Published online Jun 20, 2023. doi: 10.5662/wjm.v13.i3.79
Peer-review started: April 1, 2023
First decision: May 4, 2023
Revised: May 11, 2023
Accepted: May 31, 2023
Article in press: May 31, 2023
Published online: June 20, 2023
Processing time: 80 Days and 3.9 Hours
Gastric cancer (GC) is believed to be the fifth most common cancer and the third most common cause of death worldwide. Treatment techniques include radiation, chemotherapy, gastrectomy, and targeted treatments are often employed. Some hopeful results from the development of GC immunotherapy have already changed treatment approaches. Along with previous combination medicines, new immunotherapies have been developed that target distinct molecules. Despite ongoing studies into the current therapeutic options and significant improve
Core Tip: Throughout the globe, gastric cancer (GC) is ranked as the fifth most frequent cancer and the third most common cause of death. Chemotherapy, radiation, stomach resection, and targeted treatments are common treatment modalities. The development of immunotherapy for GC has already produced some encouraging outcomes and changed the treatment process. Currently, new immunotherapies that target novel molecules, as well as other combination treatments, have been developed. Immune checkpoint inhibitors are being used more and more often. In this review, we sought to examine the viewpoint, development, and reported clinical results of several immunotherapy treatment modalities for advanced GC patients.
- Citation: Leowattana W, Leowattana P, Leowattana T. Immunotherapy for advanced gastric cancer. World J Methodol 2023; 13(3): 79-97
- URL: https://www.wjgnet.com/2222-0682/full/v13/i3/79.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i3.79
The third leading cause of cancer-related mortality is gastric cancer (GC), which includes adenocarcinomas of the gastroesophageal junction (GEJ) and stomach. GC is the fifth most frequent cancer around the globe. Eastern Europe, Eastern Asia, and South America have the highest prevalence rates of GC. The majority of patients in Western nations receive their diagnoses at an advanced stage, which is defined by metastatic dissemination that is inoperable[1-3]. Long-term disease control has not yet been accomplished, despite the emergence of novel treatments. As a result, advanced GC (AGC) has a terrible prognosis, with a 5-year survival rate of only 10%. While cardiac GC is more common in North America, Australia, and the United Kingdom, non-cardiac GC is more common in Eastern Asia. The most well-known cause of GC is Helicobacter pylori (H. pylori) infection, while Epstein-Barr virus (EBV) infection has also been associated with the development of GC. Cigarette smoking, obesity, a high salt intake, a poor intake of fruits and vegetables, and a high intake of salted preserved foods are some lifestyle choices that have been linked to an increased risk of GC[4,5].
The prognosis for AGC is still bleak despite recent improvements in multimodal therapy. The GC's extremely complicated molecular basis is one of the factors contributing to its dismal prognosis. Numerous genetic and epigenetic changes, including differential gene expression, gene mutations, DNA/histone methylation, and somatic copy number changes, have been shown to contribute to the aggressive phenotype of GC. No encouraging and treatable causes of GC have yet been discovered, regardless of the fact that it is a diverse disease that is probably caused by several genetic and epigenetic abnormalities[6-8]. Although no successful therapies based on molecular characterization have been created yet, the creation of more effective treatment strategies based on novel molecular data may be feasible in the future. Nowadays, the median survival time with the best supportive care varies from 4 mo to 12 mo with standard cytotoxic treatment. Throughout the last several decades, advances in knowledge of cancer's molecular etiology and biology have resulted in the creation of innovative targeted therapy techniques that have led to higher survival rate in some contexts. These targeted therapies are also offered as small molecule inhibitors and monoclonal antibodies (mAbs), most of which are tyrosine kinase inhibitors (TKIs). Therefore, current systemic therapies for metastatic GC combine cytotoxic chemotherapy with first- and second-line therapies using targeted medicines such as trastuzumab and ramucirumab, respectively. Additionally, the establishment of immune checkpoint inhibition in the past ten years has been recognized as a significant medical and scientific advancement in the battle against malignancy; however, studies looking at the use of immunotherapy in GCs, either as a single agent or in conjunction with cytotoxic chemotherapy, have only produced limited authorization in the second-line setting, after the failure of the initial treatment, with comparably modest rates of response ranging between 5% and 30%[9-11]. The goal of this review is to quickly highlight some of the most promising immunotherapies now being researched while also summarizing the currently investigated and authorized treatments for GC.
GC is often asymptomatic in the early stages, making it challenging to purposefully discover. Late diagnosis is mostly to account for the high mortality of GC. In order to lower GC mortality, early identification and treatment are essential[12,13]. Some East Asian nations with high relative risks have implemented their own extensive screening programs. Regardless of whether an individual has symptoms, upper gastrointestinal endoscopy is available in these nations. Endoscopic screening can lower GC mortality by 67% compared to radiography screening, according to Japanese population-based cohort research[14]. Endoscopy was the most economical screening technique, according to data from the National Cancer Screening Program in South Korea, which may enhance survival rates[15]. In addition, the quality of endoscopic imaging has recently dramatically improved. Endoscopy using image enhancement techniques, including narrow-band imaging, can help with early GC discovery and complete endoscopic resection. Indeed, these active screening methods have resulted in earlier discovery and a higher survival rate[16,17]. The 5-year relative survival rate in Japan between 2009 and 2011 was reported at 66.6%, with more than 60% of cases of GC being discovered at stage I, according to population-based statistics collected countrywide[18]. In contrast to Asian nations, Western nations lack widespread screening programs, which causes discovery to occur later. According to the SEER-based CONCORD-2 research in the United States, only 22.1% (2001-2003) or 24.9% (2004-2009) of patients had a localized stage at diagnosis, and in comparison to Asian countries, the stated 5-year survival rate was lower (26.1% from 2001-2003 and 29.0% from 2004-2009)[19]. Western nations have a higher prevalence of GC in the proximal third. Proximal GCs are more likely to be in an advanced stage at presentation, be larger, and have a histology that is poorly differentiated. The poorer survivability in the West may be explained by this[20,21].
In particular, for intestinal-type distal carcinoma, H. pylori infection raises cancer risk. In comparison to Europe (47.0%) and North America (37.1%), Asia has a greater prevalence of H. pylori (54.7%). It is well known that the elimination of H. pylori causes the symptoms of atrophic gastritis to return. It is hypothesized that intestinal metaplasia in chronic gastritis caused by H. pylori is less likely to improve with H. pylori eradication than atrophic gastritis alone. The comparative risk of getting GC following the removal of H. pylori was 0.65, according to a meta-analysis. While extensive intestinal metaplasia occurs, there is little data to suggest that treating the H. pylori infection lowers the risk of GC[22]. Yan et al[23] recently completed a randomized, placebo-controlled study to assess the long-term impact of H. pylori eradication medication on the incidence and death of GC in a high-risk group. A total of 1630 asymptomatic H. pylori-infected people were randomly allocated to undergo conventional triple treatment for H. pylori eradication (n = 817) or a placebo (n = 813), and were then followed up for 26.5 years. There were 35 people in the placebo group (4.31%) and 21 people (2.57%) in the treatment group who tested positive for GC. H. pylori medication patients had a lower chance of developing GC in comparison to the placebo group [hazard ratio (HR): 0.57; 95%CI: 0.33-0.98]. They concluded that eradicating H. pylori may provide long-term protection against GC in high-risk groups, particularly in infected individuals who did not have precancerous gastric lesions at baseline.
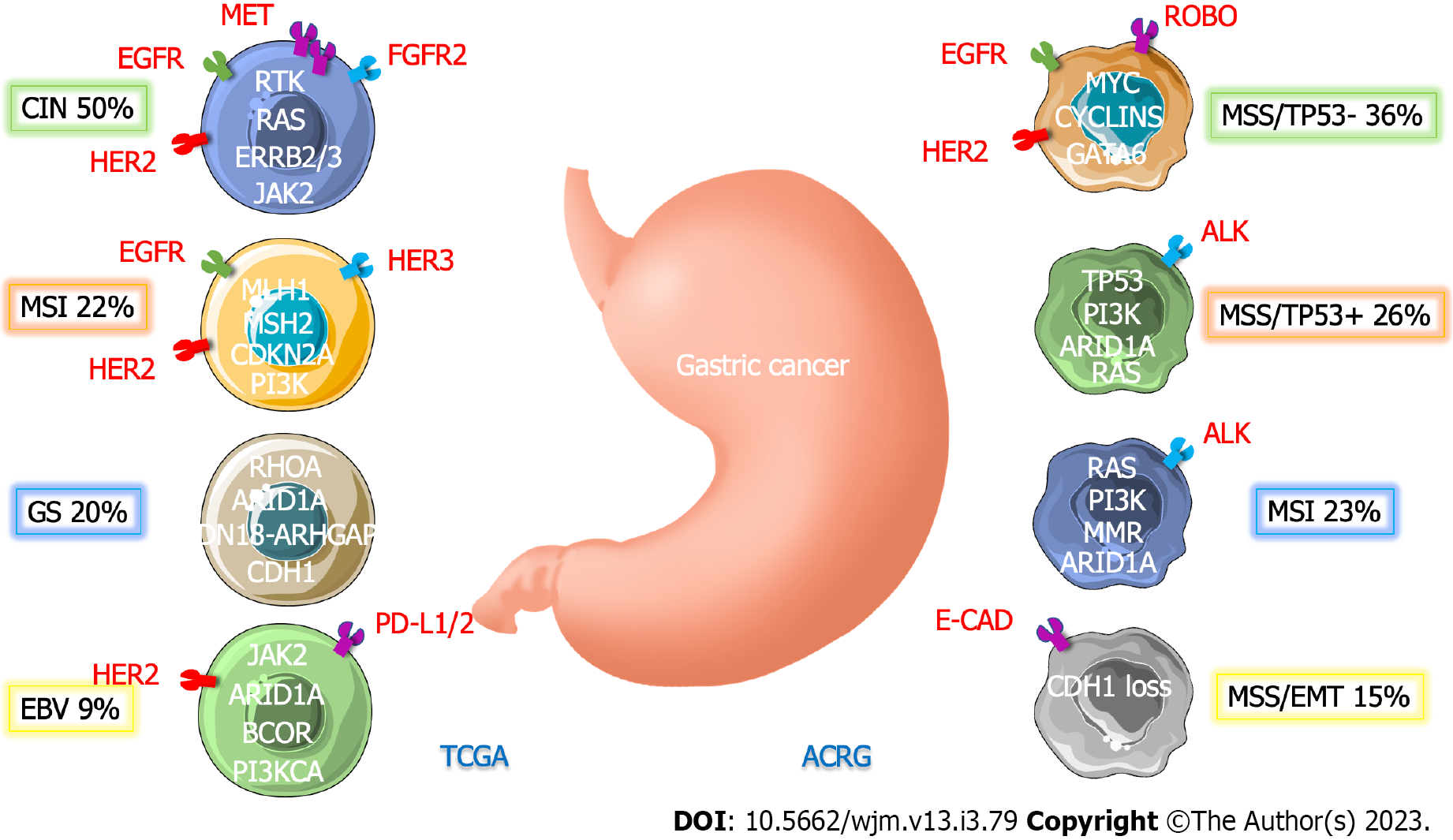
GC is a molecularly diverse malignancy with several genetic mutations. Based on histological results, the Lauren classification originally divided GC into two types (intestinal and diffuse). However, it is unable to reliably predict treatment outcomes and prognosis because it fails to take into consideration the variable nature of GC[24]. The Cancer Genome Atlas (TCGA), by classifying patients into four distinct molecular subtypes based on six different molecular subtypes, has provided a detailed depiction of the genetic underpinnings of GC: (1) Tumors positive for EBV, which display recurrent PIK3CA mutations, extreme DNA hypermethylation, and amplification of Janus-associated kinase 2, programmed cell death ligand-1 (PD-L1), and PD-L2; (2) microsatellite unstable tumors have high mutation rates, including changes in the genes producing proteins that can be targeted to cause cancer; (3) genomically stable tumors that are enriched for mutations in Ras homolog family member A or fusions involving RHO family GTPase-activating proteins as well as diffuse histological variation; and (4) chromosomally unstable tumors with pronounced aneuploidy and localized receptor tyrosine kinase amplification (Table 1)[25]. Moreover, because of the complexity of GC, the Asian Cancer Research Group (ACRG) subtypes were introduced to enhance classification[26-28]. Although the TCGA classification is extensive and provides clinically useful information, no classification method includes all clinically meaningful signals. This would be required to best lead a customized strategy (Figure 1).
| Subtypes | EBV-positive | MSI | GS | CIN |
| Frequency, % | 8.8 | 21.7 | 19.7 | 449.8 |
| Demographic | Male patients (81%) | Old age (median 72 yr) | Young age (median 59 yr) | Not specific |
| Histology | Not specific | Not specific | Diffuse | Intestinal |
| Main location | Fundus and body | Fundus, body, and antrum | Mostly diffuse subtype | Majority of tumors at the GEJ |
| Molecular | EBV-CIMP: (1) PD-L1/2, JAK2 overexpression; (2) Mutation in PIK3CA, ARID1A, and BCOR; (3) CDKN2A silencing; (4) Immune cell signaling; and (5) Rare TP53 mutations | Gastric-CIMP: (1) Hypermutation in TP53, PIK3CA, ERBB2/3, and ARID1A; (2) MLH1 silencing; (3) Mitotic pathways activation; and (4) Commune changes in the genes of CMH1 | (1) CDH1 and RHOA mutation; (2) CLDN18-ARHGAP fusion; (3) Cell adhesion; (4) Angiogenesis pathways enriched; and (5) Rare TP53 mutations | (1) TP53 mutation; (2) RTK-RAS activation; and (3) Mutations of SMAD4 and APC |
| alterations | ||||
| Potential therapeutic points | (1) PIK3CA; (2) JAK2; and (3) PD-L1/L2 | (1) PIK3CA; (2) ERBB2/3; (3) EGFR; (4) PD-L1; and (5) MLH1 silencing | (1) RHOA; and (2) CLDN18 | (1) RTKs; (2) EGFR; (3) VEGFA; (4) CCNE1; (5) CCND1; and (6) CDK6 |
Another group, ACRG, developed a different categorization scheme by dividing GC into four subgroups based on gene expression data: Microsatellite stable with tumor protein 53 (TP53) functional loss (MSS/TP53-), MSS/TP53+ (TP53 intact), MSS/Epithelial-mesenchymal transition (EMT) (EMT signatures), and microsatellite instability (MSI) (23%)[7]. The MSI group from TCGA exhibited similarities to the MSI subtype from ACRG. Despite the fact that the EBV-positive, genomically stable, and chromosomal instability subtypes in TCGA were somewhat enriched in the MSS/TP53+, MSS/EMT, and MSS/TP53-subtypes in ACRG, respectively, there were still a number of differences seen in other subtypes. This demonstrates the distinctiveness of these two classes from TCGA and ACRG. The ACRG also included survival data, which showed the prognostic efficacy of each subtype categorization, in contrast to the TCGA. Following MSS/TP53+, MSS/TP53-, and MSS/EMT GC, MSI GC was shown to have the highest overall survival (OS) and the lowest frequency of recurrence[29]. There has been evidence of ethnic influences on molecular features. Despite the fact that the TCGA data did not reveal any significant biological differences between East Asian and other populations, certain variations in pathway-level gene expression were detected. For instance, East Asian patients had elevated telomerase regulatory pathway expression and decreased hypoxia inducible factor-1-alpha transcription factor network expression[29]. Another study found that Asian and non-Asian GC patients had significantly different tumor immunity profiles. Non-Asian cases of GC were connected to an enrichment of T-cell gene expression patterns and a lesser expression of the immunosuppressive marker FOXP3 as compared to Asian cases of GC[30-32]. Further research with a sufficient sample size is required to better understand how racial variations affect molecular background.
Host immunity is now widely recognized as being important in the prevention of cancer. The findings suggest that our immune system can inhibit cancer growth through a mechanism known as immune surveillance. Dying cancer cells may generate and disseminate tumor-specific and tumor-related antigens that can be ingested and processed by tissue-resident dendritic cells (DCs). These cells then develop into antigen-presenting cells (APCs) in the presence of a favorable microenvironment, which is typically rich in activator molecules known as danger-associated molecular patterns, which are released by dying cancer cells[33,34]. Mature APCs must effectively deliver tumor antigens in the form of peptides to CD8+ T lymphocytes via major histocompatibility complex (MHC) class I molecules and CD4+ T lymphocytes via MHC class II molecules in order to trigger effective anticancer immunity. The most effective tumor antigens are non-self or altered proteins, such as those produced by somatic mutations in genes expressed by tumor cells or those encoded by viruses. Effective activation of CD8+ T cells requires both antigen presentation as a first signal and the presence of costimulatory molecules as a second signal. Once these cells are activated, they enter the tumor bed and multiply. Furthermore, T lymphocytes are able to recruit other immune cells, such as natural killer (NK) cells and M1 macrophages, that are able to further aid in the destruction of cancer cells. Additionally, T lymphocytes themselves are able to directly destroy cancer cells through the production of cytokines. This process is essential for the body to effectively fight cancer cells. The fact that not all traditional cytotoxic chemo
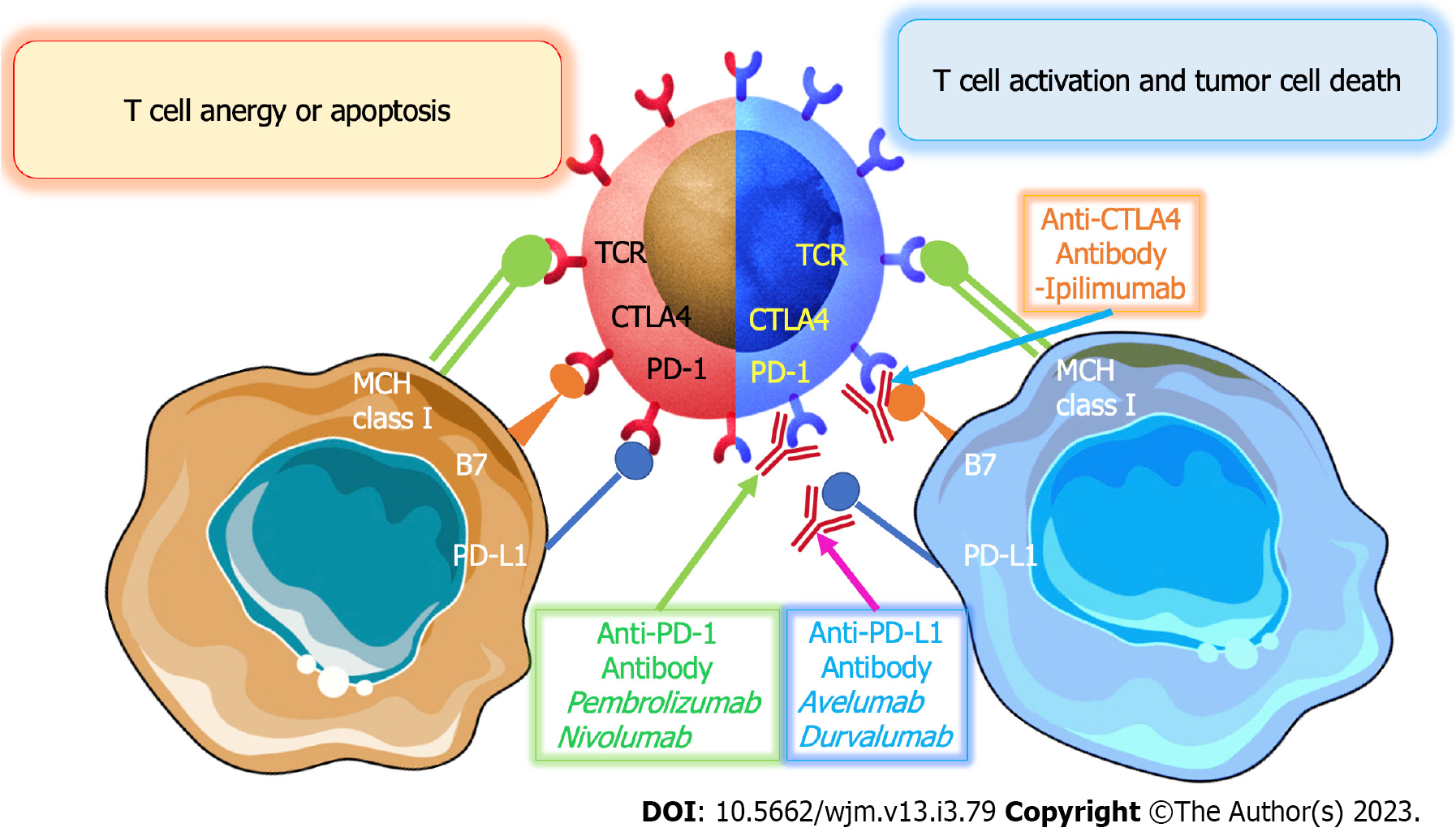
T-cell receptor (TCR), co-stimulatory molecules, including CD28, and cytokines are all required for effective activation of cytotoxic T cells. Tumor cells can resist immunosuppression through a variety of mechanisms. The production of several co-inhibitory receptors is one of the ways to prevent T cell activation in the body. PD-1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and lymphocyte-activation gene 3 (LAG-3) are examples of inhibitory receptors that bind to tumor cell ligands and block T cell activation. The overexpression of these receptors induces the inhibition of coinhibitory pathways, such as the B7-CD28-CTLA-4 family. This helps to ensure the physiological functioning of the immune system, which is essential in preventing tumor growth and the spread of cancer cells[37,38]. CTLA-4 is an important co-stimulatory molecule expressed on the surface of CD4+ and CD8+ T lymphocytes. It binds to molecules known as CD80 (B7-1) and CD86 (B7-2) on APCs, which are involved in the initiation and regulation of adaptive immunity. The binding of CTLA-4 to CD80 and CD86 on APCs results in the inhibition of T cell activation. Therefore, this molecule plays an important role in regulating the immune system. This inhibits TCR signaling by preventing APCs from binding to the CD28 co-stimulatory molecule. Additionally, CTLA-4 is continually produced in regulatory T cells (Treg) in order to control their immunosuppressive role. Tumor cells are thought to escape through downregulation of MHC class I expression. MHC class I molecules play a significant role in cytotoxic T cell-mediated immunity. Tumor cells have the ability to suppress immune cell activity by secreting immunosuppressive substances. These substances, such as interleukin-10, transforming growth factor-β1, galectins, tumor necrosis factor, prostaglandin E2, and vascular endothelial growth factor (VEGF), can inhibit the function of the immune cells, thus disrupting the body's natural defense system. The immunosuppressive substances also inhibit the receptors on the surface of the immune cells, making them unable to recognize and attack the tumor cells. This allows the tumor cells to grow and spread unchecked. Therefore, it is important to understand the role of these immunosuppressive substances in order to develop effective treatments for cancer[39].
By encouraging the polarization towards less cytotoxic T cell subsets and pro-inflammatory T cell subsets, the tumor microenvironment may compromise anti-tumor immunity. TH-2, TH-17, and Treg are T helper (TH) cells that are related to tumors. Tumor-associated macrophages (TAM) make up the majority of the immune cell population in the tumor microenvironment. Two separate subtypes of these macrophages, M1 and M2, exhibit anti-tumor and pro-tumorigenic actions, respectively. When a GC site was infiltrated with M2 TAM, the patient's prognosis was usually poor[40,41]. Myeloid-derived suppressor cells (MDSCs) are a diverse population of cells with the capacity to proliferate vigorously under pathological conditions like cancer. They are descended from the myeloid lineage. Both innate and adaptive immunity against malignancies can be suppressed by these cells. Recent studies have confirmed that patients with GC are more likely to have an increased number of MDSCs. This increased presence of MDSCs in the blood samples of GC patients has been associated with poorer clinical result[42,43]. Treg cells are a significant contributor to immune suppression in the tumor microenvironment. End-stage instances of GCs were replete with Foxp3 plus CD4 plus ICOS plus effector Tregs, also known as highly suppressive Tregs[44,45].
Patients with GC exhibit "three high and three low" characteristics: High rates of incidence, metastatic condition, and mortality; low rates of early discovery, radical resection, and five-year survival. Patients might either be early-stage or advanced-stage patients. Early-stage GCs are limited to the mucosa or submucosa, regardless of the size of the lesion or the presence of lymph node metastases. AGC is defined as cancers that infiltrate into or beyond the subserosa to surrounding organs or metastasize. Middle GC is described as cancer that extends past the submucosa to penetrate the gastric muscle layer. Tumors in the advanced stage of GC include intermediate and advanced tumors. Another classification of AGC includes local unresectable GC, distant metastasis, and postoperative recurrent GC[46,47]. The therapy’s efficacy and method are determined by the tumor's stage. At the moment, the primary objective of treating AGC is to ameliorate symptoms and extend patients' survival times using successive courses of chemotherapy. Systemic chemotherapy alone is becoming increasingly effective in treating AGC, yet the median survival duration with this approach is still only 4-13 mo. This demonstrates that, unfortunately, the prognosis for this disease is far from favorable. In spite of the progress made in treating AGC with chemotherapy, more research and development are needed to improve the outlook for those afflicted with it. As a result, both more effective chemotherapy medications and regimens with fewer hazardous side effects, as well as innovative therapeutic paradigms, such as targeted therapy and immunotherapy, should be investigated.
Immunotherapy is a form of cancer treatment that employs the body's own immune system to fight cancer cells. It is divided into two types: Passive and active. Passive immunotherapy is a form of therapy that utilizes antibody therapies to target cancer cells. On the other hand, active immunotherapy focuses on boosting the body's immune response against tumor cells. Examples of active immunotherapy include vaccinations and chimeric antigen receptors (CAR). CARs are designed to recognize and bind to tumor cells, triggering the body's immune system to attack the cancer cells. However, with passive immunotherapy, immune system components, such as mAbs, are generated outside of the body. Immunotherapies nowadays are frequently based on cytotoxic T cells, mAbs, and gene-transfected vaccines. Immune checkpoint inhibitors (ICIs) have become more popular since ipilimumab was originally approved in 2011 for the treatment of metastatic BRAF-negative melanoma. Following more than 1000 clinical investigations, ICIs are now recognized as a therapeutic approach in the management of both solid organ and hematologic malignancies[48-50].
ICIs are a type of drug used in cancer immunotherapy. They work by preventing the T cells from the immune system from being suppressed by the tumor. Targeting CTLA-4, PD-1, and PD-L1, three essential ICI drug types were previously created for preclinical and clinical research. In order to lessen or decrease CD28 signaling, CTLA-4, a CD28 homolog, may bind to B7-1 and B7-2 with a greater affinity than CD28. However, PD-1 encourages tumor cell survival by inducing apoptosis in T lymphocytes that have been activated. Pharmacological blockage of its route may damage the function of immune cells such as B cells and NK cells since PD-1 is extensively expressed in many immune cells[51-53] (Figure 2).
Pembrolizumab was the first monoclonal antibody designed to target PD-1. In 2017, the FDA authorized it for the treatment of advanced non-small cell lung cancer. In a phase I study, the anti-tumor efficacy, safety, and tolerability of pembrolizumab were assessed in 39 patients with AGC (KEYNOTE-012). According to the findings, pembrolizumab provides promising anti-tumor effectiveness with a manageable amount of toxicity in these individuals. The positive outcomes of this experiment spurred the conduct of more clinical trials testing PD-1-blocking therapy[52]. In 2018, Fuchs et al[53] performed the KEYNOTE-059 trial, a phase 2 global, open-label, single-arm, multicohort study that recruited 259 AGC patients from 16 countries to assess the safety and effectiveness of pembrolizumab. They discovered an 11.6% objective response rate (ORR) with a 2.3% full response rate. The median response time was 8.4 mo. ORR and median response duration were 15.5% and 16.3 mo in PD-L1-positive patients, respectively, compared to 6.4% and 6.9 mo in PD-L1-negative patients. One or more grade 3-5 treatment-related adverse events (TRAEs) occurred in 46 individuals (17.8%). Two patients (0.8%) stopped due to TRAEs, and two fatalities were deemed treatment-related. They concluded that in patients with AGC or advanced gastroesophageal junction cancer (AGEJC) who had previously undergone at least two lines of treatment, Pembrolizumab monotherapy displayed promising efficacy and manageable safety. Patients with PD-L1-positive malignancies experienced long-term responses. Furthermore, Shitara et al[54] undertook a randomized, open-label, phase 3 research study in 30 countries to evaluate Pembrolizumab vs Paclitaxel in 592 patients with AGC or AGEJC who had progressed on platinum- and fluoropyrimidine-based first-line treatment. With a combined positive score (CPS) of 1 or higher, they found that 326 people passed away (151 of 196 patients in the Pembrolizumab group and 175 of 199 patients in the Paclitaxel group). With Pembrolizumab, the median OS was 9.1 mo, while with Paclitaxel, it was 8.3 mo. With Pembrolizumab, the median progression-free survival (PFS) was 1.5 mo, while with Paclitaxel, it was 4.1 mo. Furthermore, grade 3-5 TRAEs occurred in 42 (14%) of the 294 patients treated with Pembrolizumab and 96 (35%) of the 276 patients treated. They concluded that Pembrolizumab, when used as a second-line treatment for AGC or AGEJC with a PD-L1 CPS of 1 or higher, did not substantially increase OS when compared to Paclitaxel. However, Paclitaxel has a worse safety profile than Pembrolizumab (Table 2).
| Ref. | Drug(s) | Number of patients | Study phase | ORR (%) | Median OS (months) | Median PFS (months) | Results |
| Muro et al[52] | Pembrolizumab | 39 | 1b | 33 | 11.4 | 1.8 | Pembrolizumab demonstrated a reasonable safety profile and potential antitumor efficacy in metastatic PD-L1-positive GC, warranting further exploration in phase 2 and 3 studies |
| Fuchs et al[53] | Pembrolizumab | 259 | 2 | 15.5 | 5.6 | 2.0 | Pembrolizumab is a potential new therapy option for AGC or AGEJC that has progressed following second-line treatment, demonstrating high and persistent responses. Pembrolizumab has a mechanism of action, duration of response, and toxicity profile that differs from and does not overlap with conventional treatment for gastroesophageal adenocarcinoma |
| Shitara et al[54] | Pembrolizumab vs Paclitaxel | 395 | 3 | - | 9.1/8.3 (Pem/Pac) | 1.5/4.1 (Pem/Pac) | When compared to Paclitaxel, Pembrolizumab did not significantly improve overall survival when administered as a second-line therapy for AGC or AGEJC with PD-L1 CPS of 1 or higher |
| Bang et al[55] | Pembrolizumab vs Pembrolizumab plus chemotherapy | 56 | 2 | 25.8/60.0 (Pem/Pem+Chem) | 13.8/20.7 (Pem/Pem+Chem) | 3.3/6.6 (Pem/Pem+Chem) | Pembrolizumab combined chemotherapy showed acceptable tolerability and potential anticancer efficacy in AGC or AGEJC, independent of PD-L1 expression. In patients with PD-L1 CPS ≥ 1, pembrolizumab monotherapy revealed good antitumor efficacy and acceptable safety |
| Kawazoe et al[56] | Pembrolizumab plus chemotherapy | 54 | 2b | 72.2 | Not reached | 9.4 | For the first-line treatment of AGC or AGEJC patients, chemotherapy with Pembrolizumab shown good effectiveness and a tolerable toxicity profile |
| Kawazoe et al[57] | Pembrolizumab plus Lenvatinib | 29 | 2 | 69.0 | Not reached | 7.1 | In patients with AGC, the combination of Lenvatinib and Pembrolizumab demonstrated promising anti-tumor effectiveness while maintaining a tolerable safety profile |
| Shitara et al[58] | Pembrolizumab vs Pembrolizumab plus chemotherapy vs chemotherapy | 763 | 3 | - | 10.6/11.1 (Pem/chem) CPS ≥ 1; 17.0/10.8 CPS ≥ 10 | 6.9/6.4 (Pem/chem) | In individuals with untreated AGC or AGEJC, Pembrolizumab was shown to be noninferior to chemotherapy, with less adverse effects. Pembrolizumab alone or in combination with chemotherapy did not outperform treatment in terms of OS and PFS |
| Kwon et al[60] | Pembrolizumab | 18 | 2 | 55.6 | - | - | A subset of MSI-H GC patients with certain immunological responses at baseline, such as stronger TMB, abundant T cell infiltration, more TCR clonal diversity, and less stem-like exhausted T cells, may not require anything more than anti-PD-1 monotherapy |
| Yamaguchi et al[63] | Pembrolizumab plus SOX vs Pembrolizumab plus SP | 100 | 2b | 72.2/80.4 | 16.9/17.1 | 9.4/8.3 | In Japanese patients with PD-L1 positive, HER-2 negative AGC or AGEJC, the combination of Pembrolizumab plus SOX or SP as first-line treatment indicated high efficacy and reasonable tolerability |
| Lee et al[64] | Pembrolizumab plus Trastuzumab plus Capecitabine plus Cisplatin | 43 | 1b/2 | 76.7 | 19.3 | 8.6 | The use of a quadruplet combination as first-line therapy (Pembrolizumab plus Trastuzumab plus Capecitabine plus Cisplatin) resulted in tumor decrease in HER-2-positive AGC |
| Satake et al[65] | Pembrolizumab vs Pembrolizumab plus chemotherapy vs chemotherapy | 187 | 3 | 22.6/37.7 (Pem/chem); 26.9/31.8 (PD-L1 CPS ≥ 10) | 22.7/13.8 (Pem/chem); 28.5/14.8 (PD-L1 CPS ≥ 10) | 4.1/6.5 (Pem/chem); 7.2/6.9 (PD-L1 CPS ≥ 10) | Pembrolizumab monotherapy was related with statistically better OS results in patients with AGC or AGEJC with PD-L1 CPS ≥ 1 and CPS ≥ 10 tumors as compared to chemotherapy alone. When compared to chemotherapy, Pembrolizumab monotherapy had a better tolerability profile |
| Janjigian et al[66] | Nivolumab plus Ipilimumab vs Nivolumab | 160 | 1/2 | 24/12 (Niv+Ipi/Niv) | 6.9/6.2 (Niv+Ipi/Niv) | 1.6/1.4 (Niv+Ipi/Niv) | Nivolumab and Nivolumab in combination with Ipilimumab provide a viable treatment option for individuals with AGEJC |
| Kang et al[67] | Nivolumab vs placebo | 493 | 3 | 11.2/0.0 | 5.32/4.14 | 1.61/1.45 | Nivolumab might be a potential therapy option for people with AGC or AGEJC who have been highly pretreated |
| Boku et al[69] | Nivolumab plus SOX vs Nivolumab plus CapeOX | 77 | 2 | 57.1/76.5 | Not reached | 9.7/10.6 | In these individuals, Nivolumab in conjunction with SOX or CapeOX was well tolerated and showed potential efficacy |
| Nakajima et al[71] | Nivolumab plus Paclitaxel plus Ramucirumab | 43 | 1/2 | 37.2 | 13.1 | 5.1 | As a second-line treatment for AGC, Nivolumab in combination with Paclitaxel and Ramucirumab shown promising antitumor activity with tolerable tolerability |
| Janjigian et al[72] | Nivolumab plus chemotherapy vs chemotherapy | 1,581 | 3 | 51/41 | 14.4/11.1 | 7.7/6.1 | Nivolumab in conjunction with chemotherapy is being considered as a new standard first-line treatment for these individuals |
| Shah et al[73] | Andecaliximab plus Nivolumab vs Nivolumab | 141 | 2 | 10/7 | 7.1/5.9 | - | When compared to Nivolumab alone, the combination of Andecaliximab and Nivolumab exhibited a favorable safety profile but did not boost efficacy in these people |
| Kang et al[74] | Nivolumab plus oxaliplatin-based chemotherapy vs placebo plus oxaliplatin-based chemotherapy | 724 | 2/3 | - | 17.45/17.15 | 10.45/8.34 | In these patients, Nivolumab in conjunction with oxaliplatin-based chemotherapy improved PFS but not OS |
| Bang et al[76] | Avelumab vs chemotherapy | 371 | 3 | 2.2/4.3 | 4.6/5.0 | 1.4/2.7 | As compared to chemotherapy, treating these patients in the third-line setting with single-agent Avelumab did not improve OS or PFS. Avelumab, on the other hand, had a more manageable toxicity profile than chemotherapy |
| Moehler et al[77] | Avelumab vs chemotherapy | 499 | 3 | - | 10.4/10.9 | - | In patients with AGC or AGEJC in general, or in a specified PD-L1-positive population, Avelumab maintenance therapy did not give a superior OS when compared to continuing chemotherapy |
Bang et al[55] conducted the KEYNOTE-059 multicohort, phase 2, non-randomized trial in 56 patients with AGC or AGEJC in 2019. They discovered that the ORR was 60.0% in patients receiving combination treatment and 25.8% in individuals receiving Pembrolizumab monotherapy. Also, in the combination therapy cohort, 19 patients (76.0%) experienced grade 3/4 treatment-related side events; none were fatal. In the monotherapy cohort, seven patients (22.6%) experienced grade 3-5 TRAEs; one fatality was ascribed to a TRAE (pneumonitis). They concluded that in patients with previously untreated AGC or AGEJC, Pembrolizumab displayed anticancer efficacy and was well tolerated as monotherapy and in combination with chemotherapy. One year later, Kawazoe et al[56] performed a non-randomized, multicenter, open-label phase IIb study, KEYNOTE-659, in 54 AGC or AGEJC patients with human epidermal growth factor receptor 2-negative and PD-L1-positive to assess the safety and efficacy of Pembrolizumab combined with chemotherapy [S-1 plus oxaliplatin (SOX)] as the first-line treatment. They observed that the ORR and disease control rate (DCR) were, respectively, 72.2% and 96.3%. In terms of DOR, time to response (TTR), PFS, and OS, the median values were not attained at 1.5 mo, 9.4 mo, or not reached at all. Patients with CPSs of 1 to 10 had an ORR of 73.9%, whereas those with CPSs of more than 10 had an ORR of 70.0%. Grade 3 TRAEs occurred in 57% of participants. The most frequent grade 3 adverse events were decreased platelet count (14.8%), decreased neutrophil count (13.0%), colitis (5.6%), and adrenal insufficiency (5.6%). They came to the conclusion that SOX and Pembrolizumab demonstrated potential efficacy and a manageable safety profile for the first management of AGC or AGEJC.
Pembrolizumab was also examined in conjunction with a targeted therapy such as Lenvatinib in an open-label, single-arm, phase 2 study in 29 patients with AGC to assess the combination of Lenvatinib and Pembrolizumab. They discovered that 69% of the patients had an ORR. Hypertension, proteinuria, and a fall in platelet count were the most frequently occurring grade 3 TRAEs, occurring in 11 (38%) of the patients, five (17%) of the patients, and two (7%) of the patients, respectively. No grade 4 adverse events, no serious adverse events, and no fatalities associated with the therapy were reported. They concluded that Lenvatinib and Pembrolizumab had potential anti-tumor effectiveness with a tolerable safety profile in AGC patients[57]. A phase 3 KEYNOTE-062 randomized, controlled, and partially blinded interventional trial was carried out by Shitara et al[58] in 2020 in 763 patients from 29 countries who had untreated AGC or AGEJC and a PD-L1 CPS of 1 or above. Every three weeks, participants were given a random choice between receiving Pembrolizumab 200 mg, chemotherapy plus placebo, or chemotherapy combined with cisplatin 80 mg/m2/d on day 1 plus fluorouracil 800 mg/m2/d on days 1 through 5. After a median follow-up of 29.4 mo (median OS, 10.6 vs 11.1 mo), they found that Pembrolizumab was non-inferior to chemotherapy for OS in patients with a CPS of 1 or higher. Chemotherapy was not better than Pembrolizumab monotherapy in individuals with a CPS of 1 or higher. Patients with a CPS of 10 or higher experienced longer OS with pembrolizumab compared to chemotherapy (median OS, 17.4 vs 10.8 mo); however, the difference was not statistically significant. In terms of OS in patients with a CPS of 1 or higher (12.5 vs 11.1 mo), CPS of 10 or greater (12.3 vs 10.8 mo), or PFS in patients with a CPS of 1 or higher (6.9 vs 6.4 mo), Pembrolizumab in combination with chemotherapy did not outperform treatment. Pembrolizumab was found to be noninferior to chemotherapy in patients with untreated AGC or AGEJC, with fewer side effects. For the OS and PFS end points assessed, Pembrolizumab or Pembrolizumab with chemotherapy were not superior to chemotherapy. Additionally, Pembrolizumab was evaluated in PD-L1-positive (CPS ≥ 10) AGC or AGEJC patients from KEYNOTE-062 (n = 182), KEYNOTE-061 (n = 108), and KEYNOTE-059 (n = 46) to better define the specificity of CPS as a predictor of clinical outcomes. This thorough study found that pembrolizumab improved clinical outcomes in patients with CPS ≥ 10 AGC or AGEJC across many lines of treatment[59].
In 2021, Kwon et al[60] conducted a phase 2 study of Pembrolizumab in 18 patients with advanced high MSI (MSI-H) GC, including serial and multi-region tissue samples as well as serial peripheral blood testing, with a median follow-up of 19.5 mo. The findings showed that 6 patients (33.3%) had stable disease, 3 patients (16.7%) had a complete response (CR), 7 patients (38.9%) had a verified partial response (PR), and 3 patients (16.7%) had a CR, giving an ORR of 55.6% and a DCR of 88.9%. They proposed that a subgroup of MSI-H GC patients with a specific immunological response, as defined by a higher tumor mutational burden (TMB), abundant T cell infiltration, larger TCR clonal diversity, and fewer stem-like exhausted T cells at baseline, may not require anything more than anti-PD-1 monotherapy. Equally significant clinically was the finding of unfavorable genomic and immunologic characteristics from the outset, revealing a subset of MSI-H GC that may require additional therapy to benefit from PD-1 blocking. These findings indicated a combination therapy aimed at lowering Treg populations and/or augmenting and growing NK-cell numbers in this fraction. Synthetic model systems that mimic MSI-H biology, as well as extensive genomic and immunologic screening for therapeutic vulnerabilities, will be critical in identifying potential combinations for future testing. To stratify MSI-H cancers for therapy with either PD-1 blockade alone or cutting-edge combination approaches, the findings, however, signal that accurate pre- and post-treatment characterizations are attainable and will probably be needed.
When used as second-line therapy in the phase 3 KEYNOTE-061 study for patients with PD-L1 CPS > 1 AGC or AGEJC, Pembrolizumab did not significantly increase OS compared with Paclitaxel. Fuchs et al[61] conducted a trial in which they randomly assigned patients to receive Pembrolizumab 200 mg Q3W for 35 cycles or standard-dose paclitaxel and presented outcomes in the CPS 1, 5, and 10 populations after two years of follow-up. The findings revealed that 366 of 395 individuals (92.7%) with CPS ≥ 1 died. In the CPS ≥ 1 cohort, Pembrolizumab showed a tendency toward increased OS vs Paclitaxel; 24-mo OS rates: 19.9% vs 8.5%. With PD-L1 enrichment, Pembrolizumab gradually increased the OS benefit (CPS > 5: 24-mo rate, 24.2% vs 8.8%; CPS > 10: 24-mo rate, 32.1% vs 10.9%). Across treatment groups, the median PFS was similar (CPS > 1: HR, 1.25; CPS > 5: 0.98; CPS > 10: 0.79). The median DOR was 19.1 vs 5.2 mo, 32.7 vs 4.8 mo, and NR vs 6.9 mo; the ORR (Pembrolizumab vs Paclitaxel) was 16.3% vs 13.6% (CPS > 1), 20.0% vs 14.3% (CPS > 5) and 24.5% vs 9.1% (CPS > 10). Pembrolizumab was associated with fewer TRAEs than Paclitaxel (53% vs 84%). In 94 Asian patients with advanced PD-L1-positive (CPS > 1) AGC or AGEJC, 36 medical centers in China, Malaysia, South Korea, and Taiwan conducted the randomized, open-label, phase 3 study KEYNOTE-063, which compared Pembrolizumab vs Paclitaxel as second-line therapy. The results revealed that the median OS in Pembrolizumab plus Paclitaxel therapy was the same as 8 mo. The median PFS with Pembrolizumab was 2 mo vs 4 mo with Paclitaxel. Pembrolizumab had a 13% ORR against Paclitaxel's 19%. Any-grade TRAEs occurred in 28 patients receiving Pembrolizumab (60%) and 42 patients receiving Paclitaxel (96%), respectively; grades 3-5 events occurred in 5 patients (11%) and 28 patients (64%). They stated that, due to inadequate power, decisive conclusions concerning the effectiveness of second-line Pembrolizumab in Asian patients with advanced PD-L1-positive AGC or AGEJC are restricted, however, Pembrolizumab was well tolerated in this patient population. Efficacy followed a similar pattern to that shown in the phase 3 KEYNOTE-061 experiment[62].
Yamaguchi et al[63] conducted an open-label phase 2b study, KEYNOTE-659, in Japan in 2022 to examine the effectiveness and safety of first-line Pembrolizumab plus SOX (cohort 1, n = 54) or S-1 and cisplatin (SP) (cohort 2, n = 46) for AGC or AGEJC. They reported that the median duration of Pembrolizumab therapy in cohorts 1 and 2 was 6.0 and 5.1 mo, respectively. SOX (cohort 1) had a median treatment length of 4.9 mo, while SP (cohort 2) had a median treatment duration of 4.4 mo. In cohort 1, 35 patients (64.8%) had their S1 dosage reduced, 47 patients (87.0%) had their oxaliplatin dose reduced, 44 patients (81.5%) had their S1 dose interrupted, and 31 patients (57.4%) had their oxaliplatin dose interrupted. In cohort 2, 33 patients (71.7%) had their S1 dosage reduced, 43 patients (93.5%) had their cisplatin dose reduced, 29 patients (63.0%) had their S1 dose interrupted, and 22 patients (47.8%) had their cisplatin dose interrupted. The ORR in cohort 1 was 72.2% (39 of 54 patients) and 80.4% (37 of 46 patients) in cohort 2. Overall, tumor reduction was observed in 52 of 54 patients (96.3%) in cohort 1 and 44 of 46 patients (95.7%) in cohort 2. DCR in cohort 1 was 96.3% (52 of 54 patients) and 97.8% (45 of 46 patients) in cohort 2. The median PFS in cohorts 1 and 2 was 9.4 mo and 8.3 mo, respectively. In cohort 1, the median OS was 16.9 mo, while in cohort 2, it was 17.1 mo. The median DOR in cohort 1 was 10.6 mo and 9.5 mo in cohort 2, whereas the median TTR in both cohorts was 1.5 mo. They proposed that the combination of Pembrolizumab with SOX or SP as first-line therapy in Japanese patients with PD-L1 positive, Human epidermal growth factor receptor (HER)-2 negative, AGC, or AGEJC demonstrated good effectiveness and tolerable safety.
Furthermore, 43 HER-2-positive AGC patients with a median follow-up of 18.2 months underwent Pembrolizumab evaluation in a single-arm, multi-institutional phase 1b/2 research to evaluate a quadruplet combination of Pembrolizumab, Trastuzumab, Capecitabine, and Cisplatin as first-line therapy. They reported an ORR of 76.7%, with 27 (62.8%) exhibiting PR and six (14.0%) patients exhibiting CR. Nine patients (20.9%) had stable disease, and the DCR was 97.7%. In 37 patients (86.0%), the total tumor burden was reduced by 30%, and in 26 (56.6%), it was reduced by 50%. The median PFS was 8.6 mo, with a 79.1% 6-mo PFS rate and a 41.9% 1-year PFS rate. The median OS was 19.3 mo, with an 80.1% 1-year OS rate. The median number of treatment cycles was 12. They concluded that utilizing a quadruplet regimen as first-line treatment resulted in tumor reduction in HER-2-positive AGC[64].
Recently, Satake et al[65] conducted a randomized control, phase 3 KEYNOTE-062 trial in 187 patients with AGC or AGEJC to compare the effectiveness of Pembrolizumab or Pembrolizumab with chemotherapy vs standard of care chemotherapy. They found that in the PD-L1 CPS ≥ 1 patients, the median OS with Pembrolizumab was 22.7 mo compared to 13.8 mo with chemotherapy. The 12-mo and 24-mo OS rates with Pembrolizumab were 69.4% and 44.8%, respectively, compared to 54.1% and 23.0% with chemotherapy. In the PD-L1 CPS ≥ 10 patients, the median OS with Pembrolizumab was 28.5 mo vs 14.8 mo with chemotherapy. The 12-mo and 24-mo OS rates with Pembrolizumab were 80.8% and 53.6%, respectively, compared to 68.2% and 27.3% with chemotherapy. They proposed that Pembrolizumab monotherapy was linked with numerically better OS results in patients with AGC or AGEJC with PD-L1 CPS ≥ 1 and CPS ≥ 10 tumors as compared to chemotherapy alone. When compared to chemotherapy, Pembrolizumab monotherapy had a better tolerability profile.
A humanized immunoglobulin G (IgG) 4 monoclonal anti-PD-1 antibody called Nivolumab is effective against a range of tumor types. The phase 1/2 CheckMate-032 trial compared the use of Nivolumab and Ipilimumab in combination with Nivolumab monotherapy in 160 patients with AGC or AGEJC. The ORR for patients who got Nivolumab and Ipilimumab together was 24% as opposed to 12% for Nivolumab alone. Only 8% of patients in the combination arms responded to the alternate dosage (Nivolumab 3 mg/kg and Ipilimumab 1 mg/kg), suggesting that the ORR in these arms was dose-dependent. Regardless of PD-L1 expression, responses were seen. Nivolumab plus Ipilimumab treatment was linked with more severe toxicity (43%) than nivolumab alone (10%), as predicted from past combination trials[66]. Among 493 patients with unresectable AGC or AGEJC who had shown resistance to or intolerance to two or more prior chemotherapy regimens, ONO-4538-12 (ATTRA
In order to assess the safety and effectiveness of Nivolumab in combination with SOX or capecitabine plus oxaliplatin (CapeOX) as first-line therapy in 77 patients with unresectable advanced or recurrent HER-2-negative AGC or AGEJC, Boku et al[69] conducted a randomized, phase 2 trial known as ATTRACTION-4. They discovered that Nivolumab with SOX resulted in an ORR of 57.1%, and Nivolumab plus CapeOX resulted in an ORR of 76.5%. In both groups, the median OS was not attained. The median PFS was 9.7 mo vs 10.6 mo. Neutropenia (14.3%) was the most common grade 3/4 TRAE in the nivolumab plus SOX group, followed by anemia (16.7%), peripheral sensory neuropathy, reduced appetite, type 1 diabetes mellitus, and nausea (11.1%) in the nivolumab with CapeOX group. They concluded that Nivolumab in combination with SOX/CapeOX was well tolerated and showed promising effectiveness in patients with unresectable advanced or recurrent HER-2-negative GC or GEJC. The ATTRACTION-2 2-year follow-up data revealed that 493 of 601 screened individuals were randomized (2:1) to receive Nivolumab (n = 330) or placebo (n = 163), and that the OS was considerably longer in the Nivolumab group compared to the placebo group (5.26 mo vs 4.14 mo) at the 2-year follow-up. At 1 year (27.3% vs 11.6%) and 2 years (10.6% vs 3.2%), the Nivolumab group had a greater OS rate than the placebo group. Regardless of tumor PD-L1 expression, the OS advantage was seen. The median OS for patients in the Nivolumab group who had a full or PR was 26.6 mo; the OS rates at 1 and 2 years were 87.1% and 61.3%, respectively. There were no new safety signals discovered[70]. In a phase 1/2 study, Japanese researchers investigated the safety and effectiveness of Nivolumab with Paclitaxel plus Ramucirumab in 43 patients with AGC resistant to first-line treatment. They discovered an ORR of 37.2% and a 6-mo PFS rate of 46.5%. The median OS was 13.1 mo: 13.8 mo in CPS ≥ 1 patients and 8.0 mo in CPS < 1 patients. They proposed that Nivolumab in combination with Paclitaxel and Ramucirumab showed potential anti-tumor efficacy with acceptable toxicity as a second-line therapy for AGC[71].
Janjigian et al[72] conducted a multicenter, randomized, open-label, phase 3 study (CheckMate 649) in 1581 patients with AGC, AGEJC, or esophageal adenocarcinoma (Nivolumab plus chemotherapy; n = 789; or chemotherapy alone; n = 792). In patients with a PD-L1 CPS greater than 5, they found that Nivolumab with chemotherapy led to substantial improvements in OS and PFS compared to chemotherapy alone. The patients with a PD-L1 CPS > 1 and all randomly assigned people showed a significant improvement in OS as well as a benefit in PFS, according to further data. Of the 782 participants in the nivolumab + chemotherapy group, 462 (59%) and the 767 patients in the chemotherapy alone group, respectively, experienced treatment-related side events. In both groups, the most prevalent any-grade treatment-related side events (25%) were nausea, diarrhea, and peripheral neuropathy. Treatment-related fatalities were determined to be 16 (2%) deaths in the nivolumab plus chemotherapy group and 4 (1%) deaths in the chemotherapy alone group. They proposed that Nivolumab in combination with chemotherapy be the new standard first-line treatment for these patients. In 2021, Shah et al[73] conducted a phase 2 open-label, randomized multicenter trial in 141 patients with pretreatment AGC or AGEJC to compare the effectiveness, safety, and pharmacodynamics of Andecaliximab plus Nivolumab vs Nivolumab alone. The ORR was 10% with Andecaliximab and Nivolumab and 7% with Nivolumab alone. The addition of Andecaliximab had no effect on response or survival. They concluded that the combination of Andecaliximab and Nivolumab had a positive safety profile but did not increase effectiveness in these individuals when compared to Nivolumab alone. Positive HER-2, greater TMB or GRB7, and lower TGF-β1 were all related to better clinical outcomes.
For the purpose of comparing the effectiveness of Nivolumab plus oxaliplatin-based chemotherapy vs placebo plus oxaliplatin-based chemotherapy as first-line therapy, Kang et al[74] conducted a randomized, multicenter, double-blind, placebo-controlled, phase 2/3 trial (ATTRACTION-4) in 724 patients with HER-2-negative, unresectable AGC or AGEJC. They discovered that the median PFS in the Nivolumab plus chemotherapy group was 10.45 mo and 8.34 mo in the placebo plus chemotherapy group. After a 26.6-mo follow-up, the median OS in the Nivolumab plus chemotherapy group was 17.45 mo and 17.15 mo in the placebo plus chemotherapy group. They hypothesized that Nivolumab in combination with oxaliplatin-based chemotherapy enhanced PFS but not OS in these patients. Shitara et al[75] conducted a randomized study to compare Nivolumab plus chemotherapy vs chemotherapy alone (n = 1581), while CheckMate-649 provided the first findings comparing Nivolumab plus Ipilimumab vs chemotherapy alone (n = 813). They observed that, after a 24-mo follow-up, Nivolumab with chemotherapy outperformed treatment in patients with PD-L1 CPS ≥ 5; the median OS was 14.4 mo vs 11.1 mo, respectively. The risk of mortality was reduced by 30%, and the proportion of patients living at 24 mo was 31% vs 19%, respectively. In patients with PD-L1 CPS ≥ 5 or all randomized participants, PFS and ORR were not improved by Nivolumab with Ipilimumab vs chemotherapy. However, in both PD-L1 CPS ≥ 5 and all randomized individuals, responses were more sustained with Nivolumab plus Ipilimumab vs chemotherapy (median DOR, 13.2 vs 6.9 mo). They proposed that the long-term clinically relevant OS and PFS benefits, enhanced and persistent responses, sustained health-related quality of life, and tolerable safety profile of Nivolumab with chemotherapy imply a favorable benefit-risk profile. These findings support the use of this regimen as a conventional first-line therapy in previously untreated AGC or AGEJC patients.
Bang et al[76] conducted a randomized, phase 3 JAVELIN Gastric 300 study in 371 patients with AGC or AGEJC in 2018 to assess the contribution of Avelumab to the physician's choice of chemotherapy as third-line treatment. The trial's primary end objectives of increasing OS (4.6 vs 5.0 mo) or secondary end criteria of PFS (1.4 vs 2.7 mo) or ORR (2.2% vs 4.3%) in the Avelumab vs chemotherapy groups, respectively, were not met. They claimed that treating these patients with single-agent Avelumab in the third-line scenario did not enhance OS or PFS when compared to chemotherapy. Avelumab, on the other hand, had a more controllable safety profile than chemotherapy. Moehler et al[77] conducted a worldwide, open-label, phase 3 JAVELIN Gastric 100 study in 499 AGC or AGEJC patients in 2021 to examine Avelumab maintenance treatment following first-line induction chemotherapy. They found that with Avelumab against chemotherapy, the median OS was 10.4 mo vs 10.9 mo, and the 24-mo OS rate was 22.1% vs 15.5% with no significant difference. They stated that in patients with AGC or AGEJC in general or in a predetermined PD-L1-positive population, JAVELIN Gastric 100 did not offer a superior OS with Avelumab maintenance vs continuing chemotherapy.
Durvalumab is a human IgG1 monoclonal antibody with a high affinity for blocking PD-L1 binding to CD80 and PD-1. According to available data, 10 mg/kg of single-agent Durvalumab administered intravenously every two weeks for 12 mo showed prospective therapeutic efficacy in gastroesophageal malignancies[78,79]. Kwon et al[80] conducted a phase 2 open-label, single-center, non-randomized research study in 31 patients with AGC to assess the effectiveness and safety of Ceralasertib in conjunction with Durvalumab. The ORR, DCR, median PFS, and OS were reported to be 22.6%, 58.1%, 3.0 mo, and 6.7 mo, respectively. Common adverse effects were treatable by adjusting the dosage. In comparison to patients with intact ataxia telangiectasia mutated (ATM) and low sig. HRD (5.60 mo vs 1.65 mo), a subset of patients with ATM expression loss and/or a large proportion of mutational signature owing to homologous repair failure (high sig. HRD) had significantly higher PFS. They proposed that Ceralasertib in combination with Durvalumab had potential anticancer efficacy, with long-term responses in patients with refractory AGC.
The ability of cancer vaccines to activate and boost anticancer immune responses, which are predominantly mediated by T cells that detect tumor-associated antigens, gives them therapeutic potential. The ideal vaccine should be easy to give, safe, cheap to produce, and able to induce a memory response that provides long-lasting immunity. By stimulating NK cells, B lymphocytes, and naïve and memory T cells, DCs, APCs, play a crucial role in orchestrating and coordinating antitumor immune responses[81-83]. For presentation to cytotoxic CD8+ T cells or to CD4+ helper T lymphocytes, tumor antigens are loaded by DCs as short peptides onto MHC class I or II molecules. These functional features prompted the development of several ways to use DCs in cancer immunotherapy. Despite these presumptions, the low in vivo viability of DC-based vaccines prevents their widespread use in clinical settings. A larger number of DCs infiltrating the tumor in GC patients was found to correspond with reduced lymph node metastases and lymphatic invasion, as well as better 5-year survival rates[84,85]. The results suggest that synthetic tumor peptides, synthetic tumor antigen mRNA, lysates, vesicles, and inactivated tumor cells can all be used as DC vaccine-loaded antigens. Potential GC vaccine antigens include the melanoma-associated antigen A3, HER-2 (p369) peptide, gastin-17 diphtheria toxoid, URLC10 or VEGFR1 epitope, and heat shock protein GP96[86]. Regrettably, there haven't been many beneficial findings using DC vaccinations in the treatment of GC. Only three patients with GC participated in the phase 1/2 clinical study, and only one of them was effective, despite showing that a Wilms tumor 1-targeted DC vaccine might be utilized to treat advanced cancer, including GC. In order to increase the effectiveness of GC vaccines, techniques to target numerous antigens have been investigated[87]. DC vaccines can be used with chemotherapy, radiation, and ICIs to increase efficacy. DC immunizations are safe for AGC patients since their toxicity and side effects include fever, flu-like symptoms, and local reactions at the injection site. DC-cytokine induced killer cell (DC-CIK) therapy is another method of using DCs to treat tumors. DC-CIK, coupled with chemotherapy, was proven in clinical trials to be effective and well tolerated in the treatment of AGC. According to a meta-analysis, patients who get DC-CIK and chemotherapy together after GC surgery have dramatically improved OS, DFS, and T cell responses. Additionally, DC-CIK combined with S-1 and cisplatin showed good PFS and OS in the treatment of AGC, and the combination therapy was safe and well tolerated in terms of toxicity[88,89]. Tumor-infiltrating DCs are linked with a better prognosis in GC; however, the tumor microenvironment contains only a few mature DCs. Therefore, DC vaccines and DC-CIK are ineffective against cancer when used as a single therapy; therefore, it is essential to determine the reasons for the ineffectiveness or combine them with other cancer therapies in order to increase the anti-tumor effects[90].
Globally, GC incidence is still high, and because early cancer screening is not extensively used and the symptoms are not frequently recognized, the majority of patients are discovered in the middle or late stages of the disease. After several years of effort, the OS has not dramatically improved the treatment of AGC. Immunotherapy, on the other hand, has given these patients hope. There are several ways to target immune cells to treat tumors, with therapy options targeting T cells having the most substantial effect and the quickest development, as well as showing strong clinical success in various solid tumors, including GC. ICIs were the most important passive immunotherapy in enhancing AGC patients' OS and PFS. These immunotherapies, however, have limitations in the treatment of GC. In certain studies, anti-PD-1/PD-L1 and anti-CTLA-4 antibodies did not improve patients' OS and PFS when compared to chemotherapy. Although encouraging results have been reported in previous clinical studies, the bulk of these populations-which do not make up the majority of AGC patients-have only benefitted from the treatment when their PD-L1 CPS, MSH-H, or TMB scores are high. Although several strategies for targeting immune cells to treat cancer have shown promise in preclinical animal models, they have not been widely used in clinical settings. Because the results of several therapy approaches' ongoing clinical trials have not yet been made public. Another explanation would be that they are only marginally effective, like with the two DC-based cancer treatment methods (vaccination and DC-CIK), which work best in combination with other therapies like chemotherapy. While others acquire primary or secondary medication resistance, the widely used ICIs only work for a portion of tumor patients. The complex microenvironment in which the tumor is located may be the cause of immunotherapy's poor effectiveness. Current immunotherapy only targets one type of cell or a specific target on a specific type of cell, whereas the immunosuppressive environment is made up of multiple cells and multiple targets. The interaction of tumor cells, immune cells, and stromal cells in the tumor microenvironment creates a massive immune suppression network that results in tumor immune escape. More than 10 distinct categories of immunosuppressive receptors expressed on T cells have been identified, and there are still other inhibitory receptors that have not yet been found. The development of therapy modalities with several targets and cells may be the following development path. On the basis of this, multi-target combination approaches for tumor therapy have been developed. Examples include the pairing of PD-1/PD-L1 inhibitors with CTLA-4 inhibitors and PD-1 inhibitors with anti-LAG-3. Moreover, therapeutic tactics targeting immune cells have shown promising outcomes when combined with other therapies, such as chemotherapy medications. As a result, anti-tumor treatment targeting immune cells has a long way to go to achieve synergy and detoxification.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medical laboratory technology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Che XF, China; Liu L, China; Qin SS, China S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Balakrishnan M, George R, Sharma A, Graham DY. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep. 2017;19:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 2. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 983] [Article Influence: 196.6] [Reference Citation Analysis (1)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 4. | Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 860] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 6. | Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 415] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 7. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1582] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 8. | Russi S, Marano L, Laurino S, Calice G, Scala D, Marino G, Sgambato A, Mazzone P, Carbone L, Napolitano G, Roviello F, Falco G, Zoppoli P. Gene Regulatory Network Characterization of Gastric Cancer's Histological Subtypes: Distinctive Biological and Clinically Relevant Master Regulators. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Xie J, Fu L, Jin L. Immunotherapy of gastric cancer: Past, future perspective and challenges. Pathol Res Pract. 2021;218:153322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 11. | Kang BW, Chau I. Current status and future potential of predictive biomarkers for immune checkpoint inhibitors in gastric cancer. ESMO Open. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Eusebi LH, Telese A, Marasco G, Bazzoli F, Zagari RM. Gastric cancer prevention strategies: A global perspective. J Gastroenterol Hepatol. 2020;35:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 13. | Januszewicz W, Turkot MH, Malfertheiner P, Regula J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Hamashima C, Shabana M, Okada K, Okamoto M, Osaki Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015;106:1744-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park EC. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev. 2013;14:2533-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Yu H, Yang AM, Lu XH, Zhou WX, Yao F, Fei GJ, Guo T, Yao LQ, He LP, Wang BM. Magnifying narrow-band imaging endoscopy is superior in diagnosis of early gastric cancer. World J Gastroenterol. 2015;21:9156-9162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 17. | Zhang J, Guo SB, Duan ZJ. Application of magnifying narrow-band imaging endoscopy for diagnosis of early gastric cancer and precancerous lesion. BMC Gastroenterol. 2011;11:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ganjoho. jp. Stomach. [cited 5 March 2023]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/cancer/5_stomach.html. |
| 19. | Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer. 2017;123 Suppl 24:4994-5013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Wu SL, Zhang Y, Fu Y, Li J, Wang JS. Gastric cancer incidence, mortality and burden in adolescents and young adults: a time-trend analysis and comparison among China, South Korea, Japan and the USA. BMJ Open. 2022;12:e061038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Davis PA, Sano T. The difference in gastric cancer between Japan, USA and Europe: what are the facts? Crit Rev Oncol Hematol. 2001;40:77-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2051] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 23. | Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, You J, Wong BCY, Chen J, Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology. 2022;163:154-162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 24. | LAUREN P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4325] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 25. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4859] [Article Influence: 441.7] [Reference Citation Analysis (2)] |
| 26. | Wang Q, Liu G, Hu C. Molecular Classification of Gastric Adenocarcinoma. Gastroenterology Res. 2019;12:275-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Lim HJ, Zhuang L, Fitzgerald RC. Current advances in understanding the molecular profile of hereditary diffuse gastric cancer and its clinical implications. J Exp Clin Cancer Res. 2023;42:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 28. | Gullo I, Carneiro F, Oliveira C, Almeida GM. Heterogeneity in Gastric Cancer: From Pure Morphology to Molecular Classifications. Pathobiology. 2018;85:50-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Serra O, Galán M, Ginesta MM, Calvo M, Sala N, Salazar R. Comparison and applicability of molecular classifications for gastric cancer. Cancer Treat Rev. 2019;77:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Sanjeevaiah A, Cheedella N, Hester C, Porembka MR. Gastric Cancer: Recent Molecular Classification Advances, Racial Disparity, and Management Implications. J Oncol Pract. 2018;14:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Chan WL, Lam KO, Lee VHF, Davidson M, So TH, Li JS, Chau I, Kwong DLW. Gastric Cancer - From Aetiology to Management: Differences Between the East and the West. Clin Oncol (R Coll Radiol). 2019;31:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Gonzalez-Pons M, Torres-Cintrón CR, Soto-Salgado M, Vargas-Ramos Y, Perez-Portocarrero L, Morgan DR, Cruz-Correa M. Racial/ethnic disparities in gastric cancer: A 15-year population-based analysis. Cancer Med. 2023;12:1860-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Finn OJ. A Believer's Overview of Cancer Immunosurveillance and Immunotherapy. J Immunol. 2018;200:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 34. | Fan X, Jin J, Yan L, Liu L, Li Q, Xu Y. The impaired anti-tumoral effect of immune surveillance cells in the immune microenvironment of gastric cancer. Clin Immunol. 2020;219:108551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2057] [Article Influence: 228.6] [Reference Citation Analysis (0)] |
| 36. | Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 755] [Article Influence: 251.7] [Reference Citation Analysis (0)] |
| 37. | Dang S, Malik A, Chen J, Qu J, Yin K, Cui L, Gu M. LncRNA SNHG15 Contributes to Immuno-Escape of Gastric Cancer Through Targeting miR141/PD-L1. Onco Targets Ther. 2020;13:8547-8556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Lei X, Lin H, Wang J, Ou Z, Ruan Y, Sadagopan A, Chen W, Xie S, Chen B, Li Q, Zhu X, Yuan X, Tian T, Lv X, Fu S, Zhou J, Pan G, Xia X, Tannous BA, Ferrone S, Fan S, Li J. Mitochondrial fission induces immunoescape in solid tumors through decreasing MHC-I surface expression. Nat Commun. 2022;13:3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 39. | Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1427] [Cited by in RCA: 1709] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 40. | Wu MH, Lee WJ, Hua KT, Kuo ML, Lin MT. Macrophage Infiltration Induces Gastric Cancer Invasiveness by Activating the β-Catenin Pathway. PLoS One. 2015;10:e0134122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló S, Roda D, Huerta M, Cervantes A, Fleitas T. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020;86:102015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 42. | Ibáñez-Vea M, Zuazo M, Gato M, Arasanz H, Fernández-Hinojal G, Escors D, Kochan G. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment: Current Knowledge and Future Perspectives. Arch Immunol Ther Exp (Warsz). 2018;66:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Tang Y, Zhou C, Li Q, Cheng X, Huang T, Li F, He L, Zhang B, Tu S. Targeting depletion of myeloid-derived suppressor cells potentiates PD-L1 blockade efficacy in gastric and colon cancers. Oncoimmunology. 2022;11:2131084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 44. | Liu X, Zhang Z, Zhao G. Recent advances in the study of regulatory T cells in gastric cancer. Int Immunopharmacol. 2019;73:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Rocha S, Basto AP, Ijsselsteijn ME, Teles SP, Azevedo MM, Gonçalves G, Gullo I, Almeida GM, Maqueda JJ, Oliveira MI, Carneiro F, Barata JT, Graça L, de Miranda NFCC, Carvalho J, Oliveira C. Immunophenotype of Gastric Tumors Unveils a Pleiotropic Role of Regulatory T Cells in Tumor Development. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 47. | Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 359] [Cited by in RCA: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 48. | Högner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol. 2022;29:1559-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 49. | Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 1341] [Article Influence: 268.2] [Reference Citation Analysis (0)] |
| 50. | Lentz RW, Colton MD, Mitra SS, Messersmith WA. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol Cancer Ther. 2021;20:961-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 51. | Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, Shalaby MN, Thangavelu L, Kamrava S, Shomali N, Sohrabi AD, Adili A, Noroozi-Aghideh A, Razeghian E. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal. 2022;20:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 52. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (1)] |
| 53. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1461] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 54. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 999] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 55. | Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, Borg C, Doi T, Yoon HH, Savage MJ, Wang J, Dalal RP, Shah S, Wainberg ZA, Chung HC. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22:828-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 56. | Kawazoe A, Yamaguchi K, Yasui H, Negoro Y, Azuma M, Amagai K, Hara H, Baba H, Tsuda M, Hosaka H, Kawakami H, Oshima T, Omuro Y, Machida N, Esaki T, Yoshida K, Nishina T, Komatsu Y, Han SR, Shiratori S, Shitara K. Safety and efficacy of pembrolizumab in combination with S-1 plus oxaliplatin as a first-line treatment in patients with advanced gastric/gastroesophageal junction cancer: Cohort 1 data from the KEYNOTE-659 phase IIb study. Eur J Cancer. 2020;129:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T, Sato A, Kuwata T, Shitara K. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 58. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 867] [Article Influence: 173.4] [Reference Citation Analysis (1)] |
| 59. | Wainberg ZA, Fuchs CS, Tabernero J, Shitara K, Muro K, Van Cutsem E, Bang YJ, Chung HC, Yamaguchi K, Varga E, Chen JS, Hochhauser D, Thuss-Patience P, Al-Batran SE, Garrido M, Kher U, Shih CS, Shah S, Bhagia P, Chao J. Efficacy of Pembrolizumab Monotherapy for Advanced Gastric/Gastroesophageal Junction Cancer with Programmed Death Ligand 1 Combined Positive Score ≥10. Clin Cancer Res. 2021;27:1923-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Kwon M, An M, Klempner SJ, Lee H, Kim KM, Sa JK, Cho HJ, Hong JY, Lee T, Min YW, Kim TJ, Min BH, Park WY, Kang WK, Kim KT, Kim ST, Lee J. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov. 2021;11:2168-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (1)] |
| 61. | Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Van Cutsem E, Elme A, Thuss-Patience P, Chau I, Ohtsu A, Bhagia P, Wang A, Shih CS, Shitara K. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer. 2022;25:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 62. | Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, Park YL, Koo DH, Lu J, Xu J, Chon HJ, Bai LY, Zeng S, Yuan Y, Chen YY, Gu K, Zhong WY, Kuang S, Shih CS, Qin SK. Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): A randomized, open-label, phase 3 trial in Asian patients. Cancer. 2022;128:995-1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 63. | Yamaguchi K, Minashi K, Sakai D, Nishina T, Omuro Y, Tsuda M, Iwagami S, Kawakami H, Esaki T, Sugimoto N, Oshima T, Kato K, Amagai K, Hosaka H, Komine K, Yasui H, Negoro Y, Ishido K, Tsushima T, Han S, Shiratori S, Takami T, Shitara K. Phase IIb study of pembrolizumab combined with S-1 + oxaliplatin or S-1 + cisplatin as first-line chemotherapy for gastric cancer. Cancer Sci. 2022;113:2814-2827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Lee CK, Rha SY, Kim HS, Jung M, Kang B, Che J, Kwon WS, Park S, Bae WK, Koo DH, Shin SJ, Kim H, Jeung HC, Zang DY, Lee SK, Nam CM, Chung HC. A single arm phase Ib/II trial of first-line pembrolizumab, trastuzumab and chemotherapy for advanced HER2-positive gastric cancer. Nat Commun. 2022;13:6002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 65. | Satake H, Lee KW, Chung HC, Lee J, Yamaguchi K, Chen JS, Yoshikawa T, Amagai K, Yeh KH, Goto M, Chao Y, Lam KO, Han SR, Shiratori S, Shah S, Shitara K. Pembrolizumab or pembrolizumab plus chemotherapy versus standard of care chemotherapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma: Asian subgroup analysis of KEYNOTE-062. Jpn J Clin Oncol. 2023;53:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 66. | Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, Dorange C, Tschaika M, Le DT. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol. 2018;36:2836-2844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 67. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1716] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 68. | Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC, Lee JS, Cheong JH, Noh SH, Aoyama T, Miyagi Y, Tsuburaya A, Yoshikawa T, Ajani JA, Boussioutas A, Yeoh KG, Yong WP, So J, Lee J, Kang WK, Kim S, Kameda Y, Arai T, Zur Hausen A, Speed TP, Grabsch HI, Tan P. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64:1721-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 69. | Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, Cho H, Kang WK, Komatsu Y, Tsuda M, Yamaguchi K, Hara H, Fumita S, Azuma M, Chen LT, Kang YK. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019;30:250-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 70. | Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Kang YK, Boku N. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer. 2020;23:510-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 71. | Nakajima TE, Kadowaki S, Minashi K, Nishina T, Yamanaka T, Hayashi Y, Izawa N, Muro K, Hironaka S, Kajiwara T, Kawakami Y. Multicenter Phase I/II Study of Nivolumab Combined with Paclitaxel Plus Ramucirumab as Second-line Treatment in Patients with Advanced Gastric Cancer. Clin Cancer Res. 2021;27:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 72. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1902] [Article Influence: 475.5] [Reference Citation Analysis (1)] |
| 73. | Shah MA, Cunningham D, Metges JP, Van Cutsem E, Wainberg Z, Elboudwarej E, Lin KW, Turner S, Zavodovskaya M, Inzunza D, Liu J, Patterson SD, Zhou J, He J, Thai D, Bhargava P, Brachmann CB, Cantenacci DVT. Randomized, open-label, phase 2 study of andecaliximab plus nivolumab versus nivolumab alone in advanced gastric cancer identifies biomarkers associated with survival. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 489] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 75. | Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, Yamaguchi K, Wyrwicz L, Skoczylas T, Bragagnoli AC, Liu T, Tehfe M, Elimova E, Bruges R, Zander T, de Azevedo S, Kowalyszyn R, Pazo-Cid R, Schenker M, Cleary JM, Yanez P, Feeney K, Karamouzis MV, Poulart V, Lei M, Xiao H, Kondo K, Li M, Janjigian YY. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603:942-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 290] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 76. | Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 414] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 77. | Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol. 2021;39:966-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 78. | Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, Chung HC, Wainberg ZA, Gibson MK, Lee KW, Bendell JC, Denlinger CS, Chee CE, Omori T, Leidner R, Lenz HJ, Chao Y, Rebelatto MC, Brohawn PZ, He P, McDevitt J, Sheth S, Englert JM, Ku GY. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients with Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2020;26:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 79. | Bang YJ, Golan T, Dahan L, Fu S, Moreno V, Park K, Geva R, De Braud F, Wainberg ZA, Reck M, Goff L, Laing N, Mi G, Oliveira JM, Wasserstrom H, Lin CC. Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ). Eur J Cancer. 2020;137:272-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 80. | Kwon M, Kim G, Kim R, Kim KT, Kim ST, Smith S, Mortimer PGS, Hong JY, Loembé AB, Irurzun-Arana I, Koulai L, Kim KM, Kang WK, Dean E, Park WY, Lee J. Phase II study of ceralasertib (AZD6738) in combination with durvalumab in patients with advanced gastric cancer. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 81. | Saeed A, Park R, Dai J, Al-Rajabi R, Kasi A, Baranda J, Williamson S, Saeed A, Ripp J, Collins Z, Mulvaney K, Shugrue M, Firth-Braun J, Godwin AK, Madan R, Phadnis M, Sun W. Cabozantinib plus durvalumab in advanced gastroesophageal cancer and other gastrointestinal malignancies: Phase Ib CAMILLA trial results. Cell Rep Med. 2023;4:100916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 82. | Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1556] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 83. | Salem ML. The use of dendritic cells for peptide-based vaccination in cancer immunotherapy. Methods Mol Biol. 2014;1139:479-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Niccolai E, Taddei A, Prisco D, Amedei A. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol. 2015;21:5778-5793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Ananiev J, Gulubova MV, Manolova IM. Prognostic significance of CD83 positive tumor-infiltrating dendritic cells and expression of TGF-beta 1 in human gastric cancer. Hepatogastroenterology. 2011;58:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 86. | Dolcetti R, De Re V, Canzonieri V. Immunotherapy for Gastric Cancer: Time for a Personalized Approach? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 87. | Zhang W, Lu X, Cui P, Piao C, Xiao M, Liu X, Wang Y, Wu X, Liu J, Yang L. Phase I/II clinical trial of a Wilms' tumor 1-targeted dendritic cell vaccination-based immunotherapy in patients with advanced cancer. Cancer Immunol Immunother. 2019;68:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 88. | Wang X, Tang S, Cui X, Yang J, Geng C, Chen C, Zhou N, Li Y. Cytokine-induced killer cell/dendritic cell-cytokine-induced killer cell immunotherapy for the postoperative treatment of gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e12230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Qiao G, Wang X, Zhou L, Zhou X, Song Y, Wang S, Zhao L, Morse MA, Hobeika A, Song J, Yi X, Xia X, Ren J, Lyerly HK. Autologous Dendritic Cell-Cytokine Induced Killer Cell Immunotherapy Combined with S-1 Plus Cisplatin in Patients with Advanced Gastric Cancer: A Prospective Study. Clin Cancer Res. 2019;25:1494-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Zhao Y, Bai Y, Shen M, Li Y. Therapeutic strategies for gastric cancer targeting immune cells: Future directions. Front Immunol. 2022;13:992762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |