Published online Sep 6, 2017. doi: 10.5527/wjn.v6.i5.221
Peer-review started: March 28, 2017
First decision: May 4, 2017
Revised: July 18, 2017
Accepted: July 21, 2017
Article in press: July 23, 2017
Published online: September 6, 2017
Processing time: 159 Days and 22.7 Hours
Chronic kidney disease is a prevalent condition that affects millions of people worldwide and is a major risk factor of cardiovascular morbidity and mortality. The main diseases that lead to chronic kidney disease are frequent entities as diabetes mellitus, hypertension and glomerulopathies. One of the clinical markers of kidney disease progression is proteinuria. Moreover, the histological hallmark of kidney disease is sclerosis, located both in the glomerular and in the interstitial compartments. Glomerulosclerosis underscores an irreversible lesion that is clinically accompanied by proteinuria. In this regard, proteinuria and glomerular sclerosis are linked by the cell that has been conserved phylogenetically not only to prevent the loss of proteins in the urine, but also to maintain the health of the glomerular filtration barrier: The podocyte. It can then be concluded that the link between proteinuria, kidney disease progression and chronic kidney disease is mainly related to the podocyte. What is this situation due to? The podocyte is unable to proliferate under normal conditions, and a complex molecular machinery exists to avoid its detachment and eventual loss. When the loss of podocytes in the urine, or podocyturia, is taking place and its glomerular absolute number decreased, glomerulosclerosis is the predominant histological feature in a kidney biopsy. Therefore, tissular podocyte shortage is the cause of proteinuria and chronic kidney disease. In this regard, podocyturia has been demonstrated to precede proteinuria, showing that the clinical management of proteinuria cannot be considered an early intervention. The identification of urinary podocytes could be an additional tool to be considered by nephrologists to assess the activity of glomerulopathies, for follow-up purposes and also to unravel the pathophysiology of podocyte detachment in order to tailor the therapy of glomerular diseases more appropriately.
Core tip: Podocytes are cells that do not proliferate under normal conditions. Their loss in the urine, a process known as podocyturia, antedates proteinuria. When the number of podocytes per glomerulus is less than 80%, glomerulosclerosis is triggered and if not stopped, it will eventually lead to chronic kidney failure and end-stage kidney disease. A prompt and standardized assessement of podocyturia should lead to better results related to glomerular disease outcomes.
- Citation: Trimarchi H. Podocyturia: Potential applications and current limitations. World J Nephrol 2017; 6(5): 221-228
- URL: https://www.wjgnet.com/2220-6124/full/v6/i5/221.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i5.221
The podocyte is a well-differentiated cell that forms the glomerular filtration barrier together with the glomerular basement membrane and the adjacent fenestrated endothelial cell. Among the main functions of podocytes, two of them arise as the most relevant: Podocytes not only offer an efficient structural support to the capillary loop in order to assure the constant filtration of plasma, but podocytes also impede the loss of proteins in the urine during the constant process of plasma filtration[1]. Podocytes perform their functions attached to the glomerular basement membrane, of which they synthesize most of its components and maintain its normal structure. Located in Bowman’s space, they perform their functions facing a constant counterstream flow of plasma. This situation implies that in order to remain in their place, under normal circumstances they can eventually undergo mitosis but cannot undergone cytokinesis. This phenomenom results in maladapted binucleated podocytes[2]. Therefore, an efficient machinery is settled in order to avoid the loss of podocytes in the urine, a phenomenom denominated podocyturia[3].
When podocytes are lost, the glomerular basement membrane becomes denuded and proteins start to leak to Bowman’s space and enter the lumen of the proximal tubule. Therefore, neighbour podocytes are to extend their foot processes in order to cover the denuded area[3]. When the number of podocytes become critical, matrix expansion and glomerulosclerosis start to take place[4,5]. It appears that glomerulosclerosis starts to occur when the loss of podocytes per glomerulus is approximately between 20% and 40%. Above this number, the glomerulus starts the point of no return and is destined to obliteration. It is well known that when the number of glomeruli is less than 50%, renal insufficiency ensues[4,5]. This means that the decreased number of podocytes is followed by proteinuria, a marker of progression of chronic kidney disease and associated with cardiovascular morbidity and mortality[6]. In summary, a prompt diagnosis of podocyturia, a better management of this situation, and more efficacious pathophysiologically-based therapeutic interventions should lead to a decrease in chronic kidney disease progression and in the prevalence of end-stage renal disease.
