Published online May 6, 2017. doi: 10.5527/wjn.v6.i3.150
Peer-review started: September 23, 2016
First decision: November 2, 2016
Revised: February 28, 2017
Accepted: March 14, 2017
Article in press: March 17, 2017
Published online: May 6, 2017
Processing time: 226 Days and 19.4 Hours
To study the clinico-pathological spectrum of snake bite-induced acute kidney injury (AKI).
A retrospective study of patients admitted at Indira Gandhi Medical College Hospital, Shimla with snake bite-induced AKI from July 2003 to June 2016. Medical records were evaluated for patient’s information on demographic, clinical characteristics, complications and outcome. Outcomes of duration of hospital stay, requirement for intensive care unit support, treatment with dialysis, survival and mortality were analyzed. The survival and non survival groups were compared to see the difference in the demographic factors, clinical characteristics, laboratory results, and complications. In patients subjected to kidney biopsy, the findings of histopathological examination of the kidney biopsies were also analyzed.
One hundred and twenty-one patients were diagnosed with snake bite-induced AKI. Mean age was 42.2 ± 15.1 years and majority (58%) were women. Clinical details were available in 88 patients. The mean duration of arrival at hospital was 3.4 ± 3.7 d with a range of 1 to 30 d. Eighty percent had oliguria and 55% had history of having passed red or brown colored urine. Coagulation defect was seen in 89% patients. The hematological and biochemical laboratory abnormalities were: Anemia (80.7%), leukocytosis (75%), thrombocytopenia (47.7%), hyperkalemia (25%), severe metabolic acidosis (39.8%), hepatic dysfunction (40.9%), hemolysis (85.2%) and rhabdomyolysis (68.2%). Main complications were: Gastrointestinal bleed (12.5%), seizure/encephalopathy (10.2%), hypertension, pneumonia/acute respiratory distress syndrome (ARDS) and disseminated intravascular coagulation (9.1% each), hypotension and multi organ failure (MOF) (4.5% each). Eighty-two percent patients required renal replacement therapy. One hundred and ten (90.9%) patient survived and 11 (9.1%) patients died. As compared to the survival group, the white blood cell count (P = 0.023) and bilirubin levels (P = 0.006) were significant higher and albumin levels were significantly lower (0.005) in patients who died. The proportion of patients with pneumonia/ARDS (P = 0.001), seizure/encephalopathy (P = 0.005), MOF (P = 0.05) and need for intensive care unit support (0.001) was significantly higher and duration of hospital stay was significantly shorter (P = 0.012) in patients who died. Kidney biopsy was done in total of 22 patients. Predominant lesion on kidney biopsy was acute tubular necrosis (ATN) in 20 (91%) cases. In 11 cases had severe ATN and in other nine (41%) cases kidney biopsy showed features of ATN associated with mild to moderate acute interstitial nephritis (AIN). One patient only had moderate AIN and one had patchy renal cortical necrosis (RCN).
AKI due to snake bite is severe and a high proportion requires renal replacement therapy. On renal histology ATN and AIN are common, RCN is rare.
Core tip: Acute kidney injury (AKI) due to snake bite is an important cause of community acquired tropical AKI. Clinicopathological spectrum of snake-induced AKI has changed. Intravascular hemolysis and rhabdomyolysis is common and is the main cause of AKI due to snake bite. AKI is severe and high proportion of patients requires renal replacement therapy. Complications of pneumonia/acute respiratory distress syndrome, seizure/encephalopathy, multi organ failure, the need for intensive care support and a shorter hospital stay are factors associated with mortality. On renal histology acute tubular necrosis and acute interstitial nephritis is common, renal cortical necrosis is rare. Early administration of anti-snake-venom and alkaline diuresis may help in prevention of AKI.
- Citation: Vikrant S, Jaryal A, Parashar A. Clinicopathological spectrum of snake bite-induced acute kidney injury from India. World J Nephrol 2017; 6(3): 150-161
- URL: https://www.wjgnet.com/2220-6124/full/v6/i3/150.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i3.150
Snake bite is a significant public health problem causing considerable morbidity and mortality worldwide, particularly in tropics. Snakebite is now recognized as a Neglected Tropical Disease by the World Health Organization (WHO)[1]. According to WHO estimates about 5 million people are bitten each year by poisonous snakes which results in 2.5 million envenomations, at least 100000 deaths, and 300000 amputations and other permanent disabilities[2]. Majority of snakebite induced deaths occur in Asia and Sub-Saharan Africa[1]. The mortality due to venomous snakebite in India is estimated between 35000-50000 per annum, which is the highest in the world[1,3]. The mortality due to venomous snakebite in India continuous to be high due to various social, economic and cultural reasons[4].
Over 3000 species of snakes are known worldwide, of which around 600 are considered to be venomous.There are 2 important groups (families) of venomous snakes in south-east Asia - Elapidae have short permanently erect fangs. This family includes the cobras, king cobra, kraits, coral snakes, and the sea snakes. Viperidae have long fangs, which are normally folded up against the upper jaw, but when the snake strikes, are erected. There are 2 subgroups, the typical vipers (Viperinae) and the pit vipers (Crotalinae)[5]. Medically Important snakes of India include the so called “Big 4”, Russel’s viper (Daboia russelli), Cobra (Naja naja), Common Krait (Bungarus caeruleus) and Saw scaled viper (Echis carinatus) that occur throughout the country. The pit viper species - Malabar, green and the hump-nosed, sea snakes and others like the king cobra (Ophiophagus hannah), monocle cobra (Naja Kaouthia), Banded Krait (Bungarus fasciatus) and Echis sochureki are important causes in certain geographical areas[5,6]. The principle effects of envenomation is on the nervous system, kidneys, heart, lungs, liver, blood coagulation system, vascular endothelium and local effects at the site of bite[7].
Acute kidney injury (AKI) is an important complication of snake bite and a major cause of mortality. AKI is common after bites from myotoxic or hemotoxic snakes. These snakes are Russell’s viper, saw-scaled viper, hump-nosed pit viper, green pit viper, and sea-snake. Renal pathologic changes include tubular necrosis, cortical necrosis, interstitial nephritis, glomerulonephritis, and vasculitis. Hemodynamic alterations caused by vasoactive mediators and cytokines and direct nephrotoxicity account significantly for the development of nephropathy. Hemorrhage, hypotension, disseminated intravascular coagulation (DIC), intravascular hemolysis, and rhabdomyolysis enhance renal ischemia leading to AKI[8]. The incidence of AKI caused by these snakes varies from 5% to 29% depending on the species of snake and the severity of envenomation[9-11]. The onset of AKI is from a few hours to as late as 96 h after the bite. The duration of AKI after snake bite generally ranges from 2 to 3 wk. Tubular necrosis is an important pathological correlate of AKI. Prolonged AKI with oligoanuria after snake bite is indicative of cortical necrosis or acute tubular necrosis associated with interstitial nephritis or extracapillary glomerulonephritis[8].
Himachal Pradesh is a mountainous state in the northern part of India. It is the least urbanized state with more than 90% of the population living in rural areas[12]. Eco-rich vegetation due to a long rainy season, abundant flora and fauna and a scattered population using paths traversing rural and forest lands makes people in these areas particularly prone to snake bite[13]. The reptilian fauna shows geographical variation in habitation of medically important venomous snake species. Existence of venomous snakes like Trimeresurus albolabris (white lipped pit viper), Gloydius himalayanus (himalayan pit viper) and Naja oxiana (black cobra) has been documented in the state of Himachal Pradesh, in addition to the common “Big 4” which are found throughout India[14].
Indira Gandhi Medical College Hospital, Shimla is the only tertiary-care hospital in the state of Himachal Pradesh. We retrospectively analyzed the records to study the clinico-pathological spectrum of Snake bite-induced AKI seen during last 13 years at this hospital.
The study was a retrospective analysis conducted on patients admitted at Indira Gandhi Medical College Hospital, Shimla with snake bite-induced AKI over a period of 13 years (July 2003-June 2016). The inclusion and exclusion criteria were defined as follows.
Inclusion criteria were: (1) definitive history of snake bite; (2) clinical picture consistent with snake bite, as presence of fang marks or cellulitis or coagulopathy or neuroparalysis; (3) presence of AKI as defined using KDIGO criteria based on serum creatinine (increase in serum creatinine by ≥ 0.3 mg/dL within 48 h or increase in serum creatinine to ≥ 1.5 times baseline, which is known or presumed to have occurred within the prior 7 d[15]. It was presumed that the patient had normal renal function if the serum creatinine was 1.5 mg/dL; and (4) presence of at least one or more indication of renal replacement therapy.
Exclusion criteria were: (1) patients with pre-existent renal disease (serum creatinine > 1.5 mg/dL prior to snake bite or ultrasonography of abdomen suggestive of bilateral small kidneys/loss of corticomedullary differentiation/obstructive nephropathy/ other renal pathology); (2) diagnosed cases of hypertension /diabetes mellitus; and (3) exposure to nephrotoxic drugs/toxins.
All the patients were subjected to detailed history and clinical examination. A bed side twenty minute whole blood clotting test (20WBCT) was performed to look for coagulation defect. Hematological and biochemical investigations were performed in all patients. Patients were administered tetanus toxoid injection, if not received previously. Polyvalent anti-snake venom (ASV) was provided where needed (each vial of serum contains 10 mL, and each 1 mL neutralizes 0.6 mg cobra, 0.6 mg Russell’s viper, 0.45 mg common krait, and 0.45 mg sawscaled viper venoms) according to the standard protocol. Patients showing signs of neuroparalysis were given injection neostigmine with prior atropine. Doses were repeated as needed based on clinical response. Supportive treatment (intravenous fluids, blood components, antibiotics and analgesics) was given. Patient subjected to dialysis as per standard hospital protocol. Ventilatory support was needed for patients with respiratory failure, either due to neuroparalysis or pulmonary edema.
Medical record were evaluated for patient’s information including age, gender, month of the bite, time from snake bite to arrival at hospital and site of the snake bite.
Laboratory results of hemoglobin, leukocyte count, platelet count, blood urea, serum creatinine, serum electrolytes of sodium and potassium, serum albumin, serum bilirubin, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), alkaline phosphatase, creatine kinase (CK), lactate dehydrogenase (LDH) and arterial blood gas analysis and the prevalence of anemia (Hemoglobin < 12 g/dL), leucocytosis, thrombocytopenia, hyperkalemia, hypoalbuminemia, hepatic dysfunction, severe metabolic acidosis (pH 7.2), hemolysis and rhabdomyolysis were recorded. Peak values or worst results of these laboratory parameters during the admission were used for analysis. The complications of hypotension, hypertension, chest infection, acute respiratory distress syndrome (ARDS), myocarditis, myocardial infarction, gastro-intestinal bleed, seizure and encephalopathy, DIC and multi organ failure (MOF) seen during the hospitalization were recorded.
Outcomes of duration of hospital stay (DOHS), requirement for intensive care unit (ICU) support, treatment with dialysis, survival and mortality were analyzed. The survival and non survival groups were compared to see the difference in the demographic factors, laboratory results, clinical characteristics and complications between the two groups.
In this study, the clinical symptoms and complications of snake envenoming were classified using WHO guidelines for the clinical management of snakebites in the southeast Asian region[5].
Coagulation defect primarily detected by 20WBCT or visible spontaneous systemic bleeding documented by the referring health institution or observed at this hospital was recorded. Evidence of neurotoxicity: Ptosis, external ophthalmoplegia, muscle paralysis, inability to lift the head, etc. Hypotension was defined as an arterial systolic blood pressure below 90 mmHg and hypertension by a blood pressure of 140/90 mmHg.
Hepatic dysfunction was defined as an AST or ALT over 60 IU/L. Intravascular hemolysis by presence of anemia, jaundice, reticulocytosis, abnormal peripheral blood smear and raised serum LDH levels. Rhabdomyolysis was characterized by elevated serum CK levels of five or more times the normal levels, with a suggestive clinical picture and without heart and cerebral injury[11,16].
To diagnose disseminated intravascular coagulation, we used the guidelines for screening tests proposed by Yu et al[17]. Patients remaining oligoanuric or whose serum creatinine did not decrease satisfactorily at the end of three weeks underwent kidney biopsy which was examined with light and immunofluorescence microscopy. Findings of histopathological examination of the kidney biopsies were also analyzed. Permission from the Institutional Review Board and the hospital authorities was obtained for the study [No. HFW(MS)G-5(Ethic)/2015-9078].
The data collected were stored using a computer program. Continuous data are expressed as means ± SD, and the means of the two study groups were compared using an unpaired t test. Nominal data are expressed as frequencies or proportions, and the χ2 test and Fisher’s exact test were used to compare the differences in frequency between the two study groups. For non-normal data, a Mann-Whitney U test was performed. Multivariate binary logistic regression analysis was done for factors predictive mortality. A P-value < 0.05 was considered statistically significant. All statistics were carried out using SPSS, version 16 (SPSS, Chicago, IL, United States).
A total of 121 patients were diagnosed with snake bite-induced AKI during the study period. Eighty-one patients had AKI out of a total of 447 patients who were admitted for snake bite during a period of 5.5 years (January 2011 to June 2016), with 18.1% incidence of Snake bite-induced AKI.
Table 1 shows the demographic and clinical characteristics of the study patients. Mean age of the patients was 42.2 ± 15.1 years. Fifty-one (42.1%) patients were male and 70 (57.9%) were female. Majority (86.8%) of the snake bite accidents occurred in summer and monsoon months. The snake bite occurred in monsoon months (July-September) in 67 (55.4%), summer months (April-June) in 38 (31.4%), and the other 16 (13.2%) in winter months (October-March). All the snake bite accidents had occurred in rural areas. Clinical details were available in total of 88 (72.7%) patients. The mean duration of arrival at hospital after snake bite was 3.4 ± 3.7 d with a range of 1 to 30 d. Seventy (79.5%) patents had oliguria and 48 (54.5%) had a history of hematuria or having passed dark brown/black urine. Majority (85.2%) of the patients had sustained bite on lower extremities. Limb swelling/cellulitis was seen in 12 (13.6%) patients and 5 (5.7%) patients had associated neurotoxicity. Coagulation defect was seen/documented in 78 (88.6%) patients.
| Gender | |
| Male, n (%) | 51 (42.1) |
| Female, n (%) | 70 (57.9) |
| Age (yr) | |
| Mean ± SD | 42.2 ± 15.1 |
| Range | 14-84 |
| Month of the bite | |
| March, n (%) | 1 (0.8) |
| April, n (%) | 8 (6.6) |
| May, n (%) | 10 (8.3) |
| June, n (%) | 20 (16.5) |
| July, n (%) | 16 (13.2) |
| August, n (%) | 22 (18.2)) |
| September, n (%) | 29 (24) |
| October, n (%) | 10 (8.3) |
| November, n (%) | 5 (4.1) |
| Duration at arrival (d) | |
| Mean ± SD | 3.4 ± 3.7a |
| Median | 3 |
| Range | 1-30 |
| Duration of hospital stay (d) | |
| Mean ± SD | 12.4 ± 7.7a |
| Median | 10 |
| Range | 1-40 |
| Bite site | |
| Lower limb, n (%) | 75 (85.2) |
| Upper limb, n (%) | 13 (14.8) |
| Oliguria, n (%) | 70 (79.5)a |
| Hematuria, n (%) | 48 (54.5)a |
| Limb swelling/cellulitis, n (%) | 12 (13.6)a |
| Neurotoxicity, n (%) | 5 (5.7)a |
| Coagulation defect, n (%) | 78 (88.6)a |
| ASV given, n (%) | 85 (96.6)a |
| Blood transfusion, n (%) | 15 (17)a |
| ICU support, n (%) | 10 (11.4)a |
| Dialysis therapy, n (%) | 99 (81.8) |
| Hemodialysis | |
| Mean ± SD | 3.5 ± 3.7 |
| Median | 3 |
| Range | 1-20 |
| Outcome | |
| Survived, n (%) | 110 (90.9) |
| Died, n (%) | 11 (9.1) |
The mean hemoglobin was 9.6 ± 2.6 g/dL, white blood cell (WBC) count was 15 ± 7.4 (× 103/mm3) and platelet count was 117 ± 58 (× 103/mm3). The mean values of peak serum urea and creatinine were 169 ± 75 mg/dL and 7.2 ± 4.2 mg/dL respectively. The serum albumin 3.2 ± 0.4 g/dL, bilirubin 1.4 ± 1.4 mg/dL, AST 152 ± 296 IU/L, and ALT was 106 ± 163 IU/L. The mean values of creatine kinase were 2072 ± 4287 U/L and lactate dehydrogenase was 1153 ± 1015 IU/L (Table 2). The hematological and biochemical laboratory abnormalities were: Anemia (80.7%), leukocytosis (75%), thrombocytopenia (47.7%), hyperkalemia (25%), severe metabolic acidosis (39.8%), hepatic dysfunction (40.9%), hemolysis (85.2%) and rhabdomyolysis (68.2%) (Table 3). Fifty-three (60.2%) patients had both intravascular hemolysis and rhabdomyolysis.
| Mean ± SD | Range | |
| Hemglobin (g/dL) | 9.6 ± 2.6 | 3.8-16 |
| WBC count (× 103/mm3) | 15 ± 7.4 | 4.8-47.9 |
| Platelets (× 103/mm3) | 117 ± 58 | 18-230 |
| Urea (mg/dL) | 169 ± 75 | 54-440 |
| Creatinine (mg/dL) | 7.2 ± 4.2 | 1.6-19.2 |
| Sodium (mEq/L) | 134 ± 8.1 | 108-145 |
| Potassium (mEq/L) | 5 ± 0.8 | 3.2-7.2 |
| Albumin (g/dL) | 3.2 ± 0.4 | 2.5-4.1 |
| Bilirubin (mg/dL) | 1.4 ± 1.4 | 0.3-7.5 |
| AST (U/L) | 152 ± 296 | 16-1606 |
| ALT (U/L) | 106 ± 163 | 10-800 |
| Alk. phosph. (U/L) | 93 ± 66 | 18-432 |
| CK (U/L) | 2072 ± 4287 | 24-22000 |
| LDH (U/L) | 1153 ± 1015 | 70-4705 |
| Anemia Hb < 12 g/dL, n (%) | 71 (80.7) |
| Leukocytosis, n (%) | 66 (75) |
| Thrombocytopenia, n (%) | 42 (47.7) |
| Hyperkalemia, n (%) | 22 (25) |
| Severe metabolic acidosis, n (%) | 35 (39.8) |
| Hepatic dysfunction, n (%) | 36 (40.9) |
| Hypoalbuminemia, n (%) | 54 (61.4) |
| Hemolysis, n (%) | 75 (85.2) |
| Rhabdomyolysis, n (%) | 60 (68.2) |
Main complications were: Gastrointestinal bleed (12.5%), seizure/encephalopathy (10.2%), hypertension, pneumonia/ARDS and DIC (9.1% each), hypotension and MOF (4.5% each), myocarditis and myocardial infarction (1.1% each) (Table 4).
| Pneumonia/ARDS | 8 (9.1) |
| Hypertension | 8 (9.1) |
| Hypotension | 4 (4.5) |
| Myocarditis | 1 (1.1) |
| Myocardial infarction | 1 (1.1) |
| Gastrointestinal bleed | 11 (12.5) |
| DIC | 8 (9.1) |
| Seizure/Encephalopathy | 9 (10.2) |
| MOF | 4 (4.5) |
Polyvalent ASV was administered to 85 (96.6%) patients. Fifteen (17%) patients required blood transfusion and 10 (11.4%) patients required ICU support. The mean DOHS were 12.4 ± 7.7 with a range of 1-40 d. Ninety-nine (81.8%) patients required dialysis. The mean number of hemodialysis (HD) treatments per patient was 3.5 ± 3.7 with a range of 1-20. 110 (90.9%) patient survived and 11 (9.1%) patients died (Table 1). Eighty-six (97.7%) AKI patients had complete renal function recovery whereas 2 patients had partial renal function recovery on follow-up.
Table 5 shows the comparison of the demographic and laboratory results between survival and non-survival groups. Patients in the non-survival group had a significantly shorter DOHS (P = 0.012) as compared to that in the survival group. As compared to the survival group, the WBC count (P = 0.023) and bilirubin levels (P = 0.006) were significant higher and albumin levels were significantly lower (0.005) in patients who died.
| Survived (n = 79) | Died (n = 9) | P value | |
| Age (yr) | 43 ± 15.7 | 39 ± 12.2 | 0.460a |
| Sex | |||
| Male, n (%) | 34 (43) | 2 (22.2) | 0.299c |
| Female, n (%) | 45 (57) | 7 (77.8) | |
| Duration at arrival (d) | 3.4 ± 3.9 | 2.9 ± 0.8 | 0.536b |
| Duration of hospital stay (d) | 12.9 ± 7.2 | 8.8 ± 11.4 | 0.012b |
| Hemoglobin (g/dL) | 9.6 ± 2.7 | 8.9 ± 2.4 | 0.435a |
| WBC count (× 103/mm3) | 14.5 ± 7.3 | 19.7 ± 7.5 | 0.023b |
| Platelets (× 103/mm3) | 119 ± 57 | 95 ± 67 | 0.218 |
| Urea (mg/dL) | 167 ± 78 | 186 ± 43 | 0.466 |
| Creatinine (mg/dL) | 7 ± 4.1 | 7 ± 2 | 0.997 |
| Sodium (mEq/L) | 135 ± 8 | 138 ± 4.6 | 0.182 |
| Potassium (mEq/L) | 4.8 ± 0.8 | 5.1 ± 1.2 | 0.392 |
| Albumin (g/dL) | 3.3 ± 0.4 | 3 ± 0.3 | 0.005 |
| Bilirubin (mg/dL) | 1.2 ± 1.1 | 3.1 ± 2.2 | 0.006b |
| AST (U/L) | 141 ± 283 | 254 ± 398 | 0.231b |
| ALT (U/L) | 101 ± 158 | 156 ± 200 | 0.102b |
| ALP (U/L) | 90 ± 67 | 122 ± 54 | 0.057b |
| CK (U/L) | 1811 ± 3762 | 4069 ± 7181 | 0.298b |
| LDH (U/L) | 1106 ± 1027 | 1684 ± 732 | 0.052b |
Table 6 shows the comparison of the clinical characteristics and complications between survival and non-survival groups. The proportion of patients with pneumonia/ARDS (P = 0.001), seizure/encephalopathy (P = 0.005), MOF (P = 0.05) and need for ICU support (0.001) was significantly higher in patients who died.
| Survived (n = 79) | Died (n = 9) | P valuea | |
| Oliguria, n (%) | 62 (78.5) | 8 (88.9) | 0.679 |
| Hematuria, n (%) | 44 (55.7) | 4 (44.4) | 0.726 |
| Anemia, n (%) | 64 (81) | 7 (77.8) | 1.000 |
| Leukocytosis, n (%) | 57 (72.2) | 9 (100) | 0.105 |
| Thrombocytopenia, n (%) | 37 (46.8) | 5 (55.6) | 0.731 |
| Hyperkalemia, n (%) | 19 (24.1) | 3 (33.3) | 0.685 |
| Metabolic acidosis, n (%) | 29 (36.7) | 6 (66.7) | 0.147 |
| Hypoalbuminemia, n (%) | 46 (58.2) | 8 (88.9) | 0.145 |
| Hepatic dysfunction, n (%) | 32 (40.5) | 4 (44.4) | 1.000 |
| Hemolysis, n (%) | 67 (84.8) | 8 (88.9) | 1.000 |
| Rhabdomyolysis, n (%) | 55 (69.6) | 5 (55.6) | 0.457 |
| Pneumonia/ARDS, n (%) | 3 (3.8) | 5 (55.6) | 0.001 |
| Seizure/Encephalopathy, n (%) | 5 (6.3) | 4 (44.1) | 0.005 |
| Multiorgan failure, n (%) | 2 (2.5) | 2 (22.2) | 0.050 |
| ICU support, n (%) | 5 (6.3) | 5 (55.6) | 0.001 |
| Dialysis, n (%) | 57 (72.2) | 9 (100) | 0.105 |
There were no significant differences in the demographic and clinical characteristics, severity of hematological and biochemical abnormalities, other complications and need for dialysis therapy between groups.
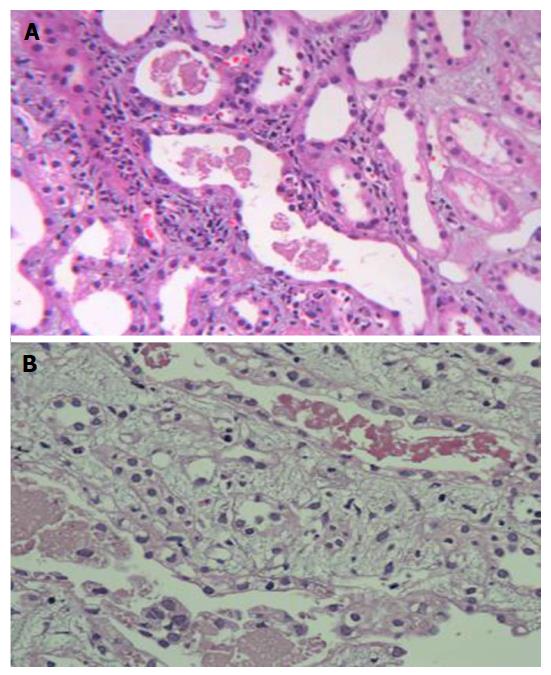
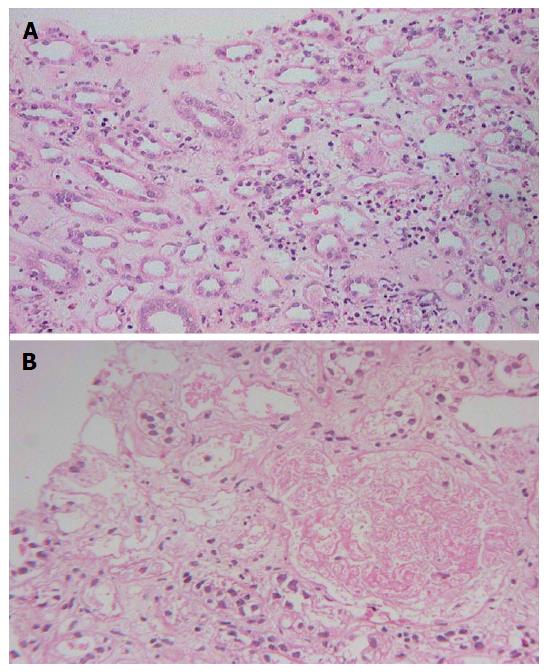
Renal histology was studied in 22 (25%) patients (Table 7). Predominant lesion on kidney biopsy was acute tubular necrosis in (ATN) 20 (91%) patients, 11 patients had severe ATN (Figure 1A) and other nine (40.9%) had ATN associated with mild to moderate acute interstitial nephritis (AIN) (Figure 1B). One (4.5%) patient only had moderate AIN (Figure 2A) on biopsy. Renal cortical necrosis (RCN) which was patchy involving 35%-40% of cortical segment (Figure 2B) was present in one patient with prolonged anuria lasting for more than 4 wk. There was a lapse of 24 h when this patient had treatment with ASV.
| Acute tubular necrosis, n (%) | 11 (50) |
| Acute tubular necrosis + acute interstitial nephritis, n (%) | 9 (40.9) |
| Acute interstitial nephritis, n (%) | 1 (4.5) |
| Acute cortical necrosis, n (%) | 1 (4.5) |
Snake bite is predominantly an occupational hazard in the rural tropics. The incidence of snake-bites depends critically on the frequency of contact between snakes and humans. Seasonal peaks of snake-bite incidence are usually associated with increases in agricultural activity or seasonal rains, perhaps coinciding with unusual movement and activity by snakes. Snakebites and snakebite fatalities peak during the monsoon season in India[18,19] and worldwide[3], probably reflecting agricultural activity, flooding, increased snake activity, and abundance of their natural prey. All the snake bite accidents in the current study had occurred in rural areas and majority of them happened during monsoon months.
The kidney, a highly vascularized organ with excretory function, is prone to venom toxicity as an innocent bystander. AKI, the most significant of all the renal manifestations, has been reported with varying frequency in different studies[8]. Many studies have appeared in literature on snake bite-induced AKI over a decade after the landmark study by Chugh[9] (Table 8). They have mainly come from Asia[9,6-30] and South America[11,25]. These studies both retrospective[9,21,22,25,27-29] and prospective[11,20,23,24,26,30] have been carried over a period of 1-25 years. All except one[26] have been in adult patients. The incidence of AKI in adults ranged from 8%-45%[9,11,20-25] where as the incidence in children is reported to be 46%[26]. An incidence of 18.1% was found in the current study. Similar to our study the mean age of the patients ranged from 24-43 years in various studies[9,11,20-25,28-30] except for one study[27] from China where the mean age of the patients was 66 years.
| Ref. | Chugh[9] | Pinho et al[11] | Athappan et al[20] | Danis et al[21] | David et al[22] | Dharod et al[23] | Harshavardhan et al[24] | Albuqerque et al[25] | Krishnamurthy et al[26] | Li et al[27] | Naqvi et al[28] | Singh et al[29] | Mukhopadhyay et al[30] | The present study |
| Year | 1989 | 2005 | 2008 | 2008 | 2012 | 2013 | 2013 | 2014 | 2015 | 2016 | 2016 | 2016 | 2016 | 2016 |
| Place | Chandigarh | São Paulo | Madurai | Diyarbakir | Vellore | Nagpur | Hassan | Fortaleza | Puducherry | Chengdu | Karachi | Barabanki | Kolkata | Shimla |
| Country | India | Brazil | India | Turkey | India | India | India | Brazil | India | China | Pakistan | India | India | India |
| Type of study | Retrospective | Prospective | Prospective | Retrospective | Retrospective | Prospective | Prospective | Retrospective | Prospective | Retrospective | Retrospective | Retrospective | Prospective | Retrospective |
| Time period (yr) | 22 | 3.6 | 2 | 7 | 10 | 1.7 | 1 | 10 | 1.6 | 1.8 | 25 | 1.5 | 1.3 | 13 |
| Total patients (n) | 246 | 100 | 1548 | 200 | 533 | 281 | 246 | 276 | 61 | 119 | - | 138 | 460 | - |
| AKI, n (%) | 70 (28) | 29 (29) | 159 (10.3) | 16 (8) | 143 (26.8) | 87 (31) | 36 (14.6) | 42 (15.2) | 28 (45.9) | 16 (13.4) | 115 | 62 (44.9) | 155 (33.7) | 121 (18.1)b |
| Age (yr) mean ± SD | 27.1 ± 15.8 | 24 | 39.1 | 40.3 ± 18.8 | 32 ± 15.9 | 36.1 ± 14.6 | 41.2 ± 12.4 | 43 ± 20 | 8 | 66 ± 7 | 35.9 ± 15 | 34.1 ± 1.6 | 36.3 ± 1.3 | 42.2 ± 15.1 |
| Male (%) | - | 83 | 56.7 | 50 | 69.4 | 70.1 | 55.5 | 76.2 | 53.6 | 50 | 62 | 80.6 | 69 | 42.1 |
| Duration at arrival (d) Mean ± SD | - | < 0.5 | 0.9 | 0.1 ± 0.1 | < 1 | 0.9 ± 1.8 | 0.9 | 1 | 0.2 | 1.3 ± 0.9 | 8.8 ± 5.6 | 2.8 ± 1.3 | 3.2a | 3.4 ± 3.7 |
| Bite site at lower limb (%) | - | 83 | 98.7 | 88 | 71.7 | 78.2 | 81.2 | - | - | - | - | - | 88.7 | 85.2 |
| Oligo-anuria, n (%) | 94 | 13.8 | 100 | 37.5 | - | 100 | 94 | - | 60.7 | - | 94 | - | 66 | 79.5 |
| Hematuria (%) | 80 | 83 | 70 | - | 64.3 | - | 80 | - | 24.6 | - | - | 58.1 | - | 54.5 |
| Limb swelling/cellulitis (%) | - | 98.7 | - | - | 92 | 91.6 | 0 | 57.1 | 39 | - | 41 | 51.6 | 63.2 | 13.6 |
| Hypotension (%) | 16 | - | 17.7 | - | - | 19.5 | 33.3 | - | - | 6.3 | - | 45.2 | 33.5 | 4.5 |
| Neurotoxicity (%) | - | 100 | - | - | - | 12.1 | - | 28.6 | - | - | 12.1 | 3.2 | - | 5.7 |
| Bleeding/coagulopathy (%) | - | 83 | 27.8 | - | - | 83 | 38.8 | 52.4 | 37.7 | - | 65.2 | 50 | 65.8 | 88.6 |
| Hemoglobin (g/dL) | 7 ± 2.6 | - | - | 13.2 ± 3.4 | - | 7.2 ± 1.4 | - | 9.4 ± 2.5 | - | 13.3 ± 1.2 | 8.3 | - | - | 9.6 ± 2.6 |
| WBC count (× 103/mL) | - | - | - | 19.3 ± 3.7 | - | - | - | 13.6 ± 5.2 | - | 11.5 ± 3.8 | 13.2 | - | - | 15 ± 7.4 |
| Urea (mg/dL) | 233 ± 107 | - | 100 | 113 ± 78 | - | 163 ± 83 | 85 ± 29.2 | 107 ± 74 | - | - | - | - | - | 169 ± 75 |
| Creatinine (mg/dL) | 9.2 ± 3.4 | - | 4.2 | 2.9 ± 0.5 | - | 4.9 ± 2.5 | 3.1 ± 1.5 | 3 ± 2.9 | - | - | - | - | 4.6 ± 0.2 | 7.2 ± 4.2 |
| Hemolysis (%) | 54 | - | 28.1 | - | - | - | 13.8 | - | - | - | - | - | - | 85.2 |
| Thrombocytopenia (%) | 14 | - | 25.2 | 60 | - | - | - | - | - | - | - | - | 9.7 | 47.7 |
| DIC (%) | 43 | - | 27.8 | 25 | - | - | 11.1 | - | - | - | - | - | 27.1 | 9.1 |
| RRT (%) | 75.7 | 24.1 | 45.3 | 25 | 57 | 55.2 | 44.4 | 30.6 | 35.7 | - | 92.2 | - | 100 | 81.8 |
| DOHS (d) | - | - | 5 | 6.7 ± 3.6 | - | - | 9 | 8 | 8 | 11.6 ± 2.7 | - | - | 11 | 10 |
| Mortality (%) | 28.6 | 10.3 | 22.6 | 18 | 21 | 39.1 | 22.2 | 0 | 14.3 | 6.3 | 13 | - | 29.7 | 9.1 |
Males are more often bitten than females, except where the work force is predominantly female (e.g., tea and coffee picking)[5]. Majority (58%) of patients in our study was found to be women in working age group whereas majority (50%-83%) of the victims in other studies was male[11,20-26,28-30]. Female preponderance in our study was due to involvement of women in hills in cutting grass for fodder more frequently.
Most snake-bites happen when the snake is trodden on, either in the dark or in undergrowth, by someone who is bare-footed or wearing only sandals. Bite site in majority (85.2%) of patients in current study was lower limb similar (72%-99%) to that in other studies[11,20-24,30]. Education on prevention of snake bite should be imparted among farmers and field workers. They should be advised to use long boots and not to walk on their bare feet or disturb snakes in the fields. Our study showed that we can prevent a large number of snake bites by protecting our feet.
Although bites from all the venomous snakes are known to cause AKI, a substantial proportion of these cases results from viper bites. Most Indian patients are victims of Russell’s viper or Echis carinatus bites[9]. Snake venom is complex poisons consisting of hundreds of different proteins: Enzymes, polypeptide toxins and non-toxic proteins. Phospholipase A2 is the most widespread and extensively studied of all venom enzymes. It damages mitochondria, red blood cells, leucocytes, platelets, peripheral nerve endings, skeletal muscle, vascular endothelium, and other membranes, produces presynaptic neurotoxic activity, opiate-like sedative effects, leads to the autopharmacological release of histamine and anti-coagulation. Viper bites cause various systemic symptoms such as: Coagulopathy; haemolysis; AKI; a generalized increase in capillary permeability; rhabdomyolysis; and neurotoxicity[5,8]. Hemoglobinuria caused by intravascular hemolysis and myoglobinuria resulting from rhabdomyolysis contribute importantly to the development of AKI after snake bite. Hemorrhage, hypotension, disseminated intravascular coagulation, intravascular hemolysis, and rhabdomyolysis enhance renal ischemia leading to renal failure. Enzymatic activities of snake venoms account for direct nephrotoxicity. Immunologic mechanism plays a minor role[8].
In various studies, the symptoms of oligoanuria has been reported to be 13.8%-100%[9,11,20,21,23,24,26,28,30] and hematuria/black colored urine 24.6%-80%[9,11,20,22,24,26,29] as compared to that of 79.5% and 54.5% respectively in our study. Cellulitis was 39%-98.7%[20,23-26,28-30] as compared to that of 13.6% observed in our study, hypotension of 4.5% observed in current study as compared to that of 6.3%-45.2% in other studies[9,20,23,24,27,29,30], neurotoxicity of 5.7% as compared to that of 3.2%-100% in other studies[11,23,25,28,29]. Bleeding/coagulopathy which is a major symptom of systemic viper poisoning was observed in 88.6% patients as compared to 27.8%-65.8% in other studies of snake bite-induced AKI[11,20,23-26,28-30].
Among laboratory investigations - hemoglobin of ranged from 7-13.3 g/dL in other studies[9,21,23,25,27,28] as compared to 9.6 g/dL in this study, WBC count ranged from 11.5-19.3 × 103/mL[21,25,27,28] as compared to 15 × 103/mL in current study, serum urea 85-233 mg/dL[9,20,21,23-25] as compared to 169 mg/dL and serum creatinine 2-9.2 mg/dL[9,20,21,23-26] as compared to 7.2 mg/dL in this study have been reported. Intravascular hemolysis has been reported in various studies to be 13.8%-54%[9,20,24], thrombocytopenia 9.7%-60%[9,20,21,30], and DIC 11.1%-43%[9,20,21,24,30] as compared to that of 85.2%, 47.7%, and 9.1 respectively in our study.
Rhabdomyolysis is well known cause of AKI[16]. Bites by sea-snakes are a common cause of rhabdomyolysis and myoglobinuria. Several Australian snakes are both myotoxic and hemotoxic. In Asia, Russell’s viper venom in certain geographic areas can cause both intravascular hemolysis and rhabdomyolysis[5,8]. Most of the studies of snake bite-induced AKI have only looked at the hematological and coagulation profile[9,20-30]. In our study all AKI patients were investigated for evidence of hemolysis and rhabdomyolysis. Rhabdomyolysis was observed in 68.2%of patients as compared to 100% of AKI following viper bite by Pinho et al[11]. It might have been missed in some of them due to delayed arrival at our hospital. Majority (60.2%) of our patients had both hemolysis and rhabdomyolysis. Both hemoglobinuria and myoglobinuria contribute to the development of AKI. Intravascular hemolysis and rhabdomyolysis were the main cause of AKI in our study patients.
Various procoagulant enzymes are found in viper venoms, which activate different steps of the clotting cascade resulting in a state of DIC. Bleeding tendencies can also cause severe blood loss resulting in hypotension, further adding to the renal insult or causing one when none existed. Fibrin thrombi in renal microvasculature glomerular capillaries, microangiopathic hemolytic anemia and thrombocytopenia in patients with cortical necrosis strongly suggest that DIC plays a major pathogenetic role in snake bite-induced cortical necrosis. Thus, bleeding tendencies secondary to DIC are a major factor in the development of AKI in patients of snake bite, especially those involving vipers[9,23]. DIC was observed in 9.1% patients in our study as compared to 11.1%-43% reported in other studies[9,20,21,24,30]. Since all patients were not investigated for evidence of DIC, we had DIC in a lower percentage of our patients.
Median duration of arrival at hospital in our study was 3 d as compared to duration of arrival from bite to the hospital or to receipt of ASV ranging from few hours to 8.8 d in other studies[11,20-30].
Incidence of complications is directly proportional to the duration of venom in the blood prior to neutralization by ASV. The early institution of ASV is beneficial in preventing complications however severe is the systemic envenomation[31]. Vijeth et al[32] found that none of the patient of snake bites with coagulopathy who received ASV within 8 h of being bitten developed renal abnormality. Patients who were left untreated due to late arrival at the hospital, the renal abnormality correlated well with the degree of coagulation abnormality.
The risk factors associated with development of AKI in snake bite are native treatment[26], bite to needle time more than 2 h[20], age[11,24,27], bite to hospital/ASV time[11,20,23,24,27,29], black or brown urine[29,33], bleeding manifestations[25,26,29], hypotension/shock[23,24], cellulitis[20,24,29], regional lymphadenopathy[20,24], abdominal pain/tenderness and vomiting[29], 20 WBCT > 20 min[33], prolonged bleeding time[23], prolonged prothrombin time[23], low hemoglobin[23], high WBC count[21], low serum albumin[21], and a high serum bilirubin[23], CK > 2000 U/L[11], albuminuria[23], intravascular hemolysis[20,24], DIC[21,24], complications of septicemia[24] and ARDS[24], and a longer DOHS[25].
The basic therapeutic approach for AKI in patients bitten by snakes is the same as that for AKI any other cause. Early administration of ASV is a vital therapeutic measure. Alkalization of urine by sodium bicarbonate helps in the prevention of AKI in the patient who has myoglobinuria or hemoglobinuria provided that this is performed early when dark urine is observed or when the snake is known to be myotoxic or hemotoxic. In patients with intravascular hemolysis and/or rhabdomyolysis, maintenance of a high urine output by increasing fluid intake and giving a diuretic, as well as rendering the urine alkaline early in the course of the disease may prevent renal damage[8,9,11,16]. Snake bite-induced AKI can be catabolic with rapid rise in blood urea and serum creatinine levels, hyperkalemia and severe metabolic acidosis. Early and frequent peritoneal dialyses and hemodialyses are life saving for AKI. Muscular symptoms caused by bites from sea snakes are improved by hemodialysis[8]. High proportion (82%) of our study patients had severe renal failure and received renal replacement therapy. Frequency of patients having received renal replacement therapy in other studies ranged from 24%-100%[9,11,20-26,30]. Each patient in our study received three sessions of hemodialysis similar that reported in the study by Harshavardhan et al[30].
Median DOHS in our study was 10 d as compared to 5-12 d reported in other studies[9,20,21,24-27,30]. AKI is a strong predictor of mortality among the in-hospital patients with snake bite[22,34]. Mortality of 9.1% was observed in our study. There was no mortality in the study from Brazil by Albuqerque et al[25]. However a mortality ranging from 6.3%-39.1% has been reported in other studies of snake bite-induced AKI[9,11,20-24,26,28,30].
The mean values of WBC counts and serum bilirubin were significantly higher and albumin levels were significantly lower in the fatal cases in our study. This suggests that the fatal cases might have had more severe envenomation. Because albumin is a relatively slow-reacting negative acute-phase reactant it may be associated with higher mortality in AKI[35]. Hypoalbuminemia was as result of a generalized increase in capillary permeability and bleeding complications. In these patients later or concomitant development of pneumonia and ARDS led to a fatal outcome. Further, complication of pneumonia/ARDS, seizure/encephalopathy, MOF, need for ICU support, and shorter DOHS were associated with mortality. Studies have found bite to needle time more than 2 h[20,24], hypotension/shock[20,24,30], bleeding manifestations[20], coagulopathy[36], thrombocytopenia[28], intravascular hemolysis[20], DIC[24,30], septicemia[24], encephalopathy[36] as risk factors/predictors for mortality in snake bite-induced AKI.
Several factors contribute to the increased mortality such as taking treatment from native healers, problems in transport, lack of availability of ASV, high cost and lack of hospital based facilities in rural areas and inadequate knowledge among medical practitioners regarding snake bite management[3,5,37]. At the community level, inappropriate first-aid measures such as incision, suction and herbal treatment are performed and vital time is lost before the victim is transported to a treatment centre[37,38]. Education strategies to inform the communities regarding appropriate first aid, rapid transport and management are necessary.
The renal lesions of clinical significance in envenomed patients are ATN, and patchy or diffuse RCN. Glomerulonephritis, interstitial nephritis, and papillary necrosis have been reported in rare patients. Tubulointerstitial lesions, predominantly ATN, are observed in 70% to 80% of patients with AKI[9]. Biopsies carried out early reveal morphological features of severe acute tubular injury with intratubular pigmented granular casts. Varying degree of acute tubulointerstitial nephritis and interstitial edema may be found. Late biopsies reveal regenerating tubular epithelium[9]. High proportion (91%) of patients subjected to kidney biopsy had ATN in our study. In addition, 41% of them had associated finding of mild to moderate AIN.
AIN was considered to be a rare after snake bite but according to recent studies AIN after snake bite is not uncommon[39,40]. AIN accounted for 5.7%-11.9% of snakebite-related AKI. All patients had severe envenomation at presentation[39,40], very high ASV requirement[39] and had prolonged renal failure[39,40]. Kidney biopsy found a mixed infiltrate composed of predominantly lymphocytes, with variable proportions of other cells including eosinophils neutrophils and plasma cells. The reason for development of this pathology is not clear, but direct venom related effects possibly lead to development of the interstitial inflammation via various cytokines, mediators and adhesions molecules[39,40]. AIN was found to be reversible with corticosteroids therapy[40]. However, the long-term outcome was found to be worse as compared with those who did not have AIN, as majority patient developed chronic kidney disease (CKD) on follow-up. Overall AIN was present in 45% of renal biopsies in our study. One patient in our study only had moderate AIN on biopsy and completely recovered after a course of corticosteroids therapy. There was no difference in the clinical picture between the patients of ATN and AIN. Patients remaining oligoanuric or whose serum creatinine did not decrease satisfactorily at the end of three weeks should be subjected to kidney biopsy. The kidney biopsy has a diagnostic, prognostic and therapeutic value in such patients. Patients with ATN or ATN associated with AIN are likely to recover. Those with AIN on kidney biopsy may be treated with a course of steroids to hasten the recovery and to prevent development of fibrosis. RCN carries a bad prognosis for renal recovery.
RCN is characterized by patchy or diffuse ischemic destruction of all the elements of renal cortex resulting from significantly diminished renal arterial perfusion due to vascular spasm and microvascular injury. In addition, direct endothelial injury particularly in setting of sepsis, eclampsia, haemolytic uremic syndrome and snake bite may lead to endovascular thrombosis with subsequent renal ischemia. It is a rare cause of AKI in developed countries whereas in developing countries it accounts for 3% of all causes of AKI[41]. The renal lesion that carries the most sinister prognosis is documented in patients bitten by Russell’s viper and Echis carinatus is RCN[5,9]. Patients with AKI who suffered oliguria for more than four weeks suggest the possibility of bilateral renal cortical necrosis. This can be confirmed by renal biopsy or contrast enhanced computed tomography (CT) scans of the kidneys. Patients with patchy cortical necrosis show delayed and partial recovery of renal function but those with diffuse cortical necrosis require regular maintenance dialysis and eventual renal transplantation[41]. RCN has been observed in 27 (24%) kidney biopsies out of total of 112 kidney biopsies carried out in patients of snake bite-induced AKI from India and Pakistan[9,28,39,40,42,43]. None had RCN on biopsy in two studies[39,40]. In our study one patient (4.5%) with patchy cortical necrosis on kidney biopsy had partial recovery of renal function and came off dialysis. Incidence of RCN in our study over 13 years was 0.8% as compared to 11.3% observed over 25 years in a recent study from Pakistan[28]. Delay in getting the ASV as in our case or lack of ASV treatment in high proportion (75%) of cases as reported in study by Chugh et al[44] might be a factor in development of this renal lesion.
Most of the patients of ATN and AIN recover by few weeks, with the help of renal replacement therapy, but who develops RCN requires renal replacement therapy on a long term basis. Further, long-term outcome of snake bite-induced severe dialysis-requiring AKI is not benign with a significant percentage of patients continuing to have features of persistent renal damage in the form of renal dysfunction, proteinuria, or hypertension[43]. CKD is an outcome of severe AKI following snake envenoming probably predicted by the length of RRT[44]. Among the patient who survived in the current study, all except two had complete renal recovery in hospital or on short term follow-up but long term outcome could not studied due to lack of such follow-up.
Our study is large series on clinico-pathological spectrum of snake bite-induced AKI from India. There are certain imitations in this study. Firstly it was a retrospective study carried out at a tertiary care renal unit where patients were referred from far flung rural areas. Most of the patient consult the traditional healers first, or wait to become severely symptomatic and the critical initial management period was lost. There were delays in seeking treatment at health centres and getting treatment with ASV due to its non-availability at peripheral health centres. Majority of the patients first received ASV at our centre. Secondly many clinical details might have been missed due to failure on the part of peripheral hospitals to observe or document them. The special investigations like complete coagulation profile as evidence for DIC were not done in all patients, identification of snake species and ELISA test to identify the snake venom are the other lacunae. Finally, we also could not assess long term residual injury in these cases of snake envenomation, although this is being increasingly recognized[43,45].
In conclusion, snake bite-induced AKI is an important cause of community acquired tropical AKI. The AKI due to snake bite was severe and a high proportion (82%) of patients required renal replacement therapy and the outcome was fatal in 9.1% of cases. A high WBC count, high total bilirubin, a low serum albumin, the complications of pneumonia/ARDS, seizure/encephalopathy, MOF, the need for ICU support and a shorter DOHS were factors associated with mortality. On renal histology ATN and AIN were common, RCN was rare.
AKI is an important complication of snake bite that may lead to mortality. Clinico-pathological spectrum of snake-induced AKI has changed. Intravascular hemolysis and rhabdomyolysis is common and is the main cause of AKI due to snake bite. AKI is severe and high proportion of patients require renal replacement therapy. Complications of pneumonia/ARDS, seizure/encephalopathy, multi organ failure, the need for intensive care support and a shorter hospital stay are factors associated with mortality. ATN and AIN are common findings on renal histology. RCN is a rare presentation. Early ASV administration and alkaline diuresis may help in prevention of AKI. Proper supportive management after ASV administration is of utmost importance, for a good patient outcome. Kidney biopsy is important and has a diagnostic, prognostic and therapeutic value for patients not showing satisfactory improvement by three weeks; ATN or AIN are likely to recover whereas RCN has poor renal recovery.
Snake bite envenomation is a major public health problem in tropical countries. Acute kidney injury (AKI) is an important complication and major cause of mortality in patients with snake bites. Although bites from all the venomous snakes are known to cause AKI, a substantial proportion of these cases results from viper bites. Exact pathogenesis of AKI following snake bite is not well established. The pathogenesis of renal lesions is multifactorial and has been attributed to the nephrotoxicity of venom, hypotension, circulatory collapse, and intravascular hemolysis with hemoglobinuria, myoglobulinuria, disseminated intravascular coagulation, hemolytic uremic syndrome, sepsis, and other factors such as hypersensitivity to venomous or antivenomous protein. On pathological investigations two main renal lesions responsible for AKI are tubular necrosis and renal cortical necrosis.
There is no study on clinicopathological spectrum of snake bite-induced AKI in the last more than quarter of a century. The authors did a retrospective study to analyze clinicopathological spectrum of snake bite-induced AKI over a 13-year period.
The authors’ study is large series on clinicopathological spectrum of snake bite-induced AKI in the last 25 years. Most of the studies of snake bite-induced AKI have only looked at the hematological and coagulation profile. In the present study all AKI patients were investigated for evidence of hemolysis and rhabdomyolysis. The authors found that the clinicopathological spectrum of snake-induced AKI has changed. Intravascular hemolysis and rhabdomyolysis is common and is the main cause of AKI due to snake bite. AKI is severe and a high proportion of patients require renal replacement therapy. Complications of pneumonia/acute respiratory distress syndrome, seizure/encephalopathy, multi organ failure, the need for intensive care support and a shorter hospital stay are factors associated with mortality. On renal histology acute tubular necrosis and acute interstitial nephritis is common, renal cortical necrosis is rare.
This study suggests that intravascular hemolysis and rhabdomyolysis is common, and is the main cause of AKI due to snake bite. Early administration anti-snake venom and alkaline diuresis may help in prevention of AKI. Dialysis and a proper supportive treatment are of utmost importance in the cases which are complicated by renal failure. The kidney biopsy has a diagnostic, prognostic and therapeutic value for patients not showing satisfactory improvement by three weeks; acute tubular necrosis or acute interstitial nephritis are likely to recover whereas renal cortical necrosis (RCN) has poor renal recovery.
RCN: It is characterized by patchy or diffuse ischemic destruction of all the elements of renal cortex resulting from significantly diminished renal arterial perfusion due to vascular spasm and microvascular injury. In addition, direct endothelial injury particularly in setting of sepsis, eclampsia, hemolytic uremic syndrome and snake bite may lead to endovascular thrombosis with subsequent renal ischemia. This renal lesion carries the most sinister prognosis.
The study is interesting and inclusive for the readers of the special field.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chang CC, Fujigaki Y S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1087] [Cited by in RCA: 1197] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 2. | WHO highlights critical need for life-saving antivenoms. [Internet]. Geneva: World Health Organization; 2014. [accessed 2014; Mar 14] Available from: http://www.who.int/mediacentre/news/notes /2010/antivenoms_20100504/en/. |
| 3. | Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, Rodriguez PS, Mishra K, Whitaker R, Jha P. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5:e1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 5. | Warrel DA. Guidelines for the management of snake-bites. World Health Organisation; WHO Library Cataloguing-in-Publication data (NLM classification: WD 410); 2010; 1-150. |
| 6. | Simpson ID, Norris RL. Snakes of medical importance in India: is the concept of the “Big 4” still relevant and useful? Wilderness Environ Med. 2007;18:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS Negl Trop Dis. 2010;4:e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Kanjanabuch T, Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol. 2008;28:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Chugh KS. Snake-bite-induced acute renal failure in India. Kidney Int. 1989;35:891-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 10. | Myint-Lwin DA, Phillips RE. Bites by Russell’s viper (Vipera russelli siamensis) in Burma: haemostatic, vascular, and renal disturbances and response to treatment. Lancet. 1985;2:1259-1264. [PubMed] |
| 11. | Pinho FM, Zanetta DM, Burdmann EA. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int. 2005;67:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Wikipedia contributors. Himachal Pradesh [Internet]. Wikipedia, The Free Encyclopedia. [retrieved 2016 Aug 28]. Available from: http://en.wikipedia.org/wiki/Himachal_Pradesh. |
| 13. | Bhardwaj A, Sokhey J. Snake bites in the hills of north India. Natl Med J India. 1998;11:264-265. [PubMed] |
| 14. | Saikia U, Sharma DK, Sharma RM. Checklist of the reptilian fauna of Himachal Pradesh, India. Reptile Rap Newsl S Asian Reptile Netw. 2007;8:6-9. |
| 15. | Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1192] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 16. | Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553-1561. [PubMed] |
| 17. | Yu M, Nardella A, Pechet L. Screening tests of disseminated intravascular coagulation: guidelines for rapid and specific laboratory diagnosis. Crit Care Med. 2000;28:1777-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Ahuja ML, Singh G. Snake bite in India. Indian J Med Res. 1954;42:661-686. [PubMed] |
| 19. | Sawai Y, Honma M. Snake bites in India. Animal, Plant and Microbial Toxins Vol 1: Biochemistry. New York: Plenum 1976; 451-460. [DOI] [Full Text] |
| 20. | Athappan G, Balaji MV, Navaneethan U, Thirumalikolundusubramanian P. Acute renal failure in snake envenomation: a large prospective study. Saudi J Kidney Dis Transpl. 2008;19:404-410. [PubMed] |
| 21. | Danis R, Ozmen S, Celen MK, Akin D, Ayaz C, Yazanel O. Snakebite-induced acute kidney injury: data from Southeast Anatolia. Ren Fail. 2008;30:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | David S, Matathia S, Christopher S. Mortality predictors of snake bite envenomation in southern India--a ten-year retrospective audit of 533 patients. J Med Toxicol. 2012;8:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Dharod MV, Patil TB, Deshpande AS, Gulhane RV, Patil MB, Bansod YV. Clinical predictors of acute kidney injury following snake bite envenomation. N Am J Med Sci. 2013;5:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Harshavardhan L, Lokesh AJ, Tejeshwari HL, Halesha BR, Siddharama SM. A study on the acute kidney injury in snake bite victims in a tertiary care centre. J Clin Diagn Res. 2013;7:853-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Albuquerque PL, Silva GB, Jacinto CN, Lima JB, Lima CB, Amaral YS, Veras Mdo S, Mota RM, Daher EF. Acute kidney injury after snakebite accident treated in a Brazilian tertiary care centre. Nephrology (Carlton). 2014;19:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Krishnamurthy S, Gunasekaran K, Mahadevan S, Bobby Z, Kumar AP. Russells Viper Envenomation-associated Acute Kidney Injury in Children in Southern India. Indian Pediatr. 2015;52:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Li W, Chen F, Wu S. The Related Risk Factors Analysis of Snake-Bite Induced Acute Kidney Injury. Med Sci Monit. 2016;22:2335-2339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Naqvi R. Snake-bite-induced Acute Kidney Injury. J Coll Physicians Surg Pak. 2016;26:517-520. [PubMed] |
| 29. | Singh RR, Uraiya D, Kumar A, Tripathi N. Early demographic and clinical predictors of developing acute kidney injury in snake bite patients: A retrospective controlled study from an Indian tertiary care hospital in North Eastern Uttar Pradesh India. Indian J Crit Care Med. 2016;20:404-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Harshavardhan L, Lokesh AJ, Tejeshwari HL, Halesha BR, Siddharama SM, Mishra R, Mukherjee D, Mishra R, Kar M. Snakebite mediated acute kidney injury, prognostic predictors, oxidative and carbonyl stress: A prospective study. Indian J Nephrol August. 2016; Epub ahead of print. |
| 31. | Narvencar K. Correlation between timing of ASV administration and complications in snake bites. J Assoc Physicians India. 2006;54:717-719. [PubMed] |
| 32. | Vijeth SR, Dutta TK, Shahapurkar J. Correlation of renal status with hematologic profile in viperine bite. Am J Trop Med Hyg. 1997;56:168-170. [PubMed] |
| 33. | Paul J, Dasgupta S. Early prediction of acute kidney injury by clinical features of snakebite patients at the time of hospital admission. N Am J Med Sci. 2012;4:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Kalantri S, Singh A, Joshi R, Malamba S, Ho C, Ezoua J, Morgan M. Clinical predictors of in-hospital mortality in patients with snake bite: a retrospective study from a rural hospital in central India. Trop Med Int Health. 2006;11:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Berbel MN, Pinto MP, Ponce D, Balbi AL. Nutritional aspects in acute kidney injury. Rev Assoc Med Bras (1992). 2011;57:600-606. [PubMed] |
| 36. | Patil TB, Bansod YV. Snake bite-induced acute renal failure: A study of clinical profile and predictors of poor outcome. Ann Trop Med Public Health. 2012;5:335-339. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Warrell DA. Snake bite. Lancet. 2010;375:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 555] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 38. | Warrell DA. Snake bite: a neglected problem in twenty-first century India. Natl Med J India. 2011;24:321-324. [PubMed] |
| 39. | Golay V, Roychowdhary A, Pandey R, Singh A, Pasari A, Abraham A. Acute interstitial nephritis in patients with viperine snake bite: single center experience of a rare presentation. Saudi J Kidney Dis Transpl. 2012;23:1262-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Priyamvada PS, Shankar V, Srinivas BH, Rajesh NG, Parameswaran S. Acute Interstitial Nephritis Following Snake Envenomation: A Single-Center Experience. Wilderness Environ Med. 2016;27:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Prakash J, Singh VP. Changing picture of renal cortical necrosis in acute kidney injury in developing country. World J Nephrol. 2015;4:480-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Merchant MR, Khanna UB, Almeida AF, Acharya VN, Mittal BV. Clinicopathological study of acute renal failure following viperine snake bite. J Assoc Physicians India. 1989;37:430-433. [PubMed] |
| 43. | Waikhom R, Sircar D, Patil K, Bennikal M, Gupta SD, Pandey R. Long-term renal outcome of snake bite and acute kidney injury: a single-center experience. Ren Fail. 2012;34:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Chugh KS, Pal Y, Chakravarty RN, Datta BN, Mehta R, Sakhuja V, Mandal AK, Sommers SC. Acute renal failure following poisonous snakebite. Am J Kidney Dis. 1984;4:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Herath HM, Wazil AW, Abeysekara DT, Jeewani ND, Weerakoon KG, Ratnatunga NV, Bandara EH, Kularatne SA. Chronic kidney disease in snake envenomed patients with acute kidney injury in Sri Lanka: a descriptive study. Postgrad Med J. 2012;88:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |