ba-Ssalamah A, Mandorfer M, Scheiner B, Reiberger T, Trauner M. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology. 2022;76:1563-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 33. | Duengelhoef P, Hartl J, Rüther D, Steinmann S, Brehm TT, Weltzsch JP, Glaser F, Schaub GM, Sterneck M, Sebode M, Weiler-Normann C, Addo MM, Lütgehetmann M, Haag F, Schramm C, Schulze Zur Wiesch J, Lohse AW. SARS-CoV-2 vaccination response in patients with autoimmune hepatitis and autoimmune cholestatic liver disease. United European Gastroenterol J. 2022;10:319-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | John BV, Bastaich D, Webb G, Brevini T, Moon A, Ferreira RD, Chin AM, Kaplan DE, Taddei TH, Serper M, Mahmud N, Deng Y, Chao HH, Sampaziotis F, Dahman B. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J Intern Med. 2023;293:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Heucke N, Keitel V. COVID-19-associated cholangiopathy: What is left after the virus has gone? Hepatology. 2022;76:1560-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Bazerbachi F, Servin-Abad LA, Nassani N, Mönkemüller K. Endosonographic and ERCP findings in COVID-19 critical illness cholangiopathy. Rev Esp Enferm Dig. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 37. | Cho JY, Lee YS, Kim SS, Song DS, Lee JH, Kim JH. Forms of cholangitis to be considered after SARS-CoV-2 infection. Clin Mol Hepatol. 2022;28:929-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Yu XQ, Zhang XX. [Concerns about COVID-19-associated liver injury]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:473-476. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Sanders D, Bomman S, Irani S. COVID-19-Induced Bile Duct Casts and Cholangitis: A Case Report. Cureus. 2021;13:e14560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | López Romero-Salazar F, Veras Lista M, Gómez-Domínguez E, Ibarrola-Andrés C, Muñoz Gómez R, Fernández Vázquez I. SARS-CoV-2 vaccine, a new autoimmune hepatitis trigger? Rev Esp Enferm Dig. 2022;114:567-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Wall AE, McKenna GJ, Onaca N, Ruiz R, Bayer J, Fernandez H, Martinez E, Gupta A, Askar M, Spak CW, Testa G. Utilization of a SARS-CoV-2-positive donor for liver transplantation. Proc (Bayl Univ Med Cent). 2022;35:62-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Ghafoor S, Germann M, Jüngst C, Müllhaupt B, Reiner CS, Stocker D. Imaging features of COVID-19-associated secondary sclerosing cholangitis on magnetic resonance cholangiopancreatography: a retrospective analysis. Insights Imaging. 2022;13:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 43. | Singh B, Kaur P, Maroules M. Autoimmune Hepatitis-Primary Biliary Cholangitis Overlap Syndrome Triggered by COVID-19. Eur J Case Rep Intern Med. 2021;8:002264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Seifert M, Kneiseler G, Dechene A. Secondary Sclerosing Cholangitis due to Severe COVID-19: An Emerging Disease Entity? Digestion. 2023;104:306-312. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Lee A, Wein AN, Doyle MBM, Chapman WC. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Cunha-Silva M, de França EVC, Greca RD, Mazo DFC, da Costa LBE, de Moraes PBS, Veiga CT, Assis-Mendonça GR, Boin IFSF, Stucchi RSB, Sevá-Pereira T. Autoimmune hepatitis and primary biliary cholangitis overlap syndrome after COVID-19. Autops Case Rep. 2023;13:e2023422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Hamid S, Alvares da Silva MR, Burak KW, Chen T, Drenth JPH, Esmat G, Gaspar R, LaBrecque D, Lee A, Macedo G, McMahon B, Ning Q, Reau N, Sonderup M, van Leeuwen DJ, Armstrong D, Yurdaydin C. WGO Guidance for the Care of Patients With COVID-19 and Liver Disease. J Clin Gastroenterol. 2021;55:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Kroepfl V, Treml B, Freund MC, Profanter C. Early detection of COVID-19 cholangiopathy using cholangioscopy-a case report of two critically ill patients. Eur Surg. 2022;54:326-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Graciolli AM, Bortoli BR, Gremelmier EMC, Henrich CF, Salgado K, Balbinot RA, Balbinot SS, Nesello RGF, Soldera J. Post-COVID-19 Cholangiopathy: a novel clinical entity. Rev AMRIGS. 2021;. |
| 50. | Keta-Cov research group. Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol. 2021;74:1243-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Zdanowicz K, Bobrus-Chociej A, Kopiczko A, Uścinowicz M, Tomczuk-Ostapczuk M, Janica J, Łotowska JM, Białokoz-Kalinowska I, Lebensztejn DM. Autoimmune sclerosing cholangitis might be triggered by SARS-CoV-2 infection in a child - a case report. Cent Eur J Immunol. 2022;47:183-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Schwarz S, Lang C, Harlander M, Štupnik T, Slambrouck JV, Ceulemans LJ, Ius F, Gottlieb J, Kuhnert S, Hecker M, Aigner C, Kneidinger N, Verschuuren EA, Smits JM, Tschernko E, Schaden E, Faybik P, Markstaller K, Trauner M, Jaksch P, Hoetzenecker K. Gamma-glutamyltransferase is a strong predictor of secondary sclerosing cholangitis after lung transplantation for COVID-19 ARDS. J Heart Lung Transplant. 2022;41:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 53. | Keskin O, Kav T, Vahabov C, Usta B, Sivri B, Parlak E. Clinical and Endoscopic Consequences of Delay in Stent Exchange Procedures With ERCP During the Covid-19 Pandemic. Surg Laparosc Endosc Percutan Tech. 2022;32:714-719. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Bartoli A, Gitto S, Sighinolfi P, Cursaro C, Andreone P. Primary biliary cholangitis associated with SARS-CoV-2 infection. J Hepatol. 2021;74:1245-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 55. | Ferreira FB, Mourato M, Bragança S, Paulo JB, Sismeiro R, Pereira A, Mónica AN, Lourenço LC, Cardoso M. COVID-19-associated secondary sclerosing cholangitis - A case series of 4 patients. Clin Res Hepatol Gastroenterol. 2022;46:102048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Bütikofer S, Lenggenhager D, Wendel Garcia PD, Maggio EM, Haberecker M, Reiner CS, Brüllmann G, Buehler PK, Gubler C, Müllhaupt B, Jüngst C, Morell B. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404-2417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 57. | Zafar M, Gordon K, Macken L, Parvin J, Heath S, Whibley M, Tibble J. COVID-19 Vaccination-Induced Cholangiopathy and Autoimmune Hepatitis: A Series of Two Cases. Cureus. 2022;14:e30304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 58. | Otani K, Watanabe T, Higashimori A, Suzuki H, Kamiya T, Shiotani A, Sugimoto M, Nagahara A, Fukudo S, Motoya S, Yamaguchi S, Zhu Q, Chan FKL, Hahm KB, Tablante MC, Prachayakul V, Abdullah M, Ang TL, Murakami K; International Gastrointestinal Consensus Symposium Study Group. A Questionnaire-Based Survey on the Impact of the COVID-19 Pandemic on Gastrointestinal Endoscopy in Asia. Digestion. 2022;103:7-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Cesar Machado MC, Filho RK, El Bacha IAH, de Oliveira IS, Ribeiro CMF, de Souza HP, Parise ER. Post-COVID-19 Secondary Sclerosing Cholangitis: A Rare but Severe Condition with no Treatment Besides Liver Transplantation. Am J Case Rep. 2022;23:e936250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Steiner J, Kaufmann-Bühler AK, Fuchsjäger M, Schemmer P, Talakić E. Secondary sclerosing cholangitis in a young COVID-19 patient resulting in death: A case report. World J Gastrointest Surg. 2022;14:1411-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 61. | Gourjault C, Tarhini H, Rahi M, Thy M, Le Pluart D, Rioux C, Parisey M, Ismael S, Aidibi AAR, Paradis V, Ghosn J, Yazdanpanah Y, Lescure FX, Gervais A. Cholangitis in three critically ill patients after a severe CoVID-19 infection. IDCases. 2021;26:e01267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Tafreshi S, Whiteside I, Levine I, D'Agostino C. A case of secondary sclerosing cholangitis due to COVID-19. Clin Imaging. 2021;80:239-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Leonhardt S, Jürgensen C, Frohme J, Grajecki D, Adler A, Sigal M, Leonhardt J, Voll JM, Kruse JM, Körner R, Eckardt KU, Janssen HJ, Gebhardt V, Schmittner MD; Pa-COVID-19 collaborative study group, Frey C, Müller-Ide H, Bauer M, Thibeault C, Kurth F, Sander LE, Müller T, Tacke F. Hepatobiliary long-term consequences of COVID-19: dramatically increased rate of secondary sclerosing cholangitis in critically ill COVID-19 patients. Hepatol Int. 2023;1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Zengarini C, Pileri A, Salamone FP, Piraccini BM, Vitale G, La Placa M. Subacute cutaneous lupus erythematosus induction after SARS-CoV-2 vaccine in a patient with primary biliary cholangitis. J Eur Acad Dermatol Venereol. 2022;36:e179-e180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Wendel-Garcia PD, Erlebach R, Hofmaenner DA, Camen G, Schuepbach RA, Jüngst C, Müllhaupt B, Bartussek J, Buehler PK, Andermatt R, David S. Long-term ketamine infusion-induced cholestatic liver injury in COVID-19-associated acute respiratory distress syndrome. Crit Care. 2022;26:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Morão B, Revés JB, Nascimento C, Loureiro R, Glória L, Palmela C. Secondary Sclerosing Cholangitis in a Critically Ill Patient with Severe SARS-CoV-2 Infection: A Possibly Emergent Entity during the Current Global Pandemic. GE Port J Gastroenterol. 2022;27:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Lee SK, Kwon JH, Yoon N, Nam SW, Sung PS. Autoimmune liver disease represented as primary biliary cholangitis after SARS-CoV-2 infection: A need for population-based cohort study. Clin Mol Hepatol. 2022;28:926-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 68. | Nikoupour H, Arasteh P, Gholami S, Nikeghbalian S. Liver transplantation and COVID-19: a case report and cross comparison between two identical twins with COVID-19. BMC Surg. 2020;20:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Arnstadt B, Zillinger C, Treitl M, Allescher HD. Corona again? SSC after a severe COVID-disease. Z Gastroenterol. 2021;59:1304-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Meersseman P, Blondeel J, De Vlieger G, van der Merwe S, Monbaliu D; Collaborators Leuven Liver Transplant program. Secondary sclerosing cholangitis: an emerging complication in critically ill COVID-19 patients. Intensive Care Med. 2021;47:1037-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Raes M, De Becker A, Blanckaert J, Balthazar T, De Ridder S, Mekeirele M, Verbrugge FH, Poelaert J, Taccone FS. Veno-venous extra-corporeal membrane oxygenation in a COVID-19 patient with cold-agglutinin haemolytic anaemia: A case report. Perfusion. 2022;2676591221127932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 72. | Fajardo J, Núñez E, Szafranska J, Poca M, Lobo D, Martín B, Hernández D, Roig C, Huerta A, Corominas H, Sánchez-Cabús S, Soriano G. We report a patient who presented intrahepatic cholangitis and cholecystitis after SARS-CoV-2 infection. J Gastroenterol Hepatol. 2021;36:2037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Pizarro Vega NM, Valer Lopez-Fando P, de la Poza Gómez G, Piqueras Alcol B, Gil Santana M, Ruiz Fuentes P, Rodríguez Amado MA, Bermejo San José F. Secondary sclerosing cholangitis: A complication after severe COVID-19 infection. Gastroenterol Hepatol. 2023;46:462-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Knooihuizen SAI, Aday A, Lee WM. Ketamine-Induced Sclerosing Cholangitis (KISC) in a Critically Ill Patient With COVID-19. Hepatology. 2021;74:519-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Zhou T, Fronhoffs F, Dold L, Strassburg CP, Weismüller TJ. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis - should we be more vigilant? J Hepatol. 2022;76:218-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 76. | Muehlenberg K, Tannapfel A, Pech O. [80-year-old patient with jaundice after a severe Covid-19 infection]. Dtsch Med Wochenschr. 2021;146:13-14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Soldera J, Salgado K. Ischemic Gastropathy in a Covid-19 pneumonia patient. Revista da AMRIGS. 65:58-59. |
| 78. | Rojas M, Rodríguez Y, Zapata E, Hernández JC, Anaya JM. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Caballero-Alvarado J, Zavaleta Corvera C, Merino Bacilio B, Ruiz Caballero C, Lozano-Peralta K. Post-COVID cholangiopathy: A narrative review. Gastroenterol Hepatol. 2023;46:474-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 80. | Franzini TAP, Guedes MMF, Rocha HLOG, Fleury CA, Bestetti AM, Moura EGH. Cholangioscopy in a post-COVID-19 cholangiopathy patient. Arq Gastroenterol. 2022;59:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Roda S, Ricciardi A, Maria Di Matteo A, Zecca M, Morbini P, Vecchia M, Chiara Pieri T, Giordani P, Tavano A, Bruno R. Post-acute coronavirus disease 2019 (COVID 19) syndrome: HLH and cholangiopathy in a lung transplant recipient. Clin Infect Pract. 2022;15:100144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Tebar DMCE, Reis LS, Mineiro GN, Pereira MLDeM, Piassa MLP, Salvajolli RR. Secondary Sclerosing Cholangitis after severe COVID-19: a new possibility in the critically ill patient. Brazilian Journal of Health Review. 2022;5:20-26. [DOI] [Full Text] |
| 83. | Santisteban Arenas MT, Osorio Castrillón LM, Guevara Casallas LG, Niño Ramírez SF. [Post-COVID-19 severe cholangiopathy: report of 6 cases]. Rev Gastroenterol Peru. 2022;42:53-57. [PubMed] |