The podocyte mass is small compared of the entire kidney mass. It is highly differentiated and as such, does not undergo cellular division. It forms part of the glomerular filtration barrier, together with the glomerular basement membrane and the adjacent fenestrated endothelium[1-3,7]. They contain a large cell body, with prominent nucleus and nucleolus, and a rich Golgi apparatus and endoplasmic reticulum, engaged in the large number of unique proteins they synthesize. Podocytes possess numerous foot processes that interdigitate with neighbouring ones. Adjacent foot processes form the slit diaphragms, structures that determine the quality of the filtered plasma flow[1-3]. They accomplish many functions, highly preserved among species.
Podocytes are coated by a sialic acid-rich polyanionic glycocalix mainly composed of podocalyxin and among its many functions contributes to the impedement of albumin filtration[8]. Considering the fact that the normal serum albumin concentration is between 3.5 and 5 g/dL, and that approximately 200 L of blood are being filtered every day, then podocytes efficiently cope with the task of avoiding the daily urinary loss of 9 kilograms of albumin. Podocytes also contribute with the synthesis of the major number of components of the glomerular basement membrane, as collagen type IV, and to its maintenance[1,7]. They also secrete vascular endothelial growth factor (VEGF) in a paracrine fashion, which is critical for the formation of fenestrae in the capillary endothelium, increasing the amount of filtered plasma[9]. Podocytes also contain toll-like receptors, as well as receptors for complement and for the Fc portion of immunoglobulins, therefore behaving as antigen presenting cells[10].
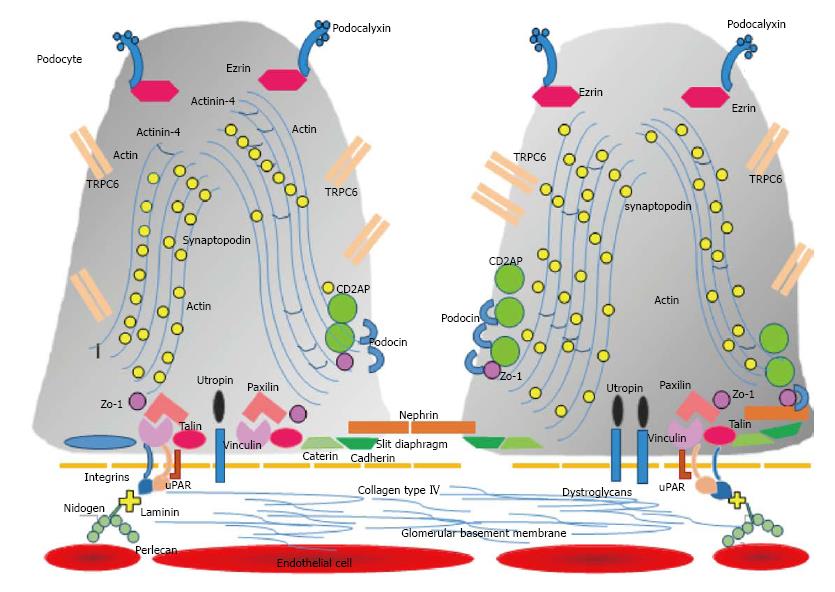
Within the cell body and the primary cell processes, microtubules and intermediate filaments dominate, while within the foot processes, longitudinally bundles of microfilaments are encountered. Actin, myosin, and α-actinin are associated with these bundles[11,12]. A chain of proteins containing vinculin and talin then link the actin filaments through integrins to fibronectin and laminin located in the glomerular basement membrane (Figure 1). The cytoskeleton of podocytes and its interaction to the glomerular basement membrane is responsible not only for the fixation of the cell to the matrix, but also for the regulation of the capillary width, a critical factor that will determine the glomerular filtration rate.
Podocytes contain a large number of proteins that exert many functions (Figure 1). For example, podocytes secrete the proteins and constituents of the slit diaphragm, and a histological and physiological unit exists between the slit diaphragm and the adjacent foot processes. As podocytes contain an important contractile machinery, their relaxation and contraction determine not only the width of the slit diaphragm, but also the kind of filtered molecules. Many proteins play this role, as actin, α-actinin 4, and synaptopodin[13]. This network of contractile proteins exert their function due to their link to surface cellular proteins located next to or on the cytoplasmic membrane. Actin is in active connection with CD2AP (CD2-associated protein), a regulatory protein that translates the information that podocin, a protein located in the foot process that also forms part of the slit diaphragm with nephrin[14]. Actin also receives the information that calcium channels receive from the outside, like TRPC6 (ion-channel protein transient receptor potential cation channel 6)[15]. Podocalyxin is a glycocalix located sialoglycoprotein that contributes to the negative charge of the cell surface and also acts a receptor[8]. Finally, actin is determined to relax or contract depending on the information that may provence from the basal side of the cell, especially that concerned with the glomerular basement membrane and the endothelial cell. This is undertaken mainly by its interaction with urokinase-type plasminogen activator receptor (uPAR), which in turn operates with certain integrins[16]. These integrins anchor podocytes to the glomerular basement membrane. When the assemblement between uPAR and integrins αVβ3 or α3β1 become activated, then actin is determined to contract. The intensity and duration of this interaction jeopardizes the attachment of the podocyte to the glomerular basement membrane. At this initial stage, foot processes efface and lose their fine digitations and the capacity to retain albumin from being lost in the urine, in order to stay attached to the surrounding matrix[11,12]. This podocyte stress is also expressed by its lower capacity to secrete the necessary amount of proteins to achieve the normal functions of the cell, and also in the lower quality of these synthesized molecules, a process known as granular endoplasmic reticulum stress[17].
As podocytes adhere to the glomerular basement membrane by their processes, the detachment of a podocyte occurs in several stages. Podocytes begin to lose their foot processes connections, while others are still anchored to the glomerular basement membrane. It is important to note that when a podocyte is damaged, the adjacent ones start to follow the same structural and functional process[18]. Concerning focal detachments, it does not necessarily imply that the cell will undergo a detachment, or that will irreversible undertake a process of disconnection from the basement membrane. Partial detachments are usually reversible to a certain degree[11,12].
During the process of detachment, podocytes regularly display shape modifications, foot process effacement being the hallmark at this stage. The vast majority of these cells appear to be viable with normal morphology and organelle distribution in the cell body[2,11,12]. However, Voguelman et al have encountered that in addition to the viable detached podocytes, apoptosis occurred in approximately 50% of the cells[2]. In this regard, Kriz et al[11,12] state that this discrepancy lies on the method employed to assess podocyturia. In any case, when a podocyte detaches, contiguous podocytes commence to build links among themselves, forming a new interconnected net. According to Kriz, binucleate cells can be encountered, confirming previous observations reporting binucleated urinary podocytes[11,19,20]. Detachment is frequently reported to occur in podocytes with effaced foot processes.
A particular kind of detachment affects podocytes that are located at the opening of Bowman’s capsule to the proximal tubule (tubular pole). This is the zone where the highest flow velocities of filtrate have been recorded. Such podocytes are in extreme danger of being lost by detachment. Of note, and opposed to what occurs with podocytes located elsewhere in the glomerulus, just podocytes protruding into the urinary pore have been encountered to display foot process effacement. Therefore, foot process effacements could be the result of a cell reaction secondary to mechanical stress that antedates and may herald a potential detachment. Podocytes found in the process of detachment employing transmission electron micrograph images of experimental models are viable and show foot process effacement. In this setting, these findings may confirm that a foot process effacement precedes detachment. The intricate and complex organization of the cytoskeleton associated with foot process effacements is in conflict with the usual thought that this morphological feature is just a reflection of certain cell injury. Foot process effacement is reversible, even when widespread. Thus, it appears to represent a specific, reactive phenotype of the podocyte, which may facilitate the atachment the glomerular basement membrane and may decrease the risk of detachment, at least transiently. Foot process effacement not always results in success, as indicated by the widespread presence of urinary podocytes in virtually all sorts of glomerular disorders, either primary or secondary[3,11].
As mentioned, podocytes attach via integrins to the glomerular basement membrane. uPAR is dispensible for normal renal function but required for podocyte motility in cell culture and for the development of foot process effacement and proteinuria in vivo[16]. Therefore, the upragulated expression of uPAR on podocytes occur under pathologic conditions. These effects of uPAR as drivers of podocyte contraction and foot process effacement are independent of its major ligand urokinase or uPA, but require the activation of αVβ3-integrin within lipid rafts and αVβ3-integrin-mediated binding to high-affinity ligands such as vitronectin[21].
Noteworthy, one of the agonists of uPAR, uPA, converts plasminogen to plasmin. Plasmin is involved not only as a fibrinolytic factor, but also acts in fibrotic processes, stimulating water and sodium reabsorption via distal tubule ENa+ channels activation[22]. In nephrotic urines, plasmin is elevated due to the upregulated action of uPA, and its receptor uPAR is highly expressed in urinary podocytes[21,22]. These findings suggest that uPAR is involved in podocyte detachment, probably via activation of αVβ3-integrin[16,23,24]. Employing immunofluorescent microscopy, we have demonstrated that in patients with IgA nephropathy and Fabry disease, uPAR is present in the cytoplasm of urinary podocytes. In the case of IgA nephropathy, the number of uPAR+ detached podocyte is significantly higher than controls and in patients with glomerular sclerotic lesions compared to those without (M1E0S1T0 vs M1E0S0T0 according to Oxford classification)[24]. Moreover, by western blot analysis as well as by PCR techniques we have confirmed the presence of elevated concentrations of uPAR in Fabry podocytes[23]. In this disease, we have found that urinary uPAR+ podocytes are significantly higher in those without therapy[23]. Finally, in Fabry subjects increased levels of urinary αVβ3-integrins have been encountered[25]. In this regard, our group suggested that a probable pathway of podocyte detachment under pathological conditions is via the interaction between uPAR and αVβ3-integrins. This pathway may not be exclusive to one glomerulopathy, while more than one pathway could also be involved in one glomerular disease[23].
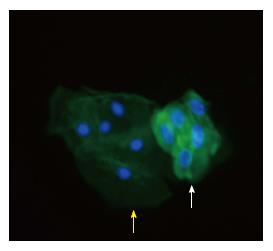
In order to avoid the deterioration of the urine, we collect a fresh three-hour retained urine on site and this sample is immediately processed as previously described[26]. Podocyturia can be diagnosed in different ways with different methods. Urinary podocytes can be identified with colorimetric qualitative methods, as immunohistochemistry or with immunofluorescence[26,27] (Figure 2). Different podocyte proteins can be targeted with monoclonal antibodies and consequently identified with the proper microscopy method employed[28]. Podocytes can also be identified by polymerase chain reaction (PCR), employing primers that belong to the different podocyte proteins. With this technique, one can quantify the levels of a certain specific podocyte protein in glomerular disorders[29]. Podocytes can also be identified by flow cytometry[29].
Our group is now studying the identification of three different kind of podocyte proteins (synaptopodin, uPAR and podocalyxin) in urinary podocytes by immunofluorescence, in parallel with their mRNA correlate detected by PCR and with flow cytometry studies. All these methods of diagnosis are not already employed as routine diagnostic tools, have not been standardized, are to be validated, and are expensive, among other limitations, as discussed below. At the present time, we believe that the main usefulness of studying podocyturia is to unveil the different mechanisms of podocyte injury and detachment. Finally, with respect to the number of podocytes collected in a urine sample, and in agreement with other authors, we believe expressing the number of podocytes per gram of urinary creatinine confers a qualitative common nominator for correction purposes.
Interestingly, podocyturia is also a physiologic process[26,30]. This statement may partially explain why human beings lose renal function as they age. However, considering the fact that glomerulosclerosis and glomerlus obliteration is a consequence of tissular depletion, the finding of podocytes in the urine above control levels heralds a grim outcome if no intervention is followed by. In this regard, one can state that glomerulosclerosis and most cases of chronic kidney disease are caused by podocyte depletion.
In the adult human kidney, it is estimated that approximately 500 podocytes populate each glomerular tuft, and their turnover rate is very slow[2,4]. As mentioned, it has been demonstrated that when a glomerulus loses between 20%-40% of its podocyte content (around 100-200 podocytes), it renders itself to sclerosis and obliteration[2,4,5]. According to histologic observations, this podocyte loss could be uneven as to the distribution of glomerular structures, being more frequently found at the sub-capsular area, where obliterated glomeruli are encountered in kidney tissue due to non-glomerular diseases. Moreover, if the loss of 100-200 podocytes can lead to one obliterated glomerulus, in one year around 1460 glomeruli would be lost, and 14600 in 10 years-time, again in normal conditions. However, in the aged general population this intriguing reality that shows a continuous physiological loss of this non-substitutable cell is not accompanied by severe proteinuria. On the other hand, renal function decline is the rule in the normal aging process. This could be due to the expansion of neighbouring podocytes covering the denuded glomerular basement membrane and tempering the degree of albumin filtration. Another provoking explanation could be- as mentioned above- the replacement of these podocytes by local pluripotential cells located in the parietal compartment of Bowman’s capsule[31]. Some parietal epithelial cells express stem cell markers as CD24 and CD133, and may act as renal progenitor cells. As they approach the vascular pole, they gradually lose these stem cell markers, acquire podocyte specific markers, and may migrate onto the glomerular basement membrane[31]. The ability of the podocyte to migrate within the glomerular tuft has been elegantly demonstrated using time lapse fluorescence microscopy[32].
However, not all replacement podocytes are derived from resident renal cells. Transplant studies in which male recipients received female kidney allografts, have demonstrated the presence of podocytes derived from the recipient male in approximately 50% of cells, suggesting a bone marrow derived stem cell source[33]. Moreover, parietal epithelial cells express podocyte markers in several experimental models of glomerulonephritis[34]. This phenotype switching may also portrend adverse consequences for the glomerulus, possibly contributing to the development of adhesions and scars between the glomerular tuft and Bowman’s basement membrane. Although some in vitro reports have identified these cells, no proven in vivo documentation has been published yet. If there is a lack of coverage, ballooning of the capillary loop ensues, synechial attachment of denuded areas to Bowman’s capsule follows and focal and segmental glomerulosclerosis develops[3,11,12].
Noteworthy, some aspects of physiologic podocyte turn over and substitution deserve to be mentioned. There is converging evidence from experimental and clinical data suggesting that adult stem cells within Bowman’s capsule can rescue some of this loss[31,35]. Glomerular epithelial stem cells generate podocytes during kidney growth and regenerate podocytes after injury, thus explaining why various glomerular disorders undergo remission occasionally. This regenerative process, however, is often inadequate because of inefficient proliferative responses by glomerular epithelial stem cells with aging or in the setting of focal segmental glomerulosclerosis. Alternatively, an excessive proliferative response by glomerular epitelial stem cells after podocyte injury can generate new lesions such as extracapillary crescentic glomerulonephritis or collapsing glomerulopathies. In addition, glomerular epithelial stem cells display a different regenerative potential at distinct stages of life, exhibiting the highest regenerative potential through adolescence[36], which might explain why glomerular disorders have a better prognosis during childhood whereas focal and segmental glomerulosclerosis is more frequent at an older age[31,35].
Pharmacological targeted at podocyte detachment mechanisms could decrease the progression and consequently the prevalence of chronic kidney diseases[16]. Once a kidney disorder in general or a glomerular disease in particular is diagnosed, a baseline level of podocyturia may prove to be useful, as a complement to the routine parameters to assess renal function and injury, such as proteinuria, hematuria, serum creatinine and glomerular filtration rate. Once therapy is started, a longitudinal profile of podocyturia could contribute to assess the case. However, a decrease in the amount of podocyturia could imply two completely different stages in a certain disease, as previously suggested. In an initial stage of a clinical setting, the amount of podocyturia could be low, and as it progresses, the amount would increase up to a point of no return, when the absolute number of cells would start to decrease due to the disease process itself and not to an improvement of the disease. In this regard, at the initial stages, a low podocyte count would be accompanied by a normal or higher glomerular filtration rate than at a more advanced stage. The same applies for proteinuria, nil or low at initial stages, and comparatively higher at later stages[26]. Podocyturia has been assessed in a wide variety of glomerulopathies, as well as in transplantation and in entities where the glomerulus is not primarily affected, as in polycystic kidney disease[26,27,29,30,37,38].
Podocyturia is ideally employed in hereditary kidney disorders like Alport of Fabry disease[26,38,39]. In these settings, an index case is usually identified and properly studied and eventually treated. However, certain relatives with apparent no clinical renal involvement, who basically present with normal renal function, no proteinuria and later proven to carry the corresponding mutation, are identified as subjects with silent subclinical kidney involvement, as they present with higher and covert podocyturia load when compared to controls[26,38,39]. Wickman et al[30] have studied the degree of podocyturia in several gomerulopathies as membranous nephropathy, IgA nephropathy, lupus nephritis, diabetic nephropathy, rapidly progressive glomerulonephritis, severe hypertension, among others. They have shown that there was a significant relationship between proteinuria and podocyturia detachment mainly in advanced diabetic nephropathy, in progressors and in active lupus nephritis or IgA nephropathy. They also demonstrated a significant correlation between the degree of podocyturia and proteinuria for all clinic patients, suggesting that proteinuria is a consequence of glomerular dysfunction. Despite proteinuria is a non-specific marker of glomerular and kidney injury, they concluded that proteinuria would persist despite effective therapies that reduce the rate of podocyte detachment and that podocyte detachment is not only related to proteinuria, but it also determines the progression process and can provide useful additional information to the nephrologist to guide response to treatment and risk for progression in the clinic[30].
However, as mentioned above, our group suggests that the correlation between podocyturia and proteinuria depends on the stage at which the process is approached. Once the mass of podocytes is low, podocyturia would decrease in contrast to a climb in proteinuria and a parallel decline in kidney function[26]. In addition, podocyturia has been widely studied in preeclampsia. The main conclusion is that podocyturia can be employed as a diagnostic tool of preeclampsia among high risk patients, and correlates with the severity of the clinical case[27]. Finally, we have just published an acute case of early-diagnosed IgA nephropathy with copious podocyturia that significantly decreased with immunosuppression and amiloride was associated with a decrease in proteinuria and an improvement in kidney function[40].
In this regard, we have reported that in several glomerular diseases the addition of amiloride contributed to the decrease in the degree of podocyturia, probably due to its interaction with uPAR, and consequently with the atachment of podocytes to the glomerular basement membrane via integrins, as explained above[16,23,38-41]. Zhang et al[41] have shown that amiloride reduces uPAR expression, inhibtis uPAR mRNA and protein synthesis in podocytes. Noteworthy, uPAR is highly expressed on cell the surface of diseased podocytes, but only scarcely on normal podocytes.
Early interventions in this asymptomatic cohort could lead to better clinical outcomes. We also believe that the study of the different podocyte proteins, particularly in each glomerulopathy, would contribute to unveil the triggered pathophysiologic mechanisms of podocyte detachment. Finally, as mentioned previously, we propose that due to the lack of biomarkers to assess kidney diseases, podocyturia ought to be a field to explore and to be standardized and validated for routine assessment of patients.
Many limitations arise when the limitations are to be discussed. The available methods to study podocyturia are time-consuming, expensive, and laborious. They also require not only of certain type of microscopes, but also of trained professionals. Molecular biology methods also require the proper reactants and antibodies, mounted laboratories and adequate technology.
Podocytes are frequently lost in clusters and erratic. This intermittent loss of podocytes in the urine may oblige physician to perform serial analyses of repeated urine samples to correctly evaluate the case. Moreover, probably different populations of podocytes could be identified, depending on the predominant proteins expressed at different stages of the disease or even at the same stage. In this regard, a set of different antibodies targeted to several proteins would be ideal to perform. Our group is now undertaking the study of proteins that belong to different cell compartments: Glycocalix, cytoplasm and basal side of podocytes.
Another important limitation is that unless proteinuria, microhematuria or an unexplained decline in renal function appear, then a kidney covert disease would not be suspected. Thus, podocyturia would be useful in clinical grounds when employed routinely for screening purposes in any patient. Urine is to be collected preferentially on site and processed without delays, in order to avoid the deterioration of the sample and the destruction of cells. The methods to assess podocyturia have not been validated yet. Finally, the cost of the study of loss podocytes is another major determinant that at this times positions this method in the research field and not also in the clinical ground.
Podocytes are highly differentiated cells unable to undergo cell division under normal conditions. Despite podocyturia exists as a physiologic phenomenom in the general population, any insult received by the podocyte either directly or indirectly, would trigger contractile mechanisms in order to retain them attached to the glomerular basement membrane, despite losing the function of avoiding the loss of protein in the urine. Under pathological situations, podocytes upregulate the synthesis of uPAR, which then couples with integrins to activate the contractile podocyte apparatus that eventually leads to podocyte detachment. Podocyte loss precedes proteinuria and loss of renal function at initial stages of kidney diseases, particularly glomerulopathies. Podocyturia has been employed as a diagnostic tool in a wide variety of glomerular disorders. Unravelling the mechanisms of podocyte detachment may lead to targeted therapeutic interventions that could delay the progression of chronic kidney diseases, as shown by the decrease of podocyte loss when amiloride is employed.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fujigaki Y, Lehtonen SH, Olowu WA, Robles NR S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Jefferson JA, Alpers CE, Shankland SJ. Podocyte biology for the bedside. Am J Kidney Dis. 2011;58:835-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40-F48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Trimarchi H. Podocyturia: What is in a name? J Transl Int Med. 2015;3:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 6. | Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3261] [Cited by in RCA: 3075] [Article Influence: 205.0] [Reference Citation Analysis (0)] |
| 7. | Jefferson JA, Nelson PJ, Najafian B, Shankland SJ. Podocyte disorders: Core Curriculum 2011. Am J Kidney Dis. 2011;58:666-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98:1591-1596. [PubMed] |
| 9. | Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 889] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 10. | Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, Hauser P, Pippin JW, Shankland SJ, Smith KD. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333-F347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Kriz W. The pathogenesis of ‘classic’ focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant. 2003;18 Suppl 6:vi39-vi44. [PubMed] |
| 13. | Welsh GI, Saleem MA. The podocyte cytoskeleton--key to a functioning glomerulus in health and disease. Nat Rev Nephrol. 2011;8:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 215] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Greka A, Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol. 2011;22:1969-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Reiser J, Gupta V, Kistler AD. Toward the development of podocyte-specific drugs. Kidney Int. 2010;77:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Piret SE, Olinger E, Reed AAC, Nesbit MA, Hough TA, Bentley L, Devuyst O, Cox RD, Thakker RV. A mouse model for inherited renal fibrosis associated with endoplasmic reticulum stress. Dis Model Mech. 2017;10:773-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Matsusaka T, Sandgren E, Shintani A, Kon V, Pastan I, Fogo AB, Ichikawa I. Podocyte injury damages other podocytes. J Am Soc Nephrol. 2011;22:1275-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Hara M, Yanagihara T, Kihara I. Significance of urinary binucleated podocytes in IgA nephropathy. Nephrology. 2001;6:A15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Trimarchi H. Primary focal and segmental glomerulosclerosis and soluble factor urokinase-type plasminogen activator receptor. World J Nephrol. 2013;2:103-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Trimarchi H, Duboscq C. Proteinuria: a cross road where complement and the plasminogen-plasmin systems meet. J Integr Nephrol Androl. 2016;3:37-47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Trimarchi H, Canzonieri R, Schiel A, Politei J, Costales-Collaguazo C, Stern A, Paulero M, Rengel T, Valiño-Rivas L, Forrester M. Expression of uPAR in Urinary Podocytes of Patients with Fabry Disease. Int J Nephrol. 2017;2017:1287289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Trimarchi H, Canzonieri R, Schiel A, Costales-Collaguazo C, Stern A, Paulero M, Rengel T, Andrews J, Iotti A, Forrester M. In IgA Nephropathy, Glomerulosclerosis Is Associated with Increased Urinary CD80 Excretion and Urokinase-Type Plasminogen Activator Receptor-Positive Podocyturia. Nephron Extra. 2017;7:52-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Utsumi K, Itoh K, Kase R, Shimmoto M, Yamamoto N, Katagiri Y, Tanoue K, Kotani M, Ozawa T, Oguchi T. Urinary excretion of the vitronectin receptor (integrin alpha V beta 3) in patients with Fabry disease. Clin Chim Acta. 1999;279:55-68. [PubMed] |
| 26. | Trimarchi H, Canzonieri R, Schiel A, Politei J, Stern A, Andrews J, Paulero M, Rengel T, Aráoz A, Forrester M. Podocyturia is significantly elevated in untreated vs treated Fabry adult patients. J Nephrol. 2016;29:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Jim B, Jean-Louis P, Qipo A, Garry D, Mian S, Matos T, Provenzano C, Acharya A. Podocyturia as a diagnostic marker for preeclampsia amongst high-risk pregnant patients. J Pregnancy. 2012;2012:984630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Maestroni S, Maestroni A, Dell’Antonio G, Gabellini D, Terzi S, Spinello A, Meregalli G, Castoldi G, Zerbini G. Viable podocyturia in healthy individuals: implications for podocytopathies. Am J Kidney Dis. 2014;64:1003-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Perez-Hernandez J, Olivares MD, Forner MJ, Chaves FJ, Cortes R, Redon J. Urinary dedifferentiated podocytes as a non-invasive biomarker of lupus nephritis. Nephrol Dial Transplant. 2016;31:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Wickman L, Afshinnia F, Wang SQ, Yang Y, Wang F, Chowdhury M, Graham D, Hawkins J, Nishizono R, Tanzer M. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol. 2013;24:2081-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 419] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 32. | Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Becker JU, Hoerning A, Schmid KW, Hoyer PF. Immigrating progenitor cells contribute to human podocyte turnover. Kidney Int. 2007;72:1468-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol. 2010;298:F702-F711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Lasagni L, Romagnani P. Glomerular epithelial stem cells: the good, the bad, and the ugly. J Am Soc Nephrol. 2010;21:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol. 2014;10:158-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 37. | Yang Y, Hodgin JB, Afshinnia F, Wang SQ, Wickman L, Chowdhury M, Nishizono R, Kikuchi M, Huang Y, Samaniego M. The two kidney to one kidney transition and transplant glomerulopathy: a podocyte perspective. J Am Soc Nephrol. 2015;26:1450-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Trimarchi H, Canzonieri R, Muryan A, Schiel A, Araoz A, Paulero M, Andrews J, Rengel T, Forrester M, Lombi F. Podocyturia: A Clue for the Rational Use of Amiloride in Alport Renal Disease. Case Rep Nephrol. 2016;2016:1492743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Trimarchi H, Canzonieri R, Muryan A, Schiel A, Araoz A, Forrester M, Karl A, Lombi F, Andrews J, Pomeranz V. Copious Podocyturia without Proteinuria and with Normal Renal Function in a Young Adult with Fabry Disease. Case Rep Nephrol. 2015;2015:257628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Trimarchi H, Paulero M, Canzonieri R, Schiel A, Iotti A, Costales-Collaguazo C, Stern A, Forrester M, Lombi F, Pomeranz V. In acute IgA nephropathy, proteinuria and creatinine are in the spot, but podocyturia operates in silence. Any place for amiloride? Case Rep Nephrol. 2017;2017:129531. |
| 41. | Zhang B, Xie S, Shi W, Yang Y. Amiloride off-target effect inhibits podocyte urokinase receptor expression and reduces proteinuria. Nephrol Dial Transplant. 2012;27:1746-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |