Published online Sep 20, 2023. doi: 10.5662/wjm.v13.i4.179
Peer-review started: April 22, 2023
First decision: May 23, 2023
Revised: June 5, 2023
Accepted: June 27, 2023
Article in press: June 27, 2023
Published online: September 20, 2023
Processing time: 151 Days and 2.8 Hours
Compensated liver cirrhosis (CLC) is defined as cirrhosis with one or more decompensating events, such as ascites, variceal haemorrhage, or hepatic encephalopathy. Patients with CLC are largely asymptomatic with preserved hepatic function. The transition from CLC to decompensated cirrhosis occurs as a result of a complex interaction between multiple predisposing and precipitating factors. The first decompensation event in CLC patients is considered a significant turning point in the progression of cirrhosis, as it signals a drastic decline in median survival rates from 10-12 years to only 1-2 years. Furthermore, early cirrhosis has the potential to regress as liver fibrosis is a dynamic condition. With the advent of effective non-invasive tools for detecting hepatic fibrosis, more and more patients with CLC are currently being recognised. This offers clinicians a unique oppor
Core Tip: Compensated liver cirrhosis might be reversible if the underlying cause is treated before the disease progresses. The median survival for these individuals is typically 10-12 years; however, after the first decompensation, it drastically drops to 1-2 years. As a result, the outcomes of such patients can be significantly improved by integrating a number of disease-modifying therapy strategies that address complex pathophysiology, risk factors, and triggering events linked with disease progression. This article discussed the natural course of compensated liver cirrhosis, risk factors for its progression, and potential therapeutic strategies to favourably influence its natural evolution and enhance outcomes.
- Citation: Kumar R, Kumar S, Prakash SS. Compensated liver cirrhosis: Natural course and disease-modifying strategies. World J Methodol 2023; 13(4): 179-193
- URL: https://www.wjgnet.com/2222-0682/full/v13/i4/179.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i4.179
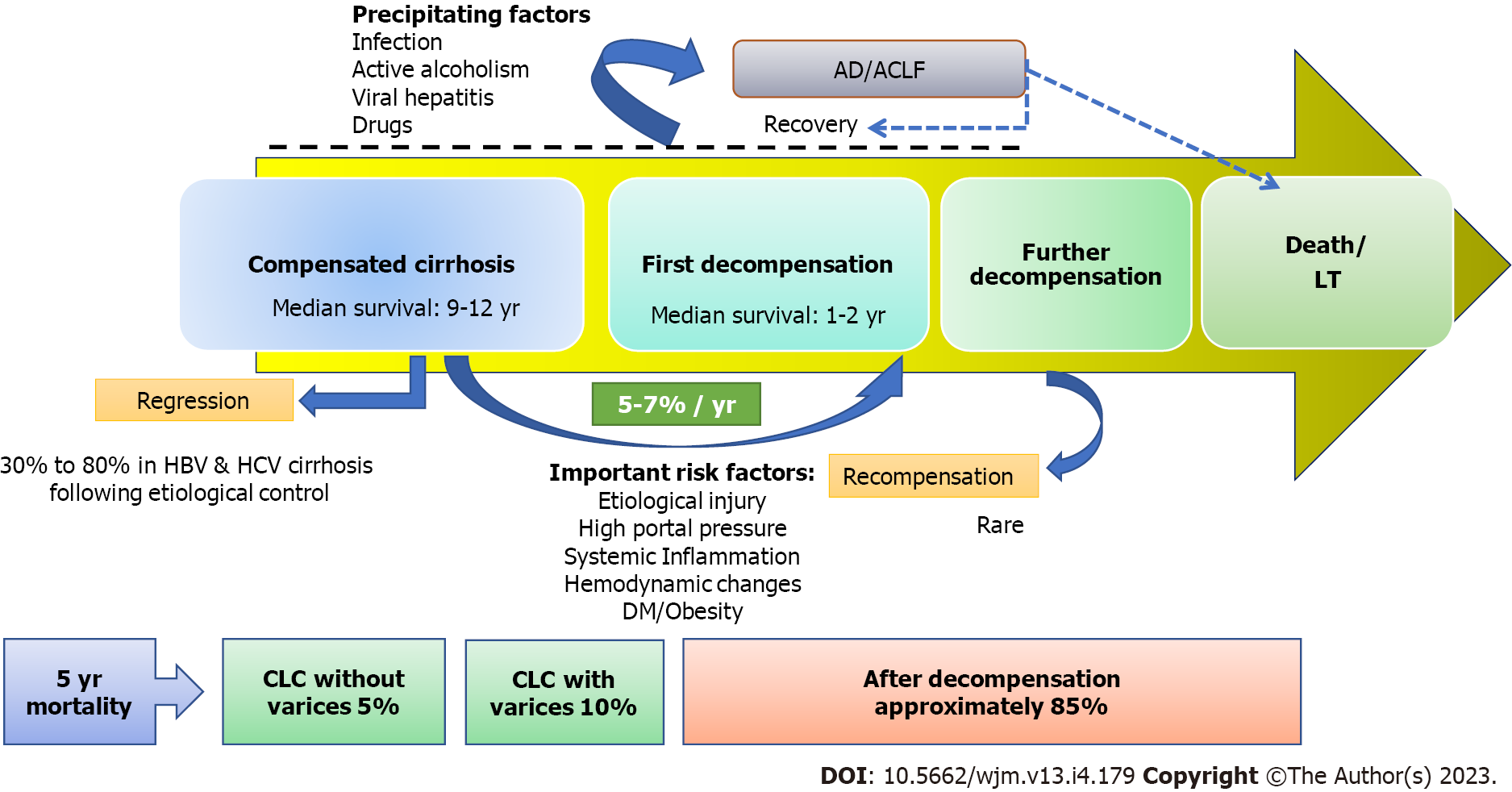
The prevalence and mortality associated with liver cirrhosis (LC) continue to increase despite improvements in knowledge and medical care. According to data from the United States, the annual number of LC-related deaths has risen by 65%, while the number of hospitalisations for LC has nearly doubled in a decade[1,2]. LC has traditionally been regarded as a singular entity with a continuum of increasing degrees of severity until death or liver transplantation. Recently, these paradigms have shifted, leading to the recognition of LC as a heterogenous condition with varying prognosis across the different stages[3,4]. The term “compensated liver cirrhosis” (CLC) is used to describe LC without one or more decompensating events, such as ascites, hepatic encephalopathy, variceal haemorrhage (VH), and jaundice[5]. After experiencing a decompensation event, LC patients are always classified as decompensated LC (DLC) because the pathogenic mechanisms that caused the decompensation persist. When LC patients were separated into two groups based on decompensating events, the median 1-year survival in CLC patients was 95% compared to 61% in DLC patients[6]. Therefore, the first decompensation in CLC patients is regarded as a prognostic watershed due to a substantial reduction in median survival from 10-12 years in CLC to only 1-2 years in DLC[6].
LC has long been viewed as the end stage of chronic liver disease (CLD). However, this perception has started to shift in the past two decades. Wanless et al[7] were the first to describe the reversal of LC, and since then numerous series of LC patients with diverse aetiologies have demonstrated the same[7,8]. Patients with CLC remain asymptomatic and undiagnosed for the first few years[9]. Despite being asymptomatic, between one-third and one-half of CLC patients have varices and clinically significant portal hypertension (CSPH) at the time of diagnosis[10-12]. Over time, CLC patients develop several risk factors that increase their susceptibility to clinical decompensation, such as rising portal pressure, systemic inflammation, and haemodynamic changes. Moreover, certain triggers including bacterial infection, medications, or alcohol can acutely precipitate decompensation. When the underlying cause of CLC is eliminated early on, a significant proportion of patients experience cirrhosis regression[7,8].
Even when regression of LC is not possible, there are variety of evolving strategies for preventing or delaying decompensation in such patients. Therefore, the prognosis of such patients can be greatly enhanced by early diagnosis of CLC. However, the medical community has predominantly focused its efforts on managing and improving the outcomes for patients with DLC, with little attention given to the medical management of CLC. In order to enhance the ease of diagnosis of advanced CLD noninvasively using transient elastography, the Baveno VI consensus introduced the new term “compensated advanced CLD” that encompasses CLC and CLD with advanced fibrosis[13]. Due to efficient noninvasive testing tools, more and more LC patients are now being recognised at an early compensated stage[14]. This offers the gastroenterologists and hepatologists greater opportunities to intervene and alter the trajectory of the natural evolution of CLC. This article discussed the natural history of CLC, risk factors for its progression and decompensation, and potential therapeutic strategies to change the course of the illness and improve the outcomes.
The natural progression of LC is characterised by a continuum from a long silent compensated phase to a more progressive symptomatic decompensation phase (Figure 1). As LC progresses over time, patients develop a variety of risk factors, including altered liver architecture, portal hypertension (PHT), systemic inflammation, and haemodynamic alterations that increase the risk for clinical decompensation. Decompensation may occur insidiously due to slowly increasing portal pressure and deteriorating hepatic function, often referred to as non-acute decompensation (AD)[5]. However, different triggering events, such as bacterial infection, alcohol, bleeding, medications, or a flare-up of liver disease, can lead to AD within days, which may progress to acute-on-chronic liver failure (ACLF). In patients with CLC, decompensation represents a turning point in terms of mortality risk, patient quality of life, and propensity for hospitalisation.
In a systematic review, pooling of data from relevant studies, revealed that the survival of patients with LC varied from 1 mo to 186 mo, with a median survival > 12 years for CLC and 1.8 years for DLC[6]. The rate of transition from a compensated to a decompensated stage is approximately 5%-7% each year[15]. Ascites is typically the first sign of decompensation in most studies. Overall, the 5-year mortality rate in CLC patients is only 1.5% for those without CSPH, 5.0% for those with CSPH but no varices, and 10.0% for those with CSPH and varices, highlighting the significance of PHT in mortality risk[3]. Therefore, CLC patients without varices and without CSPH constitute a highly compensated group with a very low mortality risk[6].
The first decompensation of CLC does not always indicate a point of no return in the natural course of LC. Emerging data suggests that although it is an uncommon occurrence, recompensation of DLC is possible if the underlying cause of LC is suppressed[16]. Recompensated cirrhosis is indeed a real condition, and the Baveno VII consensus has provided a standard definition for it[17]. Similarly, it is now more widely acknowledged that CLC can regress to a non-cirrhotic stage when etiological factors are promptly controlled[7,18]. Liver fibrosis is a dynamic condition, and early LC, which lacks extracellular matrix crosslinking and marked angiogenesis, can even revert into normal architecture[19].
The transition from CLC to DLC occurs as a result of a complex interaction between predisposing and precipitating factors (Table 1). The development of PHT is the key factor causing the switch from CLC to DLC[10,20-22]. In a study, patients with a hepatic venous pressure gradient (HVPG) < 10 mmHg had a 90% probability of not developing clinical decompensation over 4 years. As the HVPG rises above 10 mmHg, which signifies CSPH, the risk of decompensation begins to rise[10]. VH typically occurs when HVPG is higher than 12 mmHg[15]. Another study found that CLC with a baseline HVPG > 20 mmHg had a 47% risk of decompensation in mean duration of just 1.6 years[21]. There is also growing evidence that long-term use of non-selective beta-blockers (NSBBs) significantly reduces the risk of decompensation[22]. Thick fibrous septa and small nodules observed in liver biopsy specimens of CLC patients are associated with CSPH and an increased risk of decompensation[23-25]. Ongoing liver damage caused by etiological factors also increases the risk of decompensation. This is supported by the finding that attaining a sustained virological response (SVR) in hepatitis C virus (HCV)-cirrhosis and maintaining viral suppression in hepatitis B virus (HBV)-cirrhosis significantly lowers the incidence of decompensation[26-29]. The neurohormonal and inflammatory alterations in LC contribute to decompensation in the form of ascites by causing splanchnic vasodilatation and lymphatic dysfunction[30-32].
| Risk factors for non-acute decompensation | Precipitating factors for acute decompensation |
| Thick fibrous septa and micronodularity on liver biopsy | Bacterial infection |
| Persistent liver injury by etiological factor | Active alcoholism |
| High portal pressure | Gastrointestinal haemorrhage |
| Systemic inflammation & hemodynamic changes | Consumption of hepatotoxic drug/alternative medicine |
| Metabolic risk factors: DM, obesity, and dyslipidaemia | Superinfection or flare of viral hepatitis |
| Genetic risk factors: PNPLA3 G/G genotype | Major surgery and general anaesthesia |
Several metabolic factors have also been found to influence the risk of decompensation[33-36]. CLC patients with diabetes have a higher risk of developing any decompensating event than those without diabetes[33]. Obesity has a negative impact on the natural course of CLC, regardless of aetiology, and increases the risk of decompensation[34]. Sarcopenia and myosteatosis, which are common in CLD with various aetiologies, appear to promote the progression of CLD to advanced stages[35]. Another study found that the PNPLA3 G/G genotype, involved in triacylglycerol hydro
Bacterial infection can cause AD by escalating the intensity of systemic inflammation and PHT. In a large prospective study of 1672 patients with compensated HCV-related or HBV-related cirrhosis, bacterial infections preceded and precipitated decompensation in 13% of patients over a 5-year period[44]. Overall, bacterial infection is considered to be the most frequent precipitant of AD (22%-29%) and ACLF (33%-50%)[45]. Alcoholic hepatitis can cause decompensation in patients with CLC through various mechanisms[46,47]. AD and ACLF frequently develop in patients with LC undergoing surgery[48]. In a study using an animal model of CLC, it was discovered that extrahepatic surgery raises the portal pressure during the postoperative period, leading to decompensation[49]. Other situations where decompensation can develop in patients with CLC include superimposed viral hepatitis, consumption of hepatotoxic medications, and vascular thromboses[50].
The present strategy for managing patients with LC is centred on strategies intended to avoid or treat complications, without giving much thought to their effects on the natural history of LC. There is a need to pay more attention to agents that target key points in the complex pathophysiology of LC. The ideal goal of a disease-modifying medication should be the regression or reversal of cirrhosis. If this is not possible, the next goal should be to prevent or at least delay the progression of the disease. Growing evidence suggests that addressing the underlying cause of LC and reducing PHT by NSBB have positive impacts on the natural history of patients with CLC[7,8,22,26,29]. Moreover, such patients may potentially benefit from addressing a number of cofactors and precipitating factors that have a negative influence on the natural course of LC[51]. Thus, effective disease-modifying treatment strategies might include: (1) Removal of etiological factors; (2) Pathophysiology-oriented therapy; (3) Management of adverse cofactors like obesity, DM, dyslipidaemia, and alcoholism; (4) Anti-fibrotic and regenerative therapies; and (5) Elimination of precipitating factors that lead to AD/ACLF.
The main prerequisite for fibrosis regression is the cessation of liver injury, which is accomplished through therapeutic control of causal factors. Regression of LC has been extensively described in patients with HBV-related and HCV-related cirrhosis after aetiological treatment (Table 2). Nevertheless, robust data supporting the regression of non-viral causes of LC are generally lacking.
| Ref. | Study design | Drug/duration | Patients, n | Baseline LC | Main results |
| Dienstag et al[26], 2003 | Prospective, partially randomised | Lamivudin/3 yr | 63 CHB | 11 | LC regressed in 8 of 11 patients (73%) |
| Hadziyannis et al[27], 2006 | Prospective | Adefovir dipivoxil, up to 240 wk | 125 CHB | 4 | 58% had reversal of bridging fibrosis/cirrhosis; 3 of 4 LC patients had reversal |
| Marcellin et al[29], 2013 | Randomised trial | TDF/adefovir for 48 wk then open-label TDF | 641 CHB | 96 | 71 of 96 (74%) became non-cirrhotic at 5 yr |
| Poynard et al[52], 2002 | Pooled data from RCTs | IFN/PEG-IFN + RBV | 3010 CHC | 153 | The reversal of LC was observed in 75 patients (49%) |
| Mauro et al[53], 2018 | Retrospective | DAAs/IFN + RBV | 112 HCV-infected LT recipients | 37 | Regression of fibrosis in 43% of LC (16/37) |
| Lassailly et al[55], 2020 | Prospective | Bariatric surgery | 180 obese NASH | 9 | At 5 yr, fibrosis regression was seen in 68% of advanced fibrosis and 33% of patients had reversal of LC |
| Sanyal et al[54], 2022 | Data from two RCTs | Simtuzumab or selonsertib or placebo | 1135 NASH patients, 709 (62%) had Ishak stage 6 fibrosis | 709 | LC regression occurred in 16% (176/1135). Drugs were not better than placebo |
| Dufour et al[64], 1997 | Retrospective | Immunosuppressant | 8 AIH cirrhosis | 8 | LC regressed in all |
| Czaja et al[63], 2004 | Retrospective | Corticosteroid | 87 AIH | 14 | LC regressed in 4 of 14 patients |
| Bardou-Jacquet et al[66], 2020 | Retrospective | Venesection | 106 patients with haemochromatosis | 66 | LC regressed in 15 of 66 (23%) during median follow-up of 9.5 yr |
Viral cirrhosis: Dienstag et al[26] reported regression of LC in 73% (8/11) of patients following 3 years of lamivudine therapy. In another prospective study, treatment with adefovir dipivoxil for up to 240 wk resulted in regression of bridging fibrosis or cirrhosis in 58% (7/12) of patients[27]. Pegylated interferon and ribavirin combination therapy has been shown to reduce liver fibrosis following SVR. Combination therapy led to the reversal of LC in 49% of patients in a pooled data analysis from four randomised controlled trials (RCTs) that included 153 patients with HCV-cirrhosis at baseline[52]. Though data are still evolving, a recent study found that direct-acting antivirals were effective in reducing fibrosis based on FibroScan[53]. In a systematic review of 463 patients with HBV-cirrhosis, regression of LC was observed in 33% to 80% of patients following sustained viral suppression. Meanwhile, LC regressed in 33% to 100% of 58 patients with HCV-cirrhosis following SVR[28]. This suggests that once the causal element is removed early in the course of CLC, progression is halted and cirrhosis regression occurs in a significant number of patients.
Non-viral cirrhosis: Weight loss through lifestyle changes improves fibrosis in patients with nonalcoholic steatohepatitis (NASH), but its effects on NASH-cirrhosis per se are still poorly understood. In a recently published study involving 709 patients with compensated NASH-cirrhosis from two RCTs on simtuzumab and selonsertib vs placebo, regression of LC was observed in 135 patients during a median follow-up of 16.6 mo. Notably, the impact of the drug was not better than placebo, indicating the influence of lifestyle modification[54]. Another study that assessed the long-term effects of bariatric surgery in 180 obese patients with NASH found significant regression of fibrosis at 5 years after surgery. The fibrosis decreased in 70.2% of patients, disappeared in 42.0% of patients, and 33.0% of patients with baseline LC became non-cirrhotic[55]. However, the outcomes of RCTs on a variety of compounds that target the metabolic/inflammatory pathways of NASH-cirrhosis have been dismal[56-59]. In a recent RCT with emricasan, an oral pan-caspase inhibitor, there was a small treatment effect on HVPG reduction in compensated NASH cirrhosis; however, the drug was overall ineffective in improving clinical outcomes[56]. Belapectin, an inhibitor of galectin-3 that was earlier found to reduce liver fibrosis and PHT in rats, was proven to be ineffective in human NASH-cirrhosis[57].
Abstinence from alcohol improves the prognosis in all stages of alcohol-related LC; nevertheless, there is scant clinical support for fibrosis/cirrhosis regression in alcoholic liver disease[60-62]. In one study, patients who were abstinent vs those who were not showed a 3-year decompensation likelihood of 32.4% vs 60.0%, respectively[60]. Early alcohol abstinence after LC diagnosis was found to be a significant predictor in survival, with abstinent patients having a 72% survival rate at 7 years compared to 44% in patients who continued to consume alcohol[61]. Reversibility of hepatic fibrosis has also been documented in autoimmune hepatitis patients[63,64]. A study on corticosteroid-treated autoimmune hepatitis has revealed regression of histological LC from 16% to 11%[63]. Ursodeoxycholic acid treatment may halt disease progression and improve the survival of patients with primary biliary cholangitis, but it appears to be less effective in promoting fibrosis regression[65]. In a retrospective analysis of patients with haemochromatosis treated with venesection, LC regression was observed in 15 out of 66 (23%) over a median period of 9.5 years[66].
NSBB: In patients with LC, CSPH appears to be an important pathophysiologic driver of the first decompensating event[10,20-22]. The onset of PHT is primarily caused by elevated portal blood inflow resistance resulting from architectural distortion (fixed component) and increased elevated hepatic microvascular tone (dynamic component). The increased hepatic vascular tone is attributed to decreased intrahepatic vasodilators, primarily nitric oxide (NO), and increased production of vasoconstrictors such as angiotensin, endothelins, and prostanoids. Portosystemic collaterals appear later, heralding splanchnic vasodilation, increased splanchnic blood flow, and hyperkinetic circulation, all contributing to further raise portal pressure. The only pharmacological class that is still recommended for long-term treatment of PHT is NSBB. Importantly, NSBBs are only beneficial in patients with CSPH and not in those with subclinical PHT because they reduce portal venous flow, and significant hyperdynamic circulation only develops once CSPH is set[67]. Adequate responses to NSBBs are associated with a lower incidence of decompensating events and improved survival rate, indicating a positive influence on the natural history of LC (Table 3).
| Ref. | Study population | Intervention | Study design | Sample size, n | Study conclusion |
| Poynard et al[70], 1991 | LC patients with oesophageal varices | Propranolol, nadolol vs placebo | Meta-analysis of 4 RCTs | 589 | Both propranolol and nadolol were effective in preventing first VH and reducing the mortality associated with VH |
| Tripathi et al[71], 2009 | LC patients with grade II or more varices | Carvedilol vs EVL | RCT | 152 | On intention-to-treat analysis, carvedilol had lower rates of the first VH compared to EVL (10% vs 23%) |
| Gluud et al[69], 2012 | LC patients with high-risk varices without prior VH | NSBBs vs EVL | Meta-analysis of 19 RCTs | 1504 | Both EVL and NSBB reduced VH (RR: 0.69 and 0.67) without difference in mortality rates |
| Sinagra et al[75], 2014 | LC patients with PHT | Carvedilol vs propranolol | Meta-analysis of 5 studies | 175 | Carvedilol reduced PHT significantly more than propranolol |
| Bhardwaj et al[76], 2017 | LC patients with small varices | Carvedilol vs placebo | RCT | 140 | Carvedilol is safe and effective in delaying the progression of small to large oesophageal varices in LC patients |
| Zacharias et al[77], 2018 | Adults with LC and varices | NSBBs | Meta-analysis of 10 RCTs | 810 | Carvedilol was more effective at reducing the HVPG. However, it was not better than traditional NSBBs with regard to the mortality, VH, or adverse events |
| Malandris et al[72], 2019 | LC patients requiring primary or secondary prevention of VH | Carvedilol, NSBBS, EVL | Meta-analysis of 13 RCTs | 1598 | Carvedilol was as efficacious and safe as standard-of-care interventions for the primary and secondary prevention of VH. Also, carvedilol was associated with lower all-cause mortality compared to EVL |
| Sharma et al[73], 2019 | LC patients with large oesophageal varices and no prior history of VH | NSBB, isosorbide-mononitrate, carvedilol, and EVL alone or in combination | Meta-analysis of 32 RCTs | 3362 | NSBB monotherapy decreased all-cause mortality and the risk of first VH. Additionally, NSBB carried a lower risk of serious complications compared with EVL |
| Villanueva et al[22], 2019 | CLC patients and CSPH | Propranolol, carvedilol vs placebo | RCT | 201 | Long-term treatment with β blockers could increase decompensation-free survival in patients with CLC with CSPH, mainly by reducing the incidence of ascites |
| Villanueva et al[78], 2022 | LC patients with CSPH | Carvedilol vs EVL/no treatment | Meta-analysis of 4 RCTs | 352 | Long-term carvedilol therapy reduced decompensation and significantly improved survival |
According to current recommendations, patients with high-risk varices should have NSBBs or endoscopic variceal ligation (EVL) as primary prophylaxis for VH[68]. While choosing between NSBBs and EVL, the patient’s preferences, tolerance, side effect profile, and contraindications can all be taken into account[69]. Importantly, NSBB, in addition to being as effective as EVL at preventing VH, has the added benefit of lowering the risk of decompensation and mortality[22,70-73]. Among commonly used NSBBs, propranolol and nadolol reduce portal pressure by reducing portal venous inflow by blocking β1 and β2 adrenergic receptors, whereas carvedilol has additional intrinsic vasodilatory activity because of its anti-α-adrenergic activity and its ability to increase NO release[74]. Moreover, carvedilol has been observed to cause greater reduction in HVPG compared to propranolol or nadolol[75,76]. Carvedilol might be especially useful for patients with CLC, where a higher hepatic vascular resistance is the main cause of PH[76,77].
The emerging evidence strongly indicates that NSBBs can prevent decompensation in patients with CLC. The PREDESCI trial, a multicentre, double-blind, RCT, studied whether the administration of NSBBs over an extended period of time prevents the development of clinical decompensation and increase survival in CLC patients with CSPH[22]. In this study, 201 patients with CLC with CSPH were randomly assigned to receive NSBBs or a placebo. After a median follow-up of 37 mo, the NSBB arm showed considerably lower rates of clinical decompensation compared to the placebo arm [17% vs 27%, hazard ratio (HR): 0.51, 95% confidence interval (CI): 0.26-0.97]. Among decompensating events, decreased incidence of ascites (20% vs 9%) was the main effect of NSBB. However, the PREDESCI trial hinged the choice of NSBB on the HVPG response to intravenous propranolol, which is impracticable for a large population of patients. In a recent competing-risk time-to-event meta-analysis that included 352 patients with CLC, 181 carvedilol-treated patients, and 171 controls from 4 RCTs, long-term carvedilol therapy was associated with a decreased risk of decompensating events and improved survival. Carvedilol decreased the risk of decompensation, mainly ascites, with a subdistribution HR of 0.506 (95%CI: 0.289-0.887), and the risk of death with a subdistribution HR: of 0.417 (95%CI: 0.194-0.896)[78].
Statins: Several studies have been published in recent years on the beneficial effects of statins in patients with LC. The benefits include a decrease in portal pressure, favourable effects on sinusoidal endothelial function, hepatic microcirculation, and liver fibrosis[79]. In the first such study of statins in LC, simvastatin was found to increase NO generation in liver sinusoids and reduced intrahepatic vascular resistance[80]. Furthermore, it was found that simvastatin had an additive effect with NSBB on HVPG reduction[81]. Several moderate-quality studies have suggested that the use of statins in CLC lowers the risk of hepatic decompensation and mortality[82-84]. In a large study from Taiwan, including 1350 patients with LC, statin use decreased the risk of decompensation, mortality, and hepatocellular carcinoma in a dose-dependent manner. With statins, the incidence of decompensation was 61% lower in HBV-cirrhosis and 49% lower in HCV-cirrhosis[85].
In a recent systematic review and meta-analysis, statins were associated with a 46% reduced risk of hepatic decompensation and a 46% lower risk of mortality in patients with LC[86]. Notably, LC patients who benefit from statins are mainly those with Child-Pugh classes A or B but not those with Child class C. In DLC, simvastatin at higher doses may even cause rhabdomyolysis and hepatotoxicity[87,88]. Therefore, despite a strong case for statins being helpful for patients with LC, more evidence from high-quality RCTs is required before they can be regularly recommended for patients with CLC. Until then, it is safe to presume that patients with CLC with dyslipidaemia should not be denied statin therapy.
Anticoagulant: As LC progresses, endothelial damage and occlusion of small hepatic veins causes parenchymal extinction, which contributes to tissue collapse and architectural distortion[8]. Furthermore, studies have shown that the oral anticoagulant rivaroxaban and the low molecular weight heparin enoxaparin lower intrahepatic vascular resistance in cirrhotic rat models[89,90]. These beneficial effects of anticoagulants were attributed to a decreased intrahepatic microthrombosis, hepatic stellate cell deactivation, and increased NO bioavailability. In a small RCT, a 12-mo regimen of enoxaparin in patients with LC with a Child-Pugh score of 7 to 10 appeared to prolong survival and delay the emergence of hepatic decompensation[91]. In a recent meta-analysis, use of antiplatelet agents was associated with a 32% decreased risk of hepatic fibrosis[92]. Still, more data are required before a conclusive statement can be made about the usage of anticoagulants and antiplatelet agents in CLC patients.
Weight loss and glycaemic control: Obesity is a condition characterised by systemic inflammation and immunological dysregulation that is linked to poor clinical outcomes in LC patients, including decompensation[34,93]. Obese patients with LC have higher levels of inflammatory cytokines, increasing the risk of decompensation through a systemic inflammatory response[94]. Obesity has a negative impact on the course of CLC across all aetiologies, regardless of portal pressure or liver function. In a study including 161 patients with CLC, clinical decompensation occurred in 15% of lean, 31% of overweight, and 43% of obese patients during a median follow-up of 59 mo[34]. Class III obesity was found to be an independent risk factor for the development of ACLF in a recent study using data registries[95]. Hence, weight loss may be an effective therapeutic strategy for obese patients with cirrhosis[96,97]. Following bariatric surgery, the advantages of weight loss have been established in obese patients with LC[96]. Nonetheless, bariatric surgery is not generally recommended for people with CLC and is contraindicated in DLC. Patients with LC may be safely recommended to change their lifestyles under the care of a dietician with the aim to reduce body weight.
In LC patients, the prevalence of abnormal glucose regulation, including type 2 DM and hepatogenous diabetes, is quite high (20% to 70%)[98,99]. This high incidence appears to be due to poor insulin clearance and dysfunctional pancreatic beta cells[98]. Hyperinsulinemia is also implicated in vasodilatory and antinatriuretic effects in patients with CLC[100]. Impaired glycaemic indices in LC are associated with disease progression, decompensation, complications, and poor outcomes[101,102]. Hence, maintaining appropriate glycaemic control can benefit the course of LC. However, standardised diabetes management for LC patients has not been established yet. In addition to lifestyle modification, oral hypoglycaemic agents can be used in LC patients up to Child-Pugh class B, while insulin is recommended for LC at all stages.
Alcohol abstinence: Abstinence from alcohol reduces the risk of decompensation and improves outcomes in all stages of alcohol-related LC[60-62]. Early alcohol abstinence after diagnosis of LC is important for a better outcome[61]. According to a meta-analysis of seven cohort studies involving 1235 patients with alcoholic cirrhosis, at least 1.5 years of abstinence is required before a statistically significant difference in survival between the abstinent and alcohol consumption groups can be observed[103].
Diet, salt, and physical activity: Dietary interventions should include a target protein intake of 1.2-1.5 g/kg/d and regular aerobic exercise to prevent or ameliorate sarcopenia, which is associated with poor outcomes in LC patients[104-106]. In a recent prospective study, 16 wk of personalised hypocaloric normoproteic diet and 60 min per week of supervised physical activity were found to reduce body weight and portal pressure in overweight/obese patients with CLC[107].
Many observational studies have found an inverse relationship between coffee consumption and LC[108,109]. According to two recent meta-analyses, coffee drinkers had a lower risk of developing LC than non-drinkers[108,109]. Unfortunately, these meta-analyses may not have adequately controlled the bias and confounding factors due to the low quality of the observational studies. Prospective RCTs are needed to establish the impact of coffee consumption on fibrosis regression in CLC patients.
Most of the scientific societies do not recommend sodium (salt) restriction for patients with CLC. However, preascitic LC patients have been found to retain sodium when faced with a high salt intake[110]. It is believed that a new steady state of sodium balance is eventually achieved in such patients primarily because of increased levels of atrial natriuretic peptide, inhibition of the renin-angiotensin-aldosterone system, and suppression of sympathetic activity, which prevent sodium retention and the formation of ascites. Jalan et al[111] reported that the severity of PHT contributed to the abnormalities of sodium handling in patients with CLC. Therefore, it would be intriguing to determine whether salt restriction could prevent the development of ascites in CLC patients with CSPH.
Correction of vitamin D deficiency: The liver plays a crucial role in 25-hydroxyvitamin D metabolism. Vitamin D deficiency and insufficiency are highly prevalent in patients with CLD (64% to 92%), where they are associated with poor outcomes[112]. Emerging evidence suggests protective effects of vitamin D against hepatic fibrogenesis[113,114]. However, vitamin D supplementation had no beneficial effects in animal models of LC and pre-existing fibrosis[115]. In a recent RCT, vitamin D treatment did raise the serum levels of 25-hydroxyvitamin D in patients with LC, but indicators of liver fibrosis did not improve[116]. The disappointing results can be attributed to the small sample size and short study period, calling for larger and longer studies in the LC population. Nevertheless, LC patients who are vitamin D deficient should receive treatment because LC itself is associated with an increased risk of osteopenia and fracture.
The potential of cell treatments to improve liver regeneration and modify the course of liver disease has garnered a lot of attention in recent years. There are many different cell types that can be employed to treat LC or fibrosis; however, mesenchymal stem cells (MSCs) are the most often used cell source (73%). Research using animal models have demonstrated that MSC therapy can reduce liver fibrosis, improve liver function, and lessen liver injury[117]. Clinical studies using MSCs for cirrhosis have demonstrated their efficacy in improving liver function; however, large-scale, stratified studies in various CLD settings are required to draw a robust conclusion[117-120]. Also, patients with advanced LC with liver failure, rather than CLC, have been the focus of the majority of clinical trials on stem cells. There is significant heterogeneity between published studies in terms of the type, dose, and method of stem cell delivery, and data accuracy, making it a difficult interpretation[121].
Granulocyte-colony stimulating factor (G-CSF) serves as an alternative to exogenous stem cell infusions by mobilising haematopoietic stem and immune cells. Some RCTs on G-CSF have reported improved survival in patients with ACLF[122,123]. However, in a recent multicentre, open-label, RCT, G-CSF with or without haemopoietic stem cell infusions did not improve liver dysfunction or fibrosis in patients with CLC. Moreover, these medicines were associated with a higher frequency of unfavourable events[124]. Therefore, because of conflicting results, G-CSF is not currently advised for use in CLC patients.
Recent developments in important pathophysiologic pathways linked to PHT in LC have revealed a number of novel possible treatment targets. These include intrahepatic abnormalities associated with inflammation, fibrogenesis, and microvascular changes[57-59,81,125-128]. Some medications, including phosphodiesterase-5 inhibitors, farnesoid X receptor agonists, endothelin-A receptor antagonists, and amino sulphonic acid taurine, have been found to lower PHT, which may be useful in reducing decompensation in CLC patients[125-128]. However, more solid and consistent evidence is required regarding the safety and effectiveness of these medicines. Currently, there are no pharmacotherapies for fibrosis that have been licensed, but research on antifibrosis medications has made significant strides in recent years, especially with regard to medications for nonalcoholic fatty liver disease-related fibrosis[129]. It seems that treatment with a single medication may not be sufficient to treat advanced liver fibrosis due to the complexity of the hepatic fibrosis process, which involves interactions between many cell types including immune cells, hepatic stellate cells, and hepatocytes. Therefore, more studies are needed to determine combination therapies using drugs that have several modes of action.
Several precipitating events can lead to abrupt worsening of clinical condition of LC patients by causing ACLF[130,131]. Furthermore, CLC patients experience a more severe form of ACLF than patients with prior decompensation episodes. Thus, controlling such precipitating variables can thereby significantly reduce cirrhosis-related morbidity and mortality. Antibiotic prophylaxis and prompt, judicious antibiotic treatment can aid in the prevention of ACLF triggered by infection[132]. Prophylactic antibiotics in conjunction with effective gastrointestinal bleeding management can prevent precipitating ACLF.
Another important preventive strategy is vaccination for viral hepatitis. For all LC patients, hepatitis B vaccine is advised. However, compared to normal subjects, patients with cirrhosis achieve lower seroprotection rates following HBV vaccination (mean response rate of 47%)[133,134]. Hepatitis E virus (HEV) and hepatitis A virus (HAV) superinfection is another well-known cause of ACLF in endemic areas[135]. While many nations recommend HAV immunisation for CLD patients, routine vaccination is not advised in some areas, such as India, where the prevalence of HAV antibodies in CLD is apparently greater than 90%. Recombinant HEV vaccines have also been developed but are not widely available or approved across the world[136]. When vaccination is not an option, general preventive measures like improvement of sanitary conditions and the provision of clean water might decrease the incidence of HEV/HAV-induced ACLF.
The life expectancy of patients with CLC is often high, and many of them may be candidates for cirrhosis regression. Therefore, to facilitate the early detection of CLC, medical professionals should employ noninvasive techniques in all CLD patients. Early diagnosis of CLC offers the opportunity to treat underlying causes and prevent or halt the progression of liver disease. For viral cirrhosis, and to a lesser extent non-viral cirrhosis, fibrosis regression may result in cirrhosis reversal when the underlying cause of CLC is treated before progression. The transition from compensated to decompensated cirrhosis is mainly driven by PHT. This transition is accompanied by a dramatic decline in the median survival rates. NSBBs are currently the cornerstones of treatment for PHT, although a number of emerging therapies may pave the way for tailored multimodal strategies in the future. Some more recent drugs have shown promise in decreasing PHT, but more reliable and consistent data are needed to determine their safety and efficacy in LC patients. Furthermore, there are several known risk factors and triggering events for the decompensation of CLC that need to be taken care of. Regardless of the aetiology of CLC, evolving disease-modifying approaches might lead to a paradigm change and a decrease in the burden, morbidity, and mortality associated with LC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Qi XS, China; Zhu WF, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016;64:2165-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 2. | Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 580] [Article Influence: 82.9] [Reference Citation Analysis (1)] |
| 3. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 4. | Garcia-Tsao G. Natural History of Cirrhosis. In: Keaveny A, Cárdenas A. editors. Complications of Cirrhosis. Cham Springer, 2015. [DOI] [Full Text] |
| 5. | D'Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol. 2022;76:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (1)] |
| 6. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2134] [Article Influence: 112.3] [Reference Citation Analysis (3)] |
| 7. | Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 244] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 8. | Hytiroglou P, Theise ND. Regression of human cirrhosis: an update, 18 years after the pioneering article by Wanless et al. Virchows Arch. 2018;473:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 9. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 862] [Article Influence: 215.5] [Reference Citation Analysis (1)] |
| 10. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 812] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 11. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 12. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R; Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 654] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
| 13. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 14. | Roccarina D, Rosselli M, Genesca J, Tsochatzis EA. Elastography methods for the non-invasive assessment of portal hypertension. Expert Rev Gastroenterol Hepatol. 2018;12:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Samonakis DN, Koulentaki M, Coucoutsi C, Augoustaki A, Baritaki C, Digenakis E, Papiamonis N, Fragaki M, Matrella E, Tzardi M, Kouroumalis EA. Clinical outcomes of compensated and decompensated cirrhosis: A long term study. World J Hepatol. 2014;6:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Reiberger T, Hofer BS. The Baveno VII concept of cirrhosis recompensation. Dig Liver Dis. 2023;55:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 17. | Wang Q, Zhao H, Deng Y, Zheng H, Xiang H, Nan Y, Hu J, Meng Q, Xu X, Fang J, Xu J, Wang X, You H, Pan CQ, Xie W, Jia J. Validation of Baveno VII criteria for recompensation in entecavir-treated patients with hepatitis B-related decompensated cirrhosis. J Hepatol. 2022;77:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 701] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 19. | Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1005] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 20. | D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 21. | Jindal A, Bhardwaj A, Kumar G, Sarin SK. Clinical Decompensation and Outcomes in Patients With Compensated Cirrhosis and a Hepatic Venous Pressure Gradient ≥20 mm Hg. Am J Gastroenterol. 2020;115:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 453] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 23. | Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Jain D, Sreenivasan P, Inayat I, Deng Y, Ciarleglio MM, Garcia-Tsao G. Thick Fibrous Septa on Liver Biopsy Specimens Predict the Development of Decompensation in Patients With Compensated Cirrhosis. Am J Clin Pathol. 2021;156:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Rastogi A, Maiwall R, Bihari C, Ahuja A, Kumar A, Singh T, Wani ZA, Sarin SK. Cirrhosis histology and Laennec staging system correlate with high portal pressure. Histopathology. 2013;62:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 27. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, Brosgart CL, Borroto-Esoda K, Arterburn S, Chuck SL; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 28. | Manne V, Akhtar E, Saab S. Cirrhosis regression in patients with viral hepatitis B and C: a systematic review. J Clin Gastroenterol. 2014;48:e76-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1371] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 30. | Cárdenas A, Arroyo V. Mechanisms of water and sodium retention in cirrhosis and the pathogenesis of ascites. Best Pract Res Clin Endocrinol Metab. 2003;17:607-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Stadlbauer V, Wright GA, Banaji M, Mukhopadhya A, Mookerjee RP, Moore K, Jalan R. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Kumar R, Anand U, Priyadarshi RN. Lymphatic dysfunction in advanced cirrhosis: Contextual perspective and clinical implications. World J Hepatol. 2021;13:300-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Liu TL, Trogdon J, Weinberger M, Fried B, Barritt AS 4th. Diabetes Is Associated with Clinical Decompensation Events in Patients with Cirrhosis. Dig Dis Sci. 2016;61:3335-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Groszmann RJ; Portal Hypertension Collaborative Group. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 35. | Cespiati A, Meroni M, Lombardi R, Oberti G, Dongiovanni P, Fracanzani AL. Impact of Sarcopenia and Myosteatosis in Non-Cirrhotic Stages of Liver Diseases: Similarities and Differences across Aetiologies and Possible Therapeutic Strategies. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Mandorfer M, Scheiner B, Stättermayer AF, Schwabl P, Paternostro R, Bauer D, Schaefer B, Zoller H, Peck-Radosavljevic M, Trauner M, Reiberger T, Ferenci P, Ferlitsch A. Impact of patatin-like phospholipase domain containing 3 rs738409 G/G genotype on hepatic decompensation and mortality in patients with portal hypertension. Aliment Pharmacol Ther. 2018;48:451-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, Zhou J, Ni S, Liu L, Pons N, Batto JM, Kennedy SP, Leonard P, Yuan C, Ding W, Hu X, Zheng B, Qian G, Xu W, Ehrlich SD, Zheng S, Li L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1539] [Article Influence: 139.9] [Reference Citation Analysis (40)] |
| 38. | Shao L, Ling Z, Chen D, Liu Y, Yang F, Li L. Disorganized Gut Microbiome Contributed to Liver Cirrhosis Progression: A Meta-Omics-Based Study. Front Microbiol. 2018;9:3166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 39. | Horvath A, Rainer F, Bashir M, Leber B, Schmerboeck B, Klymiuk I, Groselj-Strele A, Durdevic M, Freedberg DE, Abrams JA, Fickert P, Stiegler P, Stadlbauer V. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci Rep. 2019;9:12000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, Hylemon PB, Fuchs M, Puri P, Schubert ML, Sanyal AJ, Sterling RK, Stravitz TR, Siddiqui MS, Luketic V, Lee H, Sikaroodi M, Gillevet PM. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol. 2018;113:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 41. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 851] [Article Influence: 77.4] [Reference Citation Analysis (1)] |
| 42. | Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Reichert MC, Ripoll C, Casper M, Greinert R, Vandieken E, Grünhage F, Appenrodt B, Zipprich A, Lammert F. Common NOD2 Risk Variants as Major Susceptibility Factors for Bacterial Infections in Compensated Cirrhosis. Clin Transl Gastroenterol. 2019;10:e00002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Nahon P, Lescat M, Layese R, Bourcier V, Talmat N, Allam S, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Goria O, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Hillaire S, Di Martino V, Trinchet JC, Moreau R, Roudot-Thoraval F; ANRS CO12 CirVir and Microcir Groups. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort). Gut. 2017;66:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jansen C, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Jarcuska P, Steib C, Reiberger T, Acevedo J, Gatti P, Shawcross DL, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Danielsen KV, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solé C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Kani HT, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia-Lopez E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Praktiknjo M, Curto A, Pitarch C, Putignano A, Moreno E, Bernal W, Aguilar F, Clària J, Ponzo P, Vitalis Z, Zaccherini G, Balogh B, Gerbes A, Vargas V, Alessandria C, Bernardi M, Ginès P, Moreau R, Angeli P, Jalan R, Arroyo V; PREDICT STUDY group of the EASL-CLIF CONSORTIUM. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 46. | Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 47. | Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, Jalan R. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Klein LM, Chang J, Gu W, Manekeller S, Jansen C, LingoHR: P, Praktiknjo M, Kalf JC, Schulz M, Spengler U, Strassburg C, Cárdenas A, Arroyo V, Trebicka J. The Development and Outcome of Acute-on-Chronic Liver Failure After Surgical Interventions. Liver Transpl. 2020;26:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Chang J, Meinke J, Geck M, Hebest M, Böhling N, Dolscheid-Pommerich R, Stoffel-Wagner B, Kristiansen G, Overhaus M, Peyman LO, Klein S, Uschner FE, Brol MJ, Vilz TO, LingoHR: P, Kalff JC, Jansen C, Strassburg CP, Wehner S, Trebicka J, Praktiknjo M. Extrahepatic Surgery in Cirrhosis Significantly Increases Portal Pressure in Preclinical Animal Models. Front Physiol. 2021;12:720898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Poordad FF. Presentation and complications associated with cirrhosis of the liver. Curr Med Res Opin. 2015;31:925-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 51. | Herrera JL, Rodríguez R. Medical Care of the Patient With Compensated Cirrhosis. Gastroenterol Hepatol (N Y). 2006;2:124-133. [PubMed] |
| 52. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 803] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 53. | Mauro E, Crespo G, Montironi C, Londoño MC, Hernández-Gea V, Ruiz P, Sastre L, Lombardo J, Mariño Z, Díaz A, Colmenero J, Rimola A, Garcia-Pagán JC, Brunet M, Forns X, Navasa M. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67:1683-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 54. | Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, Abdelmalek MF, Ding D, Han L, Jia C, Huss RS, Chung C, Wong VW, Okanoue T, Romero-Gomez M, Muir AJ, Afdhal NH, Bosch J, Goodman Z, Harrison SA, Younossi ZM, Myers RP. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology. 2022;75:1235-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 55. | Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. 2020;159:1290-1301.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 56. | Garcia-Tsao G, Bosch J, Kayali Z, Harrison SA, Abdelmalek MF, Lawitz E, Satapathy SK, Ghabril M, Shiffman ML, Younes ZH, Thuluvath PJ, Berzigotti A, Albillos A, Robinson JM, Hagerty DT, Chan JL, Sanyal AJ; IDN-6556-14 Investigators(‡). Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 57. | Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, Noureddin M, Pyko M, Shiffman M, Sanyal A, Allgood A, Shlevin H, Horton R, Zomer E, Irish W, Goodman Z, Harrison SA, Traber PG; Belapectin (GR-MD-02) Study Investigators. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334-1345.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 58. | Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, Kohli A, Sarin S, Caldwell SH, Alkhouri N, Shiffman ML, Camargo M, Li G, Kersey K, Jia C, Zhu Y, Djedjos CS, Subramanian GM, Myers RP, Gunn N, Sheikh A, Anstee QM, Romero-Gomez M, Trauner M, Goodman Z, Lawitz EJ, Younossi Z; STELLAR-3 and STELLAR-4 Investigators. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol. 2020;73:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 59. | Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, Lawitz EJ, Rockey DC, Schall RA, Jia C, McColgan BJ, McHutchison JG, Subramanian GM, Myers RP, Younossi Z, Ratziu V, Muir AJ, Afdhal NH, Goodman Z, Bosch J, Sanyal AJ; GS-US-321-0105 and GS-US-321-0106 Investigators. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 60. | Hofer BS, Simbrunner B, Hartl L, Jachs M, Bauer DJM, Balcar L, Paternostro R, Schwabl P, Semmler G, Scheiner B, Staettermayer AF, Trauner M, Mandorfer M, Reiberger T. Alcohol Abstinence Improves Prognosis Across All Stages of Portal Hypertension in Alcohol-Related Cirrhosis. Clin Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 61. | Verrill C, Markham H, Templeton A, Carr NJ, Sheron N. Alcohol-related cirrhosis--early abstinence is a key factor in prognosis, even in the most severe cases. Addiction. 2009;104:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Takahashi H, Shigefuku R, Maeyama S, Suzuki M. Cirrhosis improvement to alcoholic liver fibrosis after passive abstinence. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 64. | Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 213] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 65. | de Veer RC, van Hooff MC, Corpechot C, Thorburn D, Invernizzi P, Lammers WJ, Janssen HLA, Battezzati PM, Nevens F, Lindor KD, Floreani A, Ponsioen CY, Mayo MJ, Parés A, Mason AL, Kowdley KV, Trivedi PJ, Hirschfield GM, Goet JC, Bruns T, Dalekos GN, Gatselis NK, Verhelst X, Hansen BE, Harms MH, van der Meer AJ. Ursodeoxycholic acid treatment-induced GLOBE score changes are associated with liver transplantation-free survival in patients with primary biliary cholangitis. Am J Gastroenterol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 66. | Bardou-Jacquet E, Morandeau E, Anderson GJ, Ramm GA, Ramm LE, Morcet J, Bouzille G, Dixon J, Clouston AD, Lainé F, Turlin B, Powell LW, Deugnier YM. Regression of Fibrosis Stage With Treatment Reduces Long-Term Risk of Liver Cancer in Patients With Hemochromatosis Caused by Mutation in HFE. Clin Gastroenterol Hepatol. 2020;18:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Villanueva C, Albillos A, Genescà J, Abraldes JG, Calleja JL, Aracil C, Bañares R, Morillas R, Poca M, Peñas B, Augustin S, Garcia-Pagan JC, Pavel O, Bosch J. Development of hyperdynamic circulation and response to β-blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 68. | Rabiee A, Garcia-Tsao G, Tapper EB. Nonselective Beta-Blockers in Portal Hypertension: Why, When, and How? Clin Liver Dis (Hoboken). 2022;19:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 69. | Gluud LL, Krag A. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev. 2012;CD004544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, Lebrec D. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 312] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 71. | Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR, Hayes PC. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 72. | Malandris K, Paschos P, Katsoula A, Manolopoulos A, Andreadis P, Sarigianni M, Athanasiadou E, Akriviadis E, Tsapas A. Carvedilol for prevention of variceal bleeding: a systematic review and meta-analysis. Ann Gastroenterol. 2019;32:287-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Sharma M, Singh S, Desai V, Shah VH, Kamath PS, Murad MH, Simonetto DA. Comparison of Therapies for Primary Prevention of Esophageal Variceal Bleeding: A Systematic Review and Network Meta-analysis. Hepatology. 2019;69:1657-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 74. | Turco L, Reiberger T, Vitale G, La Mura V. Carvedilol as the new non-selective beta-blocker of choice in patients with cirrhosis and portal hypertension. Liver Int. 2023;43:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 75. | Sinagra E, Perricone G, D'Amico M, Tinè F, D'Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 76. | Bhardwaj A, Kedarisetty CK, Vashishtha C, Bhadoria AS, Jindal A, Kumar G, Choudhary A, Shasthry SM, Maiwall R, Kumar M, Bhatia V, Sarin SK. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2017;66:1838-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Zacharias AP, Jeyaraj R, Hobolth L, Bendtsen F, Gluud LL, Morgan MY. Carvedilol versus traditional, non-selective beta-blockers for adults with cirrhosis and gastroesophageal varices. Cochrane Database Syst Rev. 2018;10:CD011510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (18)] |
| 78. | Villanueva C, Torres F, Sarin SK, Shah HA, Tripathi D, Brujats A, Rodrigues SG, Bhardwaj A, Azam Z, Hayes PC, Jindal A, Abid S, Alvarado E, Bosch J; Carvedilol-IPD-MA-group and the Baveno Cooperation: an EASL Consortium. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatol. 2022;77:1014-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 79. | Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut. 2020;69:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 80. | Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 81. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 82. | Huang YW, Lee CL, Yang SS, Fu SC, Chen YY, Wang TC, Hu JT, Chen DS. Statins Reduce the Risk of Cirrhosis and Its Decompensation in Chronic Hepatitis B Patients: A Nationwide Cohort Study. Am J Gastroenterol. 2016;111:976-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 83. | Mohanty A, Tate JP, Garcia-Tsao G. Statins Are Associated With a Decreased Risk of Decompensation and Death in Veterans With Hepatitis C-Related Compensated Cirrhosis. Gastroenterology. 2016;150:430-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 84. | Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 85. | Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1521-1530.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 86. | Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, Rodriguez M, Castellote J, García-Pagán JC, Torres F, Calleja JL, Albillos A, Bosch J; BLEPS Study Group. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients With Cirrhosis. Gastroenterology. 2016;150:1160-1170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 87. | Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1273] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 88. | Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, Roux O, Uschner FE, de Wit K, Zaccherini G, Alessandria C, Angeli P, Bernardi M, Beuers U, Caraceni P, Durand F, Mookerjee RP, Trebicka J, Vargas V, Andrade RJ, Carol M, Pich J, Ferrero J, Domenech G, Llopis M, Torres F, Kamath PS, Abraldes JG, Solà E, Ginès P. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 89. | Cerini F, Vilaseca M, Lafoz E, García-Irigoyen O, García-Calderó H, Tripathi DM, Avila M, Reverter JC, Bosch J, Gracia-Sancho J, García-Pagán JC. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016;64:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 90. | Vilaseca M, García-Calderó H, Lafoz E, García-Irigoyen O, Avila MA, Reverter JC, Bosch J, Hernández-Gea V, Gracia-Sancho J, García-Pagán JC. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65:2031-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 91. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S, Lei B, Bernabucci V, Vukotic R, De Maria N, Schepis F, Karampatou A, Caporali C, Simoni L, Del Buono M, Zambotto B, Turola E, Fornaciari G, Schianchi S, Ferrari A, Valla D. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 534] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 92. | Iqbal U, Dennis BB, Li AA, Cholankeril G, Kim D, Khan MA, Ahmed A. Use of anti-platelet agents in the prevention of hepatic fibrosis in patients at risk for chronic liver disease: a systematic review and meta-analysis. Hepatol Int. 2019;13:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Sundaram V, Kaung A, Rajaram A, Lu SC, Tran TT, Nissen NN, Klein AS, Jalan R, Charlton MR, Jeon CY. Obesity is independently associated with infection in hospitalised patients with end-stage liver disease. Aliment Pharmacol Ther. 2015;42:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Ahn JC, Sundaram V. Obesity and Liver Decompensation. Clin Liver Dis (Hoboken). 2019;14:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Sundaram V, Jalan R, Ahn JC, Charlton MR, Goldberg DS, Karvellas CJ, Noureddin M, Wong RJ. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol. 2018;69:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 96. | Miñambres I, Rubio MA, de Hollanda A, Breton I, Vilarrasa N, Pellitero S, Bueno M, Lecube A, Marcuello C, Goday A, Ballesteros MD, Soriano G, Caixàs A. Outcomes of Bariatric Surgery in Patients with Cirrhosis. Obes Surg. 2019;29:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1630] [Article Influence: 163.0] [Reference Citation Analysis (1)] |
| 98. | Kumar R. Hepatogenous Diabetes: An Underestimated Problem of Liver Cirrhosis. Indian J Endocrinol Metab. 2018;22:552-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Grancini V, Trombetta M, Lunati ME, Zimbalatti D, Boselli ML, Gatti S, Donato MF, Resi V, D'Ambrosio R, Aghemo A, Pugliese G, Bonadonna RC, Orsi E. Contribution of β-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: Role of severity of liver disease. J Hepatol. 2015;63:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 100. | Wong F, Logan A, Blendis L. Hyperinsulinemia in preascitic cirrhosis: effects on systemic and renal hemodynamics, sodium homeostasis, forearm blood flow, and sympathetic nervous activity. Hepatology. 1996;23:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Kumar R, García-Compeán D, Maji T. Hepatogenous diabetes: Knowledge, evidence, and skepticism. World J Hepatol. 2022;14:1291-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 102. | García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8:13-20. [PubMed] |
| 103. | Xie YD, Feng B, Gao Y, Wei L. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis: A systematic review and meta-analysis. Hepatol Res. 2014;44:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Plauth M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr. 2020;39:3533-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (2)] |
| 105. | Kumar R, Prakash SS, Priyadarshi RN, Anand U. Sarcopenia in Chronic Liver Disease: A Metabolic Perspective. J Clin Transl Hepatol. 2022;10:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Naseer M, Turse EP, Syed A, Dailey FE, Zatreh M, Tahan V. Interventions to improve sarcopenia in cirrhosis: A systematic review. World J Clin Cases. 2019;7:156-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 107. | Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, Bosch J; Ciberehd SportDiet Collaborative Group. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 108. | Liu F, Wang X, Wu G, Chen L, Hu P, Ren H, Hu H. Coffee Consumption Decreases Risks for Hepatic Fibrosis and Cirrhosis: A Meta-Analysis. PLoS One. 2015;10:e0142457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Systematic review with meta-analysis: coffee consumption and the risk of cirrhosis. Aliment Pharmacol Ther. 2016;43:562-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 110. | Wong F, Liu P, Blendis L. Sodium homeostasis with chronic sodium loading in preascitic cirrhosis. Gut. 2001;49:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Jalan R, Hayes PC. Sodium handling in patients with well compensated cirrhosis is dependent on the severity of liver disease and portal pressure. Gut. 2000;46:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 112. | Konstantakis C, Tselekouni P, Kalafateli M, Triantos C. Vitamin D deficiency in patients with liver cirrhosis. Ann Gastroenterol. 2016;29:297-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 113. | Udomsinprasert W, Jittikoon J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed Pharmacother. 2019;109:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 114. | Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 115. | Abramovitch S, Sharvit E, Weisman Y, Bentov A, Brazowski E, Cohen G, Volovelsky O, Reif S. Vitamin D inhibits development of liver fibrosis in an animal model but cannot ameliorate established cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G112-G120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 116. | Pilz S, Putz-Bankuti C, Gaksch M, Spindelboeck W, Haselberger M, Rainer F, Posch A, Kreuzer P, Stojakovic T, Stadlbauer V, Obermayer-Pietsch B, Stauber RE. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations in Cirrhotic Patients: A Randomized Controlled Trial. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Jang YO, Kim MY, Cho MY, Baik SK, Cho YZ, Kwon SO. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol. 2014;14:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Han HT, Jin WL, Li X. Mesenchymal stem cells-based therapy in liver diseases. Mol Biomed. 2022;3:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, Geng H, Jin L, Lau GK, Wang FS. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 120. | Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R, Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 121. | Moore JK, Stutchfield BM, Forbes SJ. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther. 2014;39:673-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 122. | Kedarisetty CK, Kumar A, Sarin SK. Insights into the Role of Granulocyte Colony-Stimulating Factor in Severe Alcoholic Hepatitis. Semin Liver Dis. 2021;41:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Shasthry SM, Sharma MK, Shasthry V, Pande A, Sarin SK. Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial. Hepatology. 2019;70:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 124. | Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J, Corbett C, Townsend S, Thomas J, Guo K, Hull D, Beard HA, Thompson J, Atkinson A, Bienek C, McGowan N, Guha N, Campbell J, Hollyman D, Stocken D, Yap C, Forbes SJ. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 125. | Schwarzer R, Kivaranovic D, Mandorfer M, Paternostro R, Wolrab D, Heinisch B, Reiberger T, Ferlitsch M, Gerner C, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Randomised clinical study: the effects of oral taurine 6g/day vs placebo on portal hypertension. Aliment Pharmacol Ther. 2018;47:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, Neagu M, Rössle M, Zipprich A, Caca K, Ferlitsch A, Dilger K, Mohrbacher R, Greinwald R, Sauerbruch T. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 127. | Zipprich A, Gittinger F, Winkler M, Dollinger MM, Ripoll C. Effect of ET-A blockade on portal pressure and hepatic arterial perfusion in patients with cirrhosis: A proof of concept study. Liver Int. 2021;41:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 128. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 930] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 129. | Chang Y, Li H. Hepatic Antifibrotic Pharmacotherapy: Are We Approaching Success? J Clin Transl Hepatol. 2020;8:222-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 130. | Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A, Mareso S, Gambino C, Brocca A, Sticca A, Fasolato S, Angeli P. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017;67:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 131. | Cullaro G, Sharma R, Trebicka J, Cárdenas A, Verna EC. Precipitants of Acute-on-Chronic Liver Failure: An Opportunity for Preventative Measures to Improve Outcomes. Liver Transpl. 2020;26:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 132. | Garcia-Tsao G. Prophylactic Antibiotics in Cirrhosis: Are They Promoting or Preventing Infections? Clin Liver Dis (Hoboken). 2019;14:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 133. | Roni DA, Pathapati RM, Kumar AS, Nihal L, Sridhar K, Tumkur Rajashekar S. Safety and efficacy of hepatitis B vaccination in cirrhosis of liver. Adv Virol. 2013;2013:196704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 134. | Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 135. | Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 455] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 136. | Wu T, Huang SJ, Zhu FC, Zhang XF, Ai X, Yan Q, Wang ZZ, Yang CL, Jiang HM, Liu XH, Guo M, Du HL, Ng MH, Zhang J, Xia NS. Immunogenicity and safety of hepatitis E vaccine in healthy hepatitis B surface antigen positive adults. Hum Vaccin Immunother. 2013;9:2474-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |