Published online Jun 20, 2023. doi: 10.5662/wjm.v13.i3.153
Peer-review started: January 14, 2023
First decision: April 13, 2023
Revised: April 30, 2023
Accepted: May 16, 2023
Article in press: May 16, 2023
Published online: June 20, 2023
Processing time: 157 Days and 2.9 Hours
Gastrointestinal stromal tumors (GISTs) are considered the most common mesenchymal tumors of the gastrointestinal tract. Microvessel density (MVD) constitutes a direct method of vascularity quantification and has been associated with survival rates in multiple malignancies.
To appraise the effect of MVD on the survival of patients with GIST.
This study adhered to Systematic reviews and Meta-Analyses guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. Electronic scholar databases and grey literature repositories were systematically screened. The Fixed Effects or Random Effects models were used according to the Cochran Q test.
In total, 6 eligible studies were identified. The pooled hazard ratio (HR) for disease free survival (DFS) was 8.52 (95%CI: 1.69-42.84, P = 0.009). The odds ratios of disease-free survival between high and low MVD groups at 12 and 60 mo did not reach statistical significance. Significant superiority of the low MVD group in terms of DFS was documented at 36 and 120 mo (OR: 8.46, P < 0.0001 and OR: 22.71, P = 0.0003, respectively) as well as at metastases rate (OR: 0.11, P = 0.0003).
MVD significantly correlates with the HR of DFS and overall survival rates at 36 and 120 mo. Further prospective studies of higher methodological quality are required.
Core Tip: This systematic review and meta-analysis summarize all available data regarding the prognostic role of microvessel density (MVD) in gastrointestinal stromal tumors (GISTs). MVD measurement affects long term GIST survival. However, further prospective studies are necessary.
- Citation: Perivoliotis K, Baloyiannis I, Samara AA, Koutoukoglou P, Ntellas P, Dadouli K, Ioannou M, Tepetes K. Microvessel density in patients with gastrointestinal stromal tumors: A systematic review and meta-analysis. World J Methodol 2023; 13(3): 153-165
- URL: https://www.wjgnet.com/2222-0682/full/v13/i3/153.htm
- DOI: https://dx.doi.org/10.5662/wjm.v13.i3.153
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal tumours of the gastrointestinal (GI) tract. According to existing literature, the average GIST incidence is estimated at 10 to 15 cases per million, ranging from 4.3 to 22/1000000 between different geographical locations[1,2]. Furthermore, although the age of reported cases spans from 10 to 100 years, the median GIST presentation appears during the mid-60 years of age, with no discrepancies in terms of gender allocation[1,2].
Based on recent studies, the origin of these tumours can be traced to the interstitial cell of Cajal, a myenteric plexus pacemaker[2-4]. The most frequent GIST locations are the stomach (55.6%), small (31.8%) and large intestine (6%). Further primary sites include the oesophagus, the omentum, the mesentery and the retroperitoneum[1-2,5]. Regarding morphological characteristics, GISTs are classified in spindle cell, epithelioid cell and mixed type histological subgroups[6].
The majority of GISTs have been found to express KIT, a proto-oncogene protein[7]. Specifically, KIT or c-kit is positive through immunohistochemical staining in almost 95% of all GISTs[6], while KIT-negative GISTs have been demonstrated to harbour mutations of platelet-derived growth factor receptor-alpha[6,8,9]. Alteration of the function of these receptor tyrosine kinases is considered of major importance in the GIST oncogenesis, through the RAS-RAF-MAPK and PI3K-AKT-mTOR pathways[6]. Surgical excision is considered the gold standard treatment for GISTs. However, kinase inhibitor adjuvant therapy (i.e. imatinib and sunitinib) has been introduced for treatment of advanced and metastatic disease[10-15], improving the overall survival (OS) and time to progression rates. Despite this, treatment resistance and disease recurrence rates still remain a significant problem[11,13].
To prognose the therapy outcomes, various risk grading systems have emerged, including those proposed by Fletcher et al[16] and Miettinen et al[17]. Several clinical and histopathologic factors been investigated such as tumor size, mitotic activity, anatomical origin, tumor rupture, tumor mutation type, predominant cell type, cellular density, p53, Ki-67, neutrophil to lymphocyte ratio and blood vessel invasion[6,11,13,18-20].
Microvessel density (MVD) assessment technique, based on the original work of Weidner et al[21], constitutes a direct method of vascularity quantification, since it represents the number of small blood vessels in tumoral tissue. Estimation of the vasculature is achieved through the application of various immunohistochemical endothelium labelling stains, such as cluster of differentiation (CD) 31, CD34, CD105 and von Willebrand Factor (vWF). The correlation between tumoral MVD and overall survival outcome in GIST patients has been extensively researched[8-9,22-25]. However, to the best of our knowledge, there is still no study assessing overall prognostic value of MVD in these neoplasms.
Considering the above, a systematic literature review and meta-analysis of the reported outcomes was designed to estimate the pooled effect of tumor vascularity on survival of GIST patients, based on MVD measurements.
The present meta-analysis was conducted based on the Cochrane Handbook for Systematic Reviews of Interventions and Systematic reviews and Meta-Analyses (PRISMA) guidelines[26]. The study was not registered in current electronic databases.
The primary endpoint of the present meta-analysis was considered the Hazard Ratio (HR) of Disease-Free Survival (DFS) between low and high MVD measurements in patients suffering from GISTS. Pooled HR > 1 denoted a higher risk of death in patients with high MVD, compared to patients with low MVD.
The secondary endpoints included pooled odds ratios (ORs) of DFS between high and low MVD measurements, at four specific time points (12, 36, 60 and 120 mo) of follow-up. Moreover, the pooled OR between high and low MVD tumours of the presence of metastases in GIST patients was estimated. A pooled OR > 1 suggested superiority of low MVD tumours when compared to respective high MVD tumours, in terms of survival endpoints. On the contrary, concerning the metastases endpoint the opposite applied.
Eligible studies were prospective or retrospective trials with a study population consisting of GIST patients, whose outcomes of interest were reported in English and were retrievable. Specifically, the study design must have incorporated a primary tumor MVD assessment.
Exclusion criteria consisted of studies written in a language other than English, with no endpoint of interest, insufficient survival data and no human studies. Furthermore, studies in the format of a letter, conference abstract, expert opinion or duplicate trials were not incorporated in the meta-analysis.
A systematic literature search in electronic scholar databases (MEDLINE, CENTRAL, Scopus and Web of Science) and grey literature repositories (OpenGrey.eu and medRxiv) was performed to identify eligible studies. The last search date was December 2022. The literature search included the following search keywords: ‘gist’, ‘gastrointestinal stromal tumor’, ‘stromal tumor’, ‘mvd’, ‘microvessel density’ and ‘microvascular density’.
The first step of screening included removing duplicate entries. Subsequently, titles and abstracts of the remaining studies were assessed based on the inclusion criteria. A full text review of accepted entries was then performed, to validate consistency with the eligibility criteria. The electronic database screening, study selection, data extraction as well as methodological and quality assessment were performed in duplicate and blindly by two independent investigators (K.P. and P.K.). To reach consensus, disagreements were resolved by mutual revision and discussion. If discrepancies were not resolved, the opinion of a third investigator (K.D) was considered.
The Newcastle-Ottawa Scale (NOS)[27] was utilized to perform rigorous quality and methodological evaluation of eligible studies. NOS evaluates non-RCT trials in certain endpoints, such as selection and comparability of study groups and confirmation of the exposure. Each included study was rated with a score ranging from 0 to 9. Cohen’s k statistic was also calculated.
Data analysis and statistical computations were performed using Cochrane Collaboration RevMan version 5.3 and IBM SPSS version 23. The primary and secondary endpoints were reported in the form of HR and OR, respectively. Results of the analyses were presented with the corresponding 95% Confidence Interval (95%CI).
If eligible trials did not directly provide the HR or OR in the article results, they were estimated based on the algorithm proposed by Parmar et al[28] and Tierney et al[29]. Specifically, required data for the meta-analysis of trials endpoints were reconstructed from the Kaplan-Meier curves provided[30]. The precision of extracted coordinates was enhanced through utilization of a digitizing software (Digitizelt)[31].
If included trials did not provide the mean and standard deviation (SD) of continuous variables, they were estimated from the median and the Interquartile Range (IR) based on the algorithm described by Hozo et al[32]. Given a sample size of >25, the mean was considered equal to the median. For a sample of < 70, the SD was regarded as IR/4. In the other case, the SD was calculated as IR/6.
The statistical method applied was Mantel-Haenszel (MH) and the Inverse Variance for OR and HR, respectively. Both the Fixed Effects and Random Effects (RE) model were calculated. The final model that was estimated was based on the Cochran Q test. If statistically significant heterogeneity was present (Q test P < 0.1), then the RE model was applied. A further quantification of the heterogeneity was performed through the calculation of I2. Statistical significance was considered at the level of P < 0.05.
To estimate the publication bias of included studies, the funnel plot of the primary outcome was visually inspected. Regarding the primary outcome, Egger’s test was also performed.
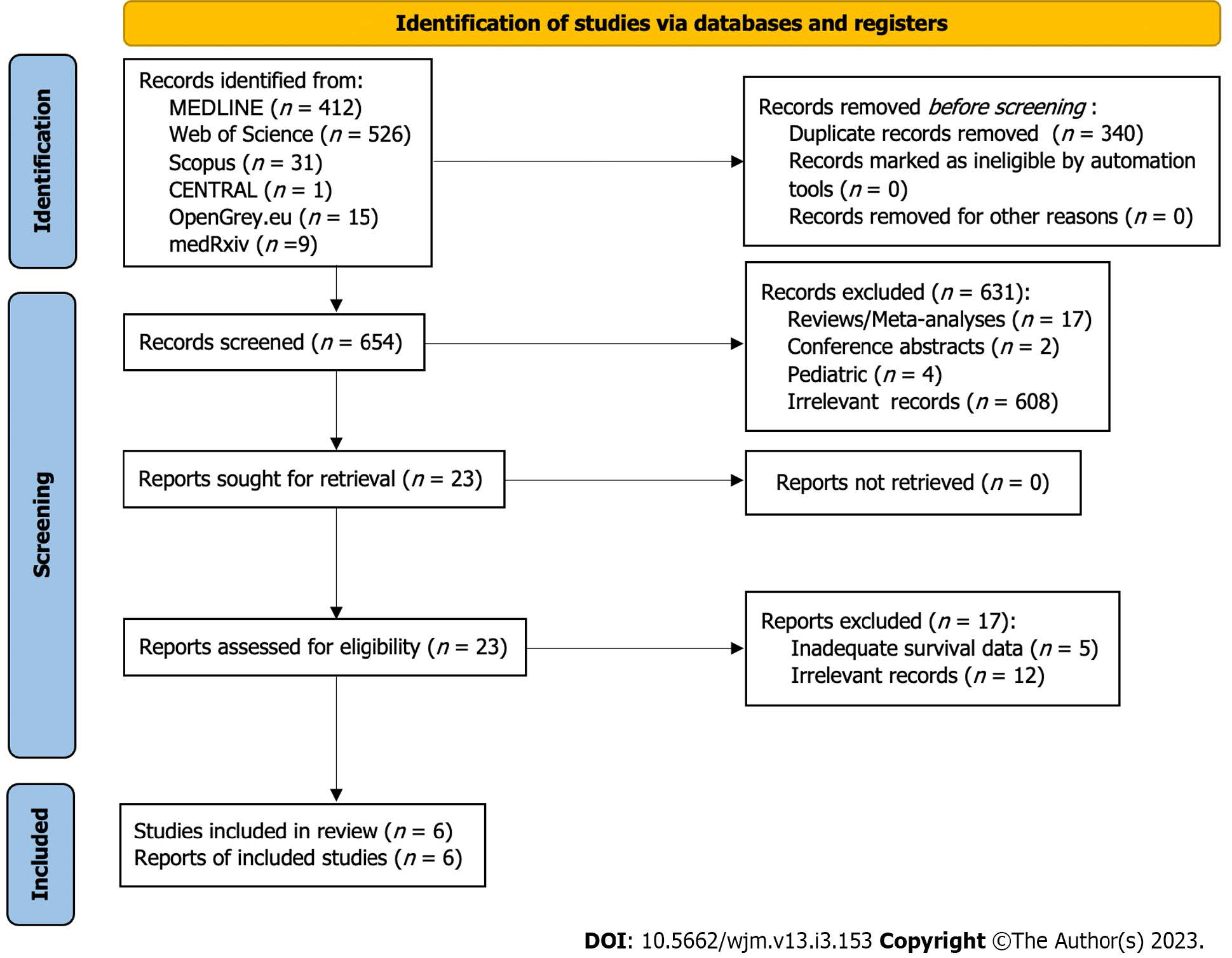
Through the above-mentioned search algorithm (Figure 1), 994 citations were retrieved (MEDLINE: 412, Web of Science: 526, Scopus: 31, CENTRAL: 1, OpenGrey.eu: 15, medRxiv: 9). The next step included the removal of 340 duplicate records. A total of 654 records underwent title and abstract screening, resulting in the exclusion of 631 entries (17 reviews/ meta-analyses, 2 conference abstracts, 4 paediatric studies, 608 irrelevant records). Examination of compliance with eligibility criteria extended to the full text articles of the previously accepted records. In total, 5 studies with inadequate survival data and 12 irrelevant studies were excluded. Subsequently, 6 studies[8-9,22-25] were included in the present meta-analysis.
Table 1 summarizes the characteristics of included studies. Regarding study type, all trials had a retrospective design. Furthermore, all except one trial[9] were single centre, with sample size ranging from 53 to 124. More specific information regarding the analysis and total specimen sample are reported in Table 1. Mean patient age and gender allocation are also displayed in Table 1. Mean follow-up period extended from 2.5 years in the study by Waengertner et al[24], up to 81.7 mo in the study by Takahashi et al[23].
| Ref. | Type of study | Country | Centre | Sample (patients) | Analysis sample | Specimens | Age | Gender (male/female) | Follow-up |
| Chen et al[22], 2005 | Retrospective | Taiwan | Single centre | 62 | 59 (3 cases lost to follow-up) | 62 | 24 (38.7) ≤ 61 yr; 38 (61.3) > 61 yr | 34 (54.8)/28 (45.2) | 50.5 (31) mo for 59 cases |
| Imamura et al[8], 2007 | Retrospective | Japan | Single centre | 95 | 95 (80 from the K-M curves) | 95 | 64 (11.667) yr | 48 (50.5)/47 (49.5) | 48.4 (26.1833) mo for 80 cases |
| Takahashi et al[23], 2003 | Retrospective | Japan | Single centre | 53 | 53 | 53 | 59.5 (13.3) yr | 32 (60.3)/21 (39.6) | 81.7 (63.2) mo |
| Waengertner et al[24], 2011 | Retrospective | Brazil | Single centre | 79 | 79 | 79 | 58.9 (13) yr | 42 (53.2)/37 (46.8) | 2.5 (2.8) yr |
| Wang et al[9], 2009 | Retrospective | China | Multicentre | 68 | 68 | 68 | 56.8 (14.75) yr | 38 (55.9)/30 (44.1) | 42.9 (14) mo for 64 patients |
| Zhao et al[25], 2012 | Retrospective | China | Single centre | 124 | 124 | 124 | 54.6 (11.667) yr | 64 (51.6)/60 (48.4) | 52 (32.333) mo |
Concerning the MVD assessment method that was applied, the majority of included trials described the use of light microscopy and immunochemistry, implementing the technique proposed by Weidner et al[21] (Table 2). Exceptions to this were trials by Imamura et al[8] and Waengertner et al[24] which reported the application of a modified Horak technique and Chalkley method, respectively. Despite the fact that the majority of eligible trials used CD31 antibodies, Zhao et al[25] utilized the CD34 antibody. Heterogeneity was identified in the reported level of magnification. More specifically, the applied magnification spanned from 40X up to 400X. Furthermore, non-uniformity was discovered in the number of spots examined, which ranged from 3 to 10 spots. Only two trials[8,9] confirmed blinded estimation of microvessel density by two independent observers, and none provided information about the existence of separate count for intratumoral and peritumoral vessels. All researchers except Zhao et al[25] included the MVD cut-off value in their study articles.
| Ref. | MVD assessment method | Antibody | Magnification used | Spots examined | Blinded reading | Observers | Separate count for intra/peritumoral vessels | MVD cut off |
| Chen et al[22] | Light microscopy, immunohistochemistry | CD31 | 10X; 20X; 100X | 3 | N/A | N/A | N/A | 15/HPF |
| Imamura et al[8] | Light microscopy, immunohistochemistry, slight modification of Horak et al technique | CD31 | 40X; 200X | 10 | Yes | 2 | N/A | 7/0.95 mm² |
| Takahashi et al[23] | Light microscopy, immunohistochemistry | CD31 | 40X; 100X; 400X | 3 | N/A | N/A | N/A | 19/HPF |
| Waengertner et al[24] | Light microscopy, immunohistochemistry, modified Chalkley method | CD31 | 200X | 3 to 5 | N/A | N/A | N/A | 6 vessels |
| Wang et al[9] | Light microscopy, immunohistochemistry | CD31 | 200X | 4 | Yes | 2 | N/A | 10.54/200HPF |
| Zhao et al[25] | Light microscopy, immunohistochemistry, Weidner technique | CD34 | 100X; 200X | 5 | N/A | N/A | N/A | N/A |
Table 3 summarizes the data regarding the risk classification of included tumours. Moreover, the localization of GISTs included: 9 in the oesophagus, 284 in the stomach, 127 in the small intestine and 28 in the anatomic area of the colon and rectum. According to Table 4, only the study group of Chen et al[22] recorded tumor complications like necrosis (37%) and haemorrhage (72.6%). Table 4 incorporates histopathologic characteristics, such as the mitotic count and the tumor size of included GISTs. From the eligible trials, tumor cell type categorization was performed in only 3[8,24,25] studies. In total, 29 epithelioid, 222 spindle and 25 mixed tumours were identified. Finally, inconsistent data were provided by the included trials in terms of the operation performed and chemotherapy type administered.
| Ref. | Risk | Location | |||||||
| Very low risk | Low risk | Intermediate risk | High risk | Stomach | Small intestine | Colon | Rectum | Esophagus | |
| Chen et al[22] | 0 (0) | 31 (50) | 0 (0) | 31 (50) | 41 (66) | 18 (29) | 3 (4.8) | 0 (0) | 0 (0) |
| Imamura et al[8] | 7 (7.3) | 22 (23.2) | 38 (40) | 28 (29.5) | 64 (67.4) | 31 (32.6) | 0 (0) | 0 (0) | 0 (0) |
| Takahashi et al[23] | 16 (30.1) | 10 (18.8) | 27(50.9) | 53 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Waengertner et al[24] | 12 (15.4) | 11 (13.8) | 18 (23.1) | 38 (47.7) | 36 (45.6) | 30 (38) | 0 (0) | 0 (0) | 0 (0) |
| Wang et al[9] | 0 (0) | 20 (29.4) | 0 (0) | 48 (70.6) | 28 (41.2) | 20 (29.4) | 11 (16.2) | 0 (0) | 0 (0) |
| Zhao et al[25] | 6 (4.8) | 20 (16.1) | 37 (29.8) | 61 (49.3) | 62 (50) | 28 (22.6) | 14 (11.3) | 9 (7.3) | |
| Necrosis | Hemorrhage | Mitotic count | Tumor size | Pcna index | Cell type | Treatment | |||||||||||
| Ref. | Yes | No | Yes | No | ≤ 10% | > 10% | Epithelioid | Spindle | Mixed | Surgery | Surgery type | Chemotherapy | Chemotherapy type | ||||
| Chen et al[22] | 23 (37) | 39 (63) | 45 (72.6) | 17 (27.4) | 36 (58) < 2/10 HPF | 26 (42) ≥ 2/10 HPF | 32 (51.6) < 5 cm | 30 (48.4) ≥ 5 cm | 32 (51.6) | 30 (48.4) | N/A | N/A | N/A | Yes | Subtotal gastrectomy, complete tumor resection or segmental enterectomy | Yes (some of them) | See comments |
| Imamura et al[8] | N/A | N/A | N/A | N/A | 55 (57.9) < 5/50 HPF | 40 (42.1) ≥ 5/50 HPF | 39 (41.05) < 5 cm | 56 (58.95) ≥ 5 cm | N/A | N/A | 1 (1.05) | 92 (96.85) | 2 (2.1) | Yes | Resection with negative margins | N/A | N/A |
| Takahashi et al[23] | N/A | N/A | N/A | N/A | 33 (62.2) < 3/50 HPF | 20 (37.7) ≥ 3/50 HPF | 21 (39.6) ≤ 3 cm | 32 (60.3) > 3 cm | N/A | N/A | N/A | N/A | N/A | Yes | Surgical resection | N/A | N/A |
| Waengertner et al[24] | N/A | N/A | N/A | N/A | N/A | N/A | N/A, varies from 0.5 to 25 cm (median 4.8 cm) | N/A | N/A | N/A | 57 (72.2%) | N/A | N/A | N/A | Yes | Adjuvant therapy with tyrosine kinase inhibitors (400mg/daily) for no longer than 3 months | |
| Wang et al[9] | N/A | N/A | N/A | N/A | 45 (66.2) < 2/10 HPF | 23 (33.8) ≥ 2/10 HPF | 24 (35.3) ≤ 5 cm | 44 (64.7) > 5 cm | N/A | N/A | N/A | N/A | N/A | Yes | N/A | No | No |
| Zhao et al[25] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 28 (22.58) | 73 (58.87) | 23 (18.55) | Yes | Only biopsy, palliative resection, radical resection | Yes | Postoperative |
Regarding the assessment based on the NOS scale, most studies achieved a 5-star score. The trial by Chen et al[22] was an exception, as it appointed a 6 star score. Inter-rater agreement was estimated to be in a very good level (Cohen’s k statistic: 86.8%, P < 0.001)
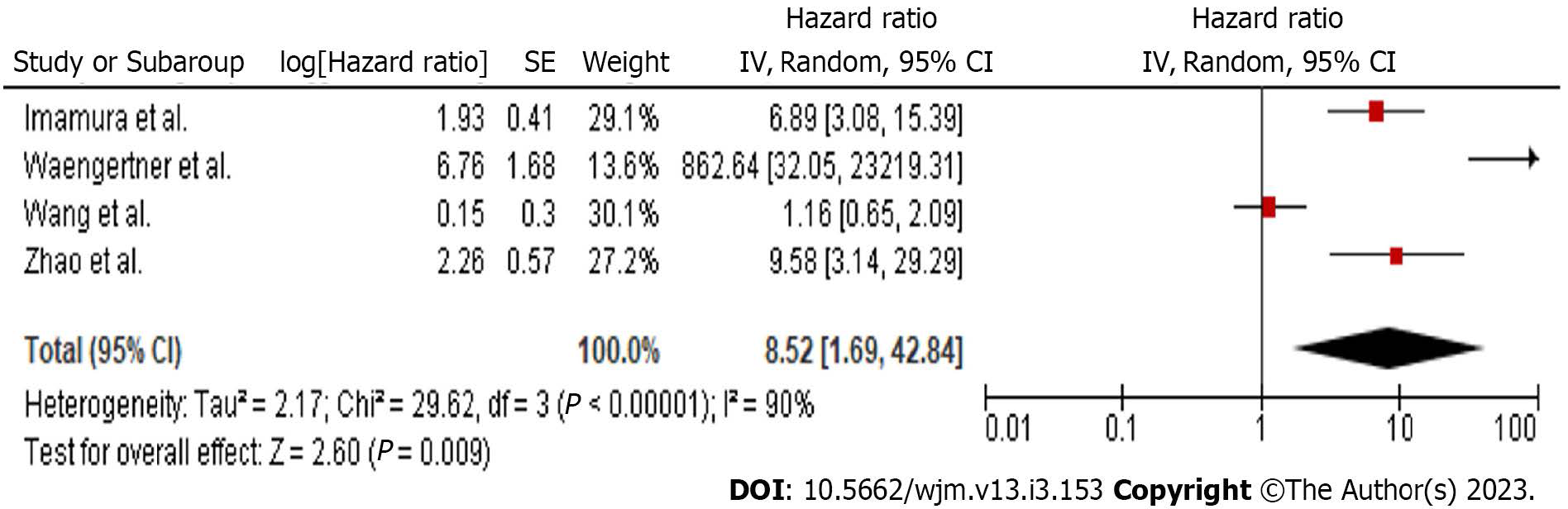
Data regarding the HR of DFS were extracted from 4 studies (Figure 2). Meta-analysis of these data showed a statistically significant (P = 0.009) hazard ratio of DFS (HR: 8.52, 95%CI: 1.69-42.84), in favour of the low MVD group. Since heterogeneity was significant (Q test P < 0.001, I2 = 90%), a RE model was applied.
Due to the high heterogeneity level, further statistical investigation was performed. The first step included a sensitivity analysis for the effect of each study separately. The overall heterogeneity level was not affected by any study. Meta-regression (Supplementary Tables) for the variables sample size, age and follow-up duration did not identify any statistically significant factor. Subgroup analysis regarding the number of study centres and the antibody used were identical to the above-mentioned sensitivity analysis. Analysis of studies implementing the Weidner MVD assessment method showed a statistically significant hazard ratio. Similarly, exclusion of the two studies which did not report blinded MVD evaluation did not influence heterogeneity. Further explanatory analyses (Supplementary Tables) included meta-regression of the primary outcome with the number of spots examined, the percentage of high-risk tumours, gastric and small intestine tumours, large size tumours (≥ 5 cm) and spindle cell malignancies. A significant correlation was not confirmed with any of the previously mentioned variables.
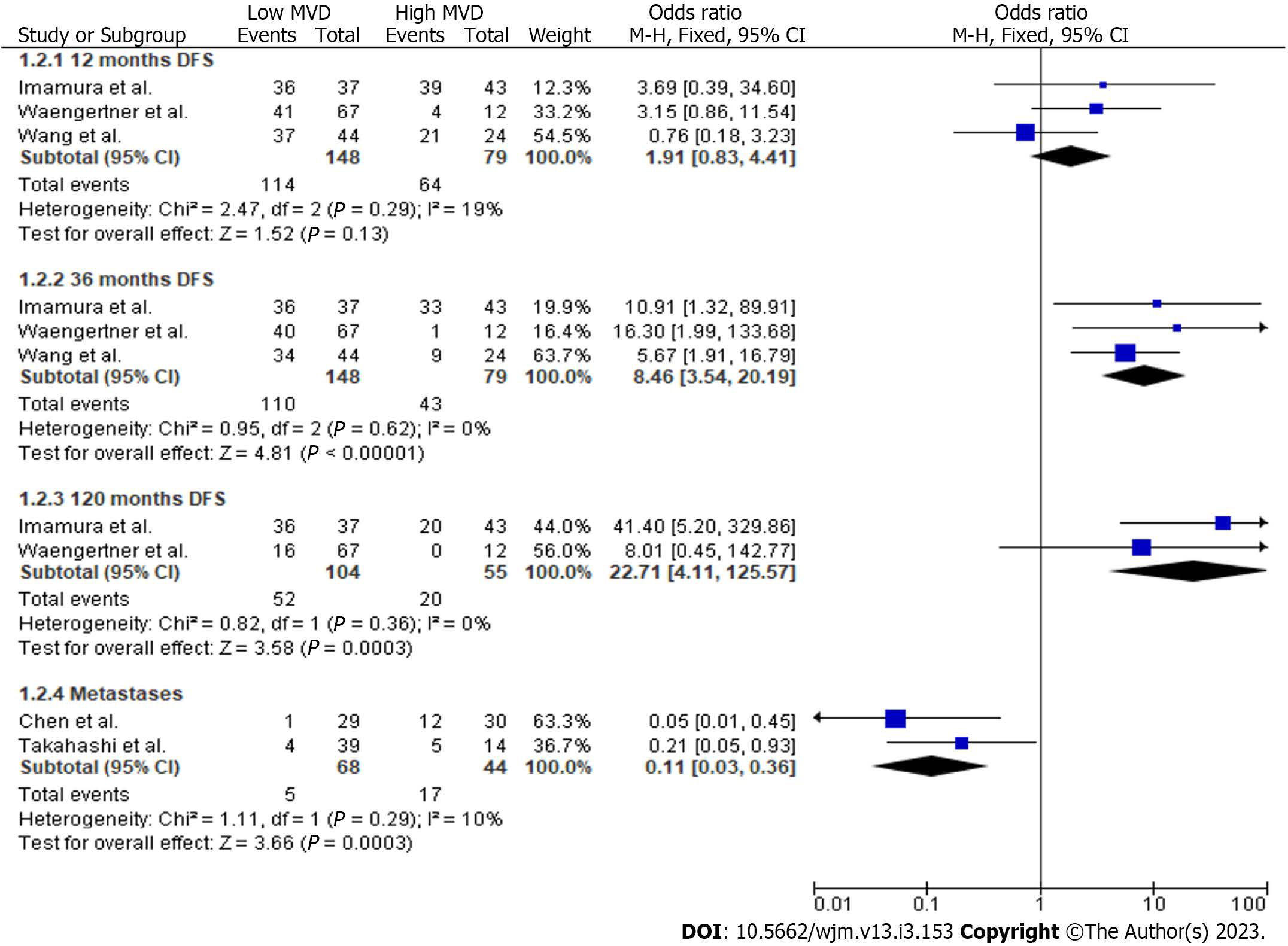
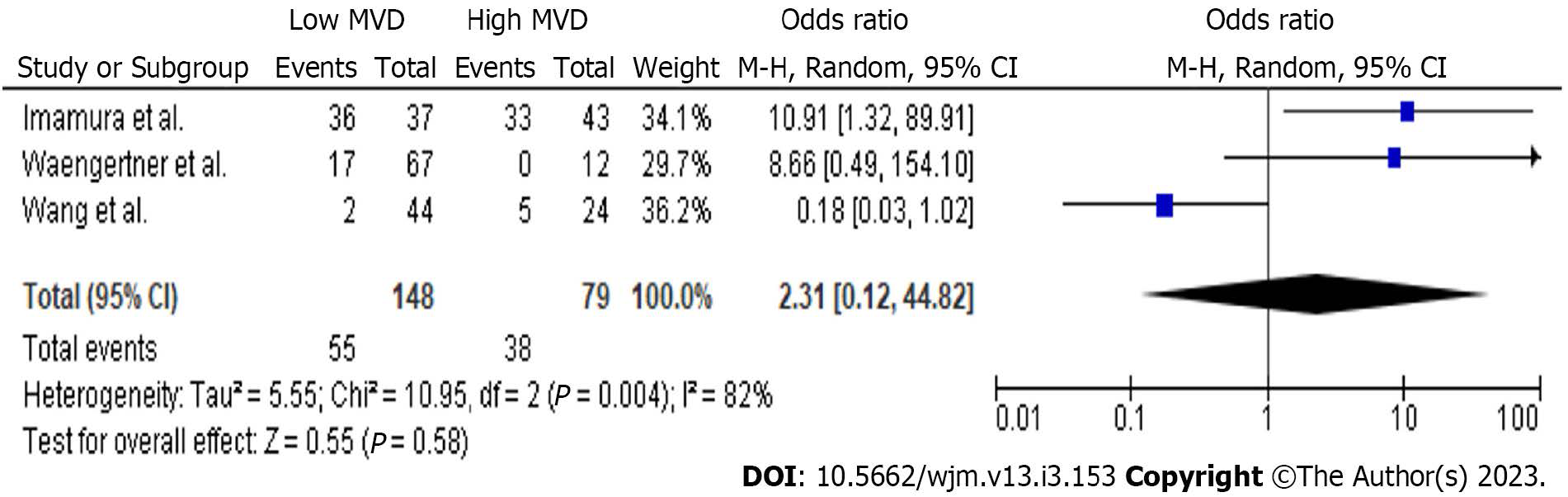
In total, 3 studies provided data concerning the comparison between high and low MVD groups for DFS at 12 mo (Figure 3). Meta-analysis of these data showed no statistically significant difference (P = 0.13) of DFS (OR: 1.91, 95%CI: 0.83-4.41) at 12 mo between the two study groups. However, a statistically significant difference (P < 0.001) of DFS (OR: 8.46, 95%CI: 3.54-20.19) in favour of the low MVD group was estimated at 36 mo. Although there was no difference (P = 0.58) of DFS rates (Figure 4) at 60 mo (OR: 2.31, 95%CI: 0.12-44.82), the low MVD group displayed a higher (P = 0.0003) DFS rate (Figure 3) at 120 mo (OR: 22.71, 95%CI: 4.11-125.57).
Finally, two studies provided data concerning the development of metastases (Figure 3). Meta-analysis of these data showed a statistically significant (P = 0.0003) lower ratio of metastases (OR: 0.11, 95%CI: 0.03-0.36) in the low MVD group. Heterogeneity was not significant in this analysis (Q test P = 0.29, I2 =10).
Visual inspection of the funnel plot suggested that studies by Wang et al[8] and Waengertner et al[23] lie beyond the 95%CI limits. Based on Egger’s test, there was no statistically significant publication bias (P = 0.517). Exclusion of the above-mentioned trials resulted in a statistically significant HR (7.71 95%CI: 4.02-14.8, P < 0.001) in favour of the low MVD group, though with a limited degree of heterogeneity (Q test P = 0.64, I2 = 0%).
Since GISTs are the most frequently occurring parenchymal neoplasms of the GI tract, research is focused on improving prognosis, introducing novel chemotherapeutic agents and refining current surgical approaches[11-15,33]. Since a few decades ago conventional chemotherapy and radiotherapy did not yield satisfactory results, a R0 resection of the tumor was considered the only therapeutic option for adequate long-term survival[33]. The discovery of the c-kit proto-oncogene mutation and ligand independent activation of the KIT receptor tyrosine kinase in GISTs, resulted in subsequent development of the tyrosine kinase inhibitors imatinib and sunitinib. This led to the onset of targeted molecular therapy of these neoplasms[11,13,33]. In a cohort study by Guller et al[34], the Surveillance, Epidemiology and End Results database was screened, with 5,138 GIST patients included. Data analysis revealed that recent advancements in treatment resulted in a significant increase in survival rates of both metastatic (3-year OS: 54.7%, cancer-specific survival: 61.9%) and non-metastatic disease (3-year OS: 88.6%, cancer-specific survival: 92.2%)[34].
It must be noted that despite the above-mentioned novelties, the mortality rate – particularly for the metastatic group - remains high. As a result, various risk grading tools have been developed to quantify the risk and provide accurate prognosis regarding survival endpoints. The study group of Fletcher et al[16] proposed the use of primary tumor size and mitotic count as grading parameters, which classified GISTs in four successive categories based on risk of aggressive behaviour. Due to a discrepancy in the metastatic risk between gastric and intestinal GISTs of different grading scores, the primary tumor location was also incorporated[17]. Exporting data from the SSG XVIII trial and using the Z9001 study as a validation tool, Joensuu et al[18] suggested that high tumor mitotic count, non-gastric location, large size and tumor rupture were significantly and independently related to a suboptimal recurrence-free survival (RFS).
Besides these grading tools, various independent tumor histopathological factors have been studied for their prognostic value. Specifically, GISTs with an epithelioid or mixed cell type have been associated with a significantly lower 5-year recurrence free survival, when compared with the respective spindle cell tumours (23% vs 49%)[35]. Moreover, according to Martin et al[36], high tumor cellularity was characterized as a significant poor RFS prognostic factor. Overexpression of Ki67, a nuclear marker abundant in proliferating cells, was found to have an increased incidence in the high risk group[19]. On the contrary, expression levels of p53 in GISTs were not significantly associated with clinical outcomes[37,38]. A pooled analysis from Luo et al[20] showed that an elevated neutrophil to lymphocyte ratio was associated with decreased DFS/RFS (HR: 2.18, 95%CI: 1.30-3.67). Furthermore, blood vessel invasion in the primary tumor was suggested as a predictor of liver metastasis and an aggressive behaviour[39].
Angiogenesis in GISTs is considered of the utmost importance for the neoplasm growth and metastasis process[8]. Proliferation of tumor vasculature is achieved via the paracrine release of angiogenic molecules and growth factors from tumor and stromal cells[8]. In a recent study by Zhao et al[25], the altered expression and secretion of proliferating and angiogenic agents like PI3K, Akt, PTEN, MMP9 and VEGF were directly associated with the DFS in GIST patients. Regarding VEGF, higher serum VEGF values were found in GIST patients when compared to healthy controls, while a positive VEGF expression rate was found in high risk groups[9]. A considerable number of clinical trials have correlated high VEGF levels with poor prognosis[9,23,25,40]. Another angiogenic factor, PDGF, has been related to GIST vasculogenesis at both theoretical and clinical levels[41,42]. As tumor angiogenesis often progresses through a hypoxic drive, researchers have correlated the expression levels of respective markers (e.g. HIF-1α) with survival outcomes[22,43]. Finally, vasculogenic mimicry (VM) which is a novel pattern of angiogenesis and defined as the formation of fluid conducting channels by highly invasive and dysregulated tumor cells, has also been studied in GISTs[44,45]. MMP-2 and MMP-9 were found to be contributing factors in VM; a significant association between VM, a high mitotic rate and liver metastases was confirmed[44].
Microvessel density is a direct method of quantifying and assessing intratumoral vasculature, and consequently angiogenesis potential. Due to the above-mentioned correlation between tumor vascularity and clinicopathological endpoints, various trials investigated GIST MVD. According to Imamura et al[8] and Waengertner et al[24], a statistically significant difference of survival rates in favour of low MVD GISTs was reported. Furthermore, Wang et al[9] stated that higher MVD values were found in high mitotic count and recurrence groups. Similar results were published by Zhao et al[25], where a significant hazard ratio for DFS was found. A retrospective study by Takahashi et al[23] suggested that while high MVD displayed a significant relationship with liver metastases, it did not influence the survival outcome at 10 years.
The results of our meta-analysis validated the significance of the MVD value effect on survival. Specifically, higher intratumoral MVD measurements were associated with a lower DFS rate at 36 and 120 mo of follow-up. These were not confirmed at the intermediate endpoints of 12 and 60 mo. The enhanced malignant potential of high vascularized GISTs was also depicted by the significant association among metastatic rate and MVD values.
The usefulness of these results involve extensive approaches in the clinical outcome prognosis[8-9,23,25,43]. Consolino et al[46] showed that in dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), imatinib-resistant tumors had an increased vessel density and permeability, with these attributes significantly correlated with MVD and MDD, respectively. Contrast enhanced endoscopic ultrasound has also demonstrated the ability to assess GIST vascularity, and subsequently, malignant potential[47]. Furthermore, since MVD is a direct tumor vasculature marker, it has been used as an indicator of the angiogenesis inhibition, as well as the overall response to novel medical treatment[48].
Besides GIST, MVD assessment has been extensively researched as a means of solid tumor vasculature quantification. Researchers have attempted to identify and estimate the presence of a correlation between microvessel density and survival outcomes in malignancies of the prostate[49], cervix[50], ovaries[51], breast[52], pancreas[53], kidney[54] and lung[55]. Moreover, in two recent meta-analyses from our study group concerning cutaneous melanoma and patients with differentiated thyroid cancer, high intratumoral MVD was related to poor survival outcomes[56,57]. According to current literature, the majority of studies validate the presence of a significant correlation between intratumoral MVD and prognosis in solid tumors[58]. However, a discrepancy exists since a minority of publications question the significance of the above-mentioned correlation[58].
Heterogeneity of various clinicopathological endpoints (survival, metastasis, local recurrence, response to treatment, etc.) in the reported results has been widely attributed to certain methodological variations[58]. Among these, selection of the hot-spot examination technique is the most important, due to the variability rates and dependence on the assessor training and experience[59,60]. Furthermore, the MVD assessment technique includes various modifications, such as Weidner’s hot-spot method[21], the lumen method[61], Chalkley’s method[62] and the computerized image analysis system[63]. Another field of methodological diversity is considered the selection of the endothelial marker, where a variety of choices such as pan-endothelial cell markers (CD31, CD34, vWF) and selective for the activated endothelium factors (CD105) are described. Finally, technical discrepancies are also reported in other methodological fields, such as type of fixative, vasculature estimation, the MVD cut-off value, level of magnification and overall field size[58]. Our study highlighted this heterogeneity; the use of different assessment methods and definitions of high and low MVD tumours prohibited the calculation of a pooled cut-off point.
Certain limitations should be taken into consideration, prior to appraising results of the present meta-analysis. Firstly, significant levels of heterogeneity were identified; despite conducting explanatory analyses, the validity of study conclusions may be compromised. Furthermore, all eligible studies were designed using a retrospective methodology and included a small sample size, thus allowing the introduction of bias. Moreover, diversity among included studies regarding methodological characteristics of the MVD assessment technique should be also acknowledged. The implementation of different assessment methods and different cut-off points prohibited the strict definition of high and low MVD GISTs. Furthermore, heterogeneity in terms of tumor location, risk classification, histopathological characteristics and cell subtype jeopardized the significance of our outcomes. Inconsistency in surgical or medical treatment could also be an influencing factor on survival endpoints. Finally, since in most trials the raw survival data had to be extracted and reconstructed from the provided Kaplan-Meier curves, a certain amount of bias was introduced, although this procedure has been extensively described and applied in the literature.
To the best of our knowledge, the present study is the first attempt to provide an overall estimation of the impact of MVD on survival rates of GIST patients. According to the pooled results of the meta-analysis, GIST allocation between high and low MVD values significantly influenced the DFS hazard ratio. Moreover, high MVD GISTs demonstrated a statistically significant lower DFS at 36 and 120 mo of follow-up, while no difference was found at 12 and 60 mo. Moreover, high MVD tumours were associated with a significantly higher rate of metastases. Based on the above-mentioned results and given several limitations, further studies with a larger sample size and adequate methodology are required.
Several clinical and histopathologic factors have been investigated as prognostic indicators of survival in patients with gastrointestinal stromal tumours (GISTs).
Microvessel density (MVD) has been extensively applied as a direct method of tumour vascularity assessment.
This meta-analysis attempted to estimate the pooled effect of tumoral vascularity based on MVD assessment on the survival of patients with GISTs.
The present meta-analysis adhered to the Systematic reviews and Meta-Analyses guidelines and the Cochrane Handbook for Systematic Reviews of Interventions.
Low vascularized tumours were associated with improved pooled disease-free survival. GISTs with lower MVD values displayed a reduced risk of metastases.
MVD is significantly associated with the survival outcomes of GIST patients.
Further prospective randomized controlled trials are required to delineate the exact correlation between MVD and prognosis outcomes in GIST patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo F, China; Shi H, China S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 2. | Diamantis A, Samara AA, Symeonidis D, Baloyiannis I, Vasdeki D, Tolia M, Volakakis G, Mavrovounis G, Tepetes K. Gastrointestinal stromal tumors (GISTs) and synchronous intra-abdominal malignancies: case series of a single institution's experience. Oncotarget. 2020;11:4813-4821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Min KW, Leabu M. Interstitial cells of Cajal (ICC) and gastrointestinal stromal tumor (GIST): facts, speculations, and myths. J Cell Mol Med. 2006;10:995-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 5. | Laurini JA, Carter JE. Gastrointestinal stromal tumors: a review of the literature. Arch Pathol Lab Med. 2010;134:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Yamamoto H, Oda Y. Gastrointestinal stromal tumor: recent advances in pathology and genetics. Pathol Int. 2015;65:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 8. | Imamura M, Yamamoto H, Nakamura N, Oda Y, Yao T, Kakeji Y, Baba H, Maehara Y, Tsuneyoshi M. Prognostic significance of angiogenesis in gastrointestinal stromal tumor. Mod Pathol. 2007;20:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Wang TB, Qiu WS, Wei B, Deng MH, Wei HB, Dong WG. Serum vascular endothelial growth factor and angiogenesis are related to the prognosis of patients with gastrointestinal stromal tumors. Ir J Med Sci. 2009;178:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Poveda A, García Del Muro X, López-Guerrero JA, Cubedo R, Martínez V, Romero I, Serrano C, Valverde C, Martín-Broto J; GEIS (Grupo Español de Investigación en Sarcomas/Spanish Group for Sarcoma Research). GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev. 2017;55:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Duffaud F, Le Cesne A. Recent advances in managing gastrointestinal stromal tumor. F1000Res. 2017;6:1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | El-Menyar A, Mekkodathil A, Al-Thani H. Diagnosis and management of gastrointestinal stromal tumors: An up-to-date literature review. J Cancer Res Ther. 2017;13:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 13. | Szucs Z, Thway K, Fisher C, Bulusu R, Constantinidou A, Benson C, van der Graaf WT, Jones RL. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21-iii26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 15. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Conrad EU 3rd, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM 3rd, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Schuetze S, Schupak KD, Schwartz HS, Tap WD, Wayne JD, Bergman MA, Scavone J. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:758-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 229] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 16. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 17. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 18. | Joensuu H, Eriksson M, Hall KS, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Sarlomo-Rikala M, Nilsson B, Sihto H, Ballman KV, Leinonen M, DeMatteo RP, Reichardt P. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer. 2014;120:2325-2333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Zhou Y, Hu W, Chen P, Abe M, Shi L, Tan SY, Li Y, Zong L. Ki67 is a biological marker of malignant risk of gastrointestinal stromal tumors: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e7911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Luo XF, Zhou LH. Prognostic significance of neutrophil to lymphocyte ratio in patients with gastrointestinal stromal tumors: A meta-analysis. Clin Chim Acta. 2018;477:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4088] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 22. | Chen WT, Huang CJ, Wu MT, Yang SF, Su YC, Chai CY. Hypoxia-inducible factor-1alpha is associated with risk of aggressive behavior and tumor angiogenesis in gastrointestinal stromal tumor. Jpn J Clin Oncol. 2005;35:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Takahashi R, Tanaka S, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Expression of vascular endothelial growth factor and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncology. 2003;64:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Waengertner LE, Meurer L, Cerski MR. Microvessel Density (Chalkley Method) in a Series of 79 Gastrointestinal Stromal Tumors. Gastroenterology Res. 2011;4:252-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Wang Q, Deng X, Zhao Y. Altered angiogenesis gene expression in gastrointestinal stromal tumors: potential use in diagnosis, outcome prediction, and treatment. Neoplasma. 2012;59:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47077] [Article Influence: 2942.3] [Reference Citation Analysis (0)] |
| 27. | Wells G, Shea B, O’Connell D, Peterson je, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. [DOI] [Full Text] |
| 28. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 29. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4946] [Article Influence: 274.8] [Reference Citation Analysis (0)] |
| 30. | Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 1705] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 31. | Bormann I. DigitizeIt 2.2. Digitizer Software—Digitize a Scanned Graph or Chart Into (x, y) Data, 2016. |
| 32. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6874] [Article Influence: 343.7] [Reference Citation Analysis (0)] |
| 33. | Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 428] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 34. | Güller U, Tarantino I, Cerny T, Schmied BM, Warschkow R. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer. 2015;15:557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20:3898-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 36. | Martín J, Poveda A, Llombart-Bosch A, Ramos R, López-Guerrero JA, García del Muro J, Maurel J, Calabuig S, Gutierrez A, González de Sande JL, Martínez J, De Juan A, Laínez N, Losa F, Alija V, Escudero P, Casado A, García P, Blanco R, Buesa JM; Spanish Group for Sarcoma Research. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol. 2005;23:6190-6198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Wallander ML, Layfield LJ, Tripp SR, Schmidt RL. Gastrointestinal stromal tumors: clinical significance of p53 expression, MDM2 amplification, and KIT mutation status. Appl Immunohistochem Mol Morphol. 2013;21:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Neves LR, Oshima CT, Artigiani-Neto R, Yanaguibashi G, Lourenço LG, Forones NM. Ki67 and p53 in gastrointestinal stromal tumors--GIST. Arq Gastroenterol. 2009;46:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Yamamoto H, Kojima A, Miyasaka Y, Imamura M, Nakamura N, Yao T, Tsuneyoshi M, Oda Y. Prognostic impact of blood vessel invasion in gastrointestinal stromal tumor of the stomach. Hum Pathol. 2010;41:1422-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Basilio-de-Oliveira RP, Pannain VL. Prognostic angiogenic markers (endoglin, VEGF, CD31) and tumor cell proliferation (Ki67) for gastrointestinal stromal tumors. World J Gastroenterol. 2015;21:6924-6930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Trairatphisan P, Wiesinger M, Bahlawane C, Haan S, Sauter T. A Probabilistic Boolean Network Approach for the Analysis of Cancer-Specific Signalling: A Case Study of Deregulated PDGF Signalling in GIST. PLoS One. 2016;11:e0156223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Nannini M, Astolfi A, Paterini P, Urbini M, Santini D, Catena F, Indio V, Casadio R, Pinna AD, Biasco G, Pantaleo MA. Expression of IGF-1 receptor in KIT/PDGF receptor-α wild-type gastrointestinal stromal tumors with succinate dehydrogenase complex dysfunction. Future Oncol. 2013;9:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Takahashi R, Tanaka S, Hiyama T, Ito M, Kitadai Y, Sumii M, Haruma K, Chayama K. Hypoxia-inducible factor-1alpha expression and angiogenesis in gastrointestinal stromal tumor of the stomach. Oncol Rep. 2003;10:797-802. [PubMed] |
| 44. | Sun B, Qie S, Zhang S, Sun T, Zhao X, Gao S, Ni C, Wang X, Liu Y, Zhang L. Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol. 2008;39:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Zhao H, Gu XM. Study on vasculogenic mimicry in malignant esophageal stromal tumors. World J Gastroenterol. 2008;14:2430-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Consolino L, Longo DL, Sciortino M, Dastrù W, Cabodi S, Giovenzana GB, Aime S. Assessing tumor vascularization as a potential biomarker of imatinib resistance in gastrointestinal stromal tumors by dynamic contrast-enhanced magnetic resonance imaging. Gastric Cancer. 2017;20:629-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Sakamoto H, Kitano M, Matsui S, Kamata K, Komaki T, Imai H, Dote K, Kudo M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc. 2011;73:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Wang TB, Wei XQ, Lin WH, Shi HP, Dong WG. The inhibition of Endostar on the angiogenesis and growth of gastrointestinal stromal tumor xenograft. Clin Exp Med. 2012;12:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Miyata Y, Sakai H. Reconsideration of the clinical and histopathological significance of angiogenesis in prostate cancer: Usefulness and limitations of microvessel density measurement. Int J Urol. 2015;22:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Saijo Y, Furumoto H, Yoshida K, Nishimura M, Irahara M. Clinical Significance of Vascular Endothelial Growth Factor Expression and Microvessel Density in Invasive Cervical Cancer. J Med Invest. 2015;62:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | He L, Wang Q, Zhao X. Microvessel density as a prognostic factor in ovarian cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Mohammed ZM, Orange C, McMillan DC, Mallon E, Doughty JC, Edwards J, Going JJ. Comparison of visual and automated assessment of microvessel density and their impact on outcome in primary operable invasive ductal breast cancer. Hum Pathol. 2013;44:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Jureidini R, da Cunha JE, Takeda F, Namur GN, Ribeiro TC, Patzina R, Figueira ER, Ribeiro U Jr, Bacchella T, Cecconello I. Evaluation of microvessel density and p53 expression in pancreatic adenocarcinoma. Clinics (Sao Paulo). 2016;71:315-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Cheng SH, Liu JM, Liu QY, Luo DY, Liao BH, Li H, Wang KJ. Prognostic role of microvessel density in patients with renal cell carcinoma: a meta-analysis. Int J Clin Exp Pathol. 2014;7:5855-5863. [PubMed] |
| 55. | Zhai XJ, Cheng HR, Long HL, Mao WK, Cao L, Xiao BR, Li RQ. Effects of docetaxel plus three-dimensional conformal radiation therapy on microvessel density and apoptosis expression in local advanced squamous non-small-cell lung cancer. Genet Mol Res. 2015;14:5399-5406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Perivoliotis K, Ntellas P, Dadouli K, Koutoukoglou P, Ioannou M, Tepetes K. Microvessel Density in Patients with Cutaneous Melanoma: An Up-to-Date Systematic Review and Meta-Analysis. J Skin Cancer. 2017;2017:2049140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Perivoliotis K, Samara AA, Koutoukoglou P, Ntellas P, Dadouli K, Sotiriou S, Ioannou M, Tepetes K. Microvessel density in differentiated thyroid carcinoma: A systematic review and meta-analysis. World J Methodol. 2022;12:448-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 58. | Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566-1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E, Weidner N, Harris AL, Dirix LY. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 347] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 60. | Ranieri G, Gasparini G. Surrogate markers of angiogenesis and metastasis. Methods Mol Med. 2001;57:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Takagi K, Takada T, Amano H. A high peripheral microvessel density count correlates with a poor prognosis in pancreatic cancer. J Gastroenterol. 2005;40:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL. Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol. 1995;177:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 306] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 63. | Beliën JA, Somi S, de Jong JS, van Diest PJ, Baak JP. Fully automated microvessel counting and hot spot selection by image processing of whole tumour sections in invasive breast cancer. J Clin Pathol. 1999;52:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |