Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.482
Peer-review started: March 22, 2016
First decision: April 20, 2016
Revised: April 23, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: September 6, 2016
Processing time: 166 Days and 3.9 Hours
To assess red blood cell (RBC) transfusion effects on acute kidney injury (AKI) after transcatheter aortic valve replacement (TAVR).
A literature search was performed using MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, and clinicaltrials.gov from the inception of the databases through December 2015. Studies that reported relative risk, odds ratio or hazard ratio comparing the risks of AKI following TAVR in patients who received periprocedural RBC transfusion were included. Pooled risk ratio (RR) and 95%CI were calculated using a random-effect, generic inverse variance method.
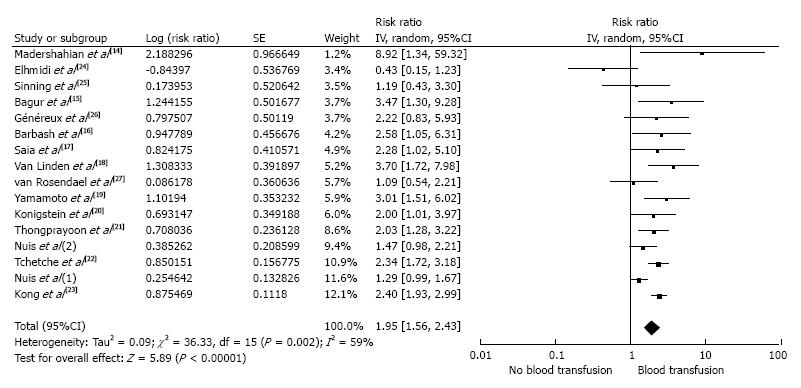
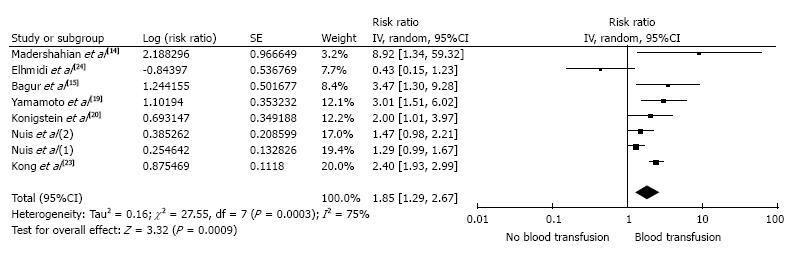
Sixteen cohort studies with 4690 patients were included in the analyses to assess the risk of AKI after TAVR in patients who received a periprocedural RBC transfusion. The pooled RR of AKI after TAVR in patients who received a periprocedural RBC transfusion was 1.95 (95%CI: 1.56-2.43) when compared with the patients who did not receive a RBC transfusion. The meta-analysis was then limited to only studies with adjusted analysis for confounders assessing the risk of AKI after TAVR; the pooled RR of AKI in patients who received periprocedural RBC transfusion was 1.85 (95%CI: 1.29-2.67).
Our meta-analysis demonstrates an association between periprocedural RBC transfusion and a higher risk of AKI after TAVR. Future studies are required to assess the risks of severe AKI after TAVR requiring renal replacement therapy and mortality in the patients who received periprocedural RBC transfusion.
Core tip: We performed this meta-analysis to assess the impact of periprocedural red blood cell (RBC) transfusion on the risk of acute kidney injury (AKI) after transcatheter aortic valve replacement (TAVR). We verified a significant association between peri-procedural RBC transfusion and AKI after a TAVR with an overall 1.95-fold increased risk of AKI compared to those who did not receive transfusion. This study highlights the importance of vigilance when considering transfusions and should impact the clinical management of the high-risk group of patients undergoing TAVR.
- Citation: Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB. Association of blood transfusion with acute kidney injury after transcatheter aortic valve replacement: A meta-analysis. World J Nephrol 2016; 5(5): 482-488
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/482.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.482
Patients with severe symptomatic aortic stenosis have destitute prognosis with medical treatment alone[1]. Transcatheter aortic valve replacement (TAVR), also known as transcatheter aortic valve implantation, is an exciting new approach to the treatment of high-risk or inoperable patients with severe aortic stenosis[2-6]. Despite advances in TAVR procedures, acute kidney injury (AKI) is one of the most frequent complications of TAVR, ranging in the literature from 15% to 57%[3,7-9]. Notably, the subset of patients, who develop AKI after TAVR, also have a high mortality rate of 9%-44% at 30 d and 32%-56% at 1 year[7,8].
Perioperative anemia has been shown to be independently associated with AKI after cardiac surgery[10,11]. Anemia can result in decreased renal oxygen delivery, increased oxidative stress and impaired hemostasis[10]. Thus, perioperative red blood cell (RBC) transfusion is used to improve oxygen delivery. However, stored RBC transfusion can also promote a pro-inflammatory state, impair tissue oxygen delivery, and induce tissue oxidative stress[12,13]. The association of AKI with RBC transfusion after TAVR is conflicting. While a few studies have demonstrated a higher incidence of AKI among patients who received periprocedural RBC transfusion[14-23], the others have shown no such association[24-29]. Thus, we conducted this meta-analysis to assess the impact of periprocedural RBC transfusion on the risk of AKI after TAVR.
Two investigators (Thongprayoon C and Cheungpasitporn W) independently searched published studies and conference abstracts indexed in MEDLINE, EMBASE, Cochrane Database of Systematic Reviews and clinicaltrials.gov from the inception of the databases through December 2015. The search strategy used is described in the supplementary material. A manual search for additional relevant studies using the references from these retrieved articles was also performed. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for a systematic review and meta-analysis[30].
We included studies that: (1) enrolled adult (≥ 18 years old) patients; (2) provided information about periprocedural RBC transfusion and comparator patients who did not receive RBC transfusion; (3) included AKI after TAVR as an outcome; (4) were randomized clinical trials or observational studies (case-control, cross-sectional or cohort studies) published as original studies or conference abstracts; and (5) provided data to calculate odds ratios (ORs), relative risks, hazard ratios (HRs) or standardized incidence ratios with 95%CIs. No language limits were applied.
Study eligibility was independently determined by the two investigators noted previously. Differing decisions were resolved by mutual consensus. The quality of each study was evaluated using the Newcastle-Ottawa quality assessment scale[31].
A standardized data collection form was used to extract the following information: Last name of the first author, article title, study design, year of study, country of origin, year of publication, sample size, AKI definition, blood transfusion, confounder adjustment, and the adjusted effect estimate with 95%CI.
Review Manager software (Version 5.3, Copenhagen, Denmark) from the Cochrane Collaboration was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird[32]. Given the high likelihood of between-study variances, a random-effect model was used. Statistical heterogeneity was assessed using Cochran’s Q test. This statistic was complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. An I2 of 0%-25% represents insignificant heterogeneity, 26%-50% low heterogeneity, 51%-75% moderate heterogeneity and > 75% high heterogeneity[33]. The presence of publication bias was assessed by funnel plots of the logarithm of ORs vs their standard errors[34].
Our search strategy yielded 1327 articles. Of these, 1169 articles were excluded based on their relevance and the eligibility criteria, following the review of their title and abstract. The remaining 158 articles underwent full-length review and an additional 142 were excluded for failing to meet the criteria: 114 articles did not report the outcome of interest; and 28 articles were not observational studies or radomized clinical trials. Sixteen cohort studies were included in the meta-analysis to assess the risk of AKI after TAVR in patients who received periprocedural RBC transfusion (Table 1). Of the 16 cohort studies, eight studies performed adjusted analysis for known risk factors for AKI[14,15,19,20,23,24,28,29]. Supplementary Item 2 outlines our search methodology and selection process.
| Ref. | Country1 | Year | n | Transfusion definition | AKI definition (changes in baseline) | RR for AKI | Confounder adjustment | S, C, O2 |
| Sinning et al[25] | Germany | 2010 | 77 | RBC in 2 d post-procedure | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% or U output < 0.5 mL/kg per hour for > 6 h in 48 h post procedure | 1.19 (0.43-3.31) | None | 3, 0, 3 |
| Bagur et al[15] | Canada | 2010 | 213 | Peri-procedural blood | Decrease in eGFR of > 25% at 48 h post procedure or hemodialysis needed during index hospitalization | 3.47 (1.30-9.29) | HTN, COPD | 3, 1, 3 |
| Van Linden et al[18] | Germany | 2011 | 261 | Blood > 4 u in 7 d post-operative | Decrease in eGFR of > 25% or increase in SCr of 50% in 7 d post procedure | AKI 3.7 (1.7-7.9) RRT 8.8 (1.7-45.6) | None | 3, 0, 3 |
| Nuis et al[29] | Netherlands | 2011 | 118 | Peri-procedural RBC | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 1.29 (1.01-1.70) | Previous MI, leukocyte count, logistic EuroScore | 3, 1, 3 |
| Elhmidi et al[24] | Germany | 2011 | 234 | Post-operative blood | Decrease in eGFR of > 25% or increase in SCr of 50% in 7 d post procedure | 0.43 (0.15-1.23) | Baseline creatinine, STS score, DM | 3, 2, 3 |
| Madershahian et al[14] | Germany | 2012 | 50 | RBC | Increase in SCr of ≥ 0.5 mg/dL or ≥ 25% from baseline within 48 h post procedure | 8.92 (1.34-59.26) | COPD and contrast amount | 3, 1, 3 |
| Kong et al[23] | Australia | 2012 | 52 | Peri-procedural RBC | SCr criteria of RIFLE classification in 48 h post procedure | 2.4 (2.0-3.1) | TA, history of HTN | 3, 1, 3 |
| Tchetche et al[22] | France, Netherlands, Italy | 2012 | 743 | RBC | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 2.34 (1.72-3.18) | None | 3, 0, 3 |
| Barbash et al[16] | United States | 2012 | 165 | Post procedure blood | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 2.58 (1.05-6.29) | None | 3, 0, 3 |
| Nuis et al[28] | Netherlands, Canada, Germany, Belgium, Columbia | 2012 | 995 | RBC in 24 h post procedure | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 1-2 u, 1.47 (0.98-2.22); 3-4 u, 3.05 (1.24-7.53); ≥ 5 u, 4.81 (1.45-15.95) | PVD, CHF, maximal leukocyte count, logistic EuroScore | 3, 2, 3 |
| Saia et al[17] | Italy | 2013 | 102 | Peri-procedural RBC | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 2.28 (1.02-5.10) | None | 3, 0, 3 |
| Konigstein et al[20] | Israel | 2013 | 251 | Blood | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 2.00 (1.01-3.97) | Gender, HTN, DM, dyslipidemia, PVD, CHF, stroke, COPD, PHTN, VC, CKD, valve type and size | 3, 2, 3 |
| Yamamoto et al[19] | France | 2013 | 415 | RBC | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 72 h post procedure | 3.01 (1.54-6.15) | Contrast amount and LVEF | 3, 1, 3 |
| Généreux et al[26] | United States | 2013 | 218 | Blood | VARC-modified RIFLE stage 2 or 3 until discharge | 2.22 (0.83-5.92) | None | 3, 0, 3 |
| Thongprayoon et al[21] | United States | 2015 | 386 | Intra-operative RBC | Increase in SCr of ≥ 0.3 mg/dL in 48 h or ≥ 50% in 7 d post procedure | 2.03 (1.28-3.23) | None | 3, 0, 3 |
| van Rosendael et al[27] | Netherlands | 2015 | 210 | Peri-procedural RBC | Increase in SCr of ≥ 0.3 mg/dL or ≥ 50% in 7 d post procedure | 1.09 (0.54-2.22) | None | 3, 0, 3 |
All observational studies were considered moderate to high quality, with a median Newcastle-Ottawa quality assessment scale of 6.5 (range: 6-8) as shown in Table 1.
All included studies identified the AKI occurrence, based on the change in serum creatinine (SCr) or GFR after TAVR. One of the included studies also used urine output criteria for the AKI diagnosis[25]. Twelve[16,17,19-23,25-29] of the 16 included studies used standard AKI definitions (modified Risk, Injury, Failure, Loss of kidney function[35]; Acute Kidney Injury Network[36]; or Kidney Disease Improving Global Outcomes criteria[37]), as shown in Table 1.
The pooled risk ratio (RR) of AKI following TAVR in patients who received a RBC transfusion was 1.95 (95%CI: 1.56-2.43; I2 = 59%). Figure 1 shows the forest plot of the included studies. When meta-analysis was limited to the studies using standard AKI definitions, the pooled RRs were 1.89 (95%CI: 1.55-2.31; I2 = 50%). To minimize the effects of confounders, we performed a sensitivity analysis and excluded studies that did not include an adjusted analysis for known risk factors for AKI. The pooled RR of AKI after TAVR remained significant in patients who received periprocedural RBC transfusions (RR = 1.85; 95%CI: 1.29-2.67; I2 = 75%), shown in Figure 2.
Nuis et al[28] assessed the dose response relationship of a RBC transfusion and AKI, and demonstrated an increased risk of AKI with a higher number of RBC transfusions with ORs of 1.47 (95%CI: 0.98-2.22), 3.05 (95%CI: 1.24-7.53), 4.81 (95%CI: 1.45-15.95) for 1-2 units, 3-4 units, and ≥ 5 units of RBC transfusion, respectively. Reporting of severe AKI requiring renal replacement therapy (RRT) was limited. Van Linden et al[18] reported a higher risk of AKI requiring RRT with an OR of 8.8 (95%CI: 1.7-45.6; Table 1).
In this meta-analysis, we verified a significant association between peri-procedural RBC transfusion and AKI after a TAVR with an overall 1.95-fold increased risk of AKI compared to those who did not receive transfusion. This association remained significant when adjusting for potential confounders.
The mechanism for the higher incidence of AKI after TAVR in patients with a periprocedural RBC transfusion is not well-elucidated. Analysis has shown that preserved RBCs used in transfusions undergo progressive structural and functional changes during storage, such as reduced deformability and increased tendency to aggregate. These changes result in the deterioration of RBC function and viability, and the resultant accumulation of free iron and pro-inflammatory agents[38] leads to AKI[8,39-41]. Studies have also shown an association between RBC transfusions and increased leukocyte count in patients who developed AKI after TAVR[28,29].
Nuis et al[28] reported that the number of RBC transfusions was an independent predictor of AKI following TAVR. In their study, a higher number of RBC transfusions were found to be associated with a higher AKI incidence. Interestingly, the investigators did not find significant associations between AKI and the clinical indications for transfusion (i.e., baseline anemia, bleeding complications, or blood loss).
The risks of transfusion-associated AKI is not limited to TAVR; patients undergoing coronary artery bypass grafting or surgical aortic valve replacements who require transfusions also have a higher frequency of AKI[41-43].
Although the included studies in our meta-analysis were all of moderate to high quality, there are some limitations of this study that bear mentioning. First, there were statistical heterogeneities among the enrolled studies. The potential sources of these heterogeneities include the variations in the diagnostic methodology of AKI after TAVR and the differences in confounder adjustment methods. Second, the data on severe AKI requiring RRT after TAVR is lacking. Further studies are certainly warranted to further delineate the impact of transfusions after TAVR with specific regard to the severity of AKI. Third, the data on valve size and approaches for TAVR procedure were limited. These factors might have affected the risk of AKI following TAVR. Lastly, this is a meta-analysis of observational studies with the inherent limitation that a causal relationship cannot be inferred.
The threshold for transfusions is constantly changing. The deleterious effects of transfusions are well documented, and many institutions have worked hard to create protocols to diminish unnecessary transfusions. Our meta-analysis demonstrates an association between periprocedural RBC transfusion and a higher risk of AKI following TAVR. In many cases, patients undergoing TAVR have considerable debility and comorbid conditions. This study highlights the importance of vigilance when considering transfusions and should impact the clinical management of the high-risk group of patients undergoing TAVR.
Transcatheter aortic valve replacement (TAVR) is an exciting new approach to the treatment of high-risk or inoperable patients with severe aortic stenosis. Despite advances in TAVR procedures, acute kidney injury (AKI) is one of the most frequent complications of TAVR, associated with significant morbidity and mortality following the procedures.
The association of AKI with red blood cell (RBC) transfusion after TAVR is conflicting in the findings of previous literature. It is thus necessary to assess the impact of periprocedural RBC transfusion on the risk of AKI after TAVR.
In this study, the authors verified a significant association between peri-procedural RBC transfusion and AKI after a TAVR with an overall 1.95-fold increased risk of AKI compared to those who did not receive transfusion.
The data in this study highlights the importance of vigilance when considering transfusions and should impact the clinical management of the high-risk group of patients undergoing TAVR.
PRISMA: Preferred reporting items for systematic reviews and meta-analyses, etc.
This is a reasonable first meta-analysis of association of blood transfusion with AKI after transcatheter aortic valve replacement. The results have potential clinical applications.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koudoumas D, Yong D S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 682] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 2. | O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 936] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 3. | Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1971] [Cited by in RCA: 2166] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 4. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521-e643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 894] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 5. | Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB, Chetcuti S. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2015;66:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 6. | Cheungpasitporn W, Thongprayoon C, Kashani K. Transcatheter Aortic Valve Replacement: a Kidney’s Perspective. J Renal Inj Prev. 2016;5:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Greason KL, Kashani KB. Acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. Am J Nephrol. 2015;41:372-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, Piazza N. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Arch Cardiovasc Dis. 2014;107:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Thongprayoon C, Cheungpasitporn W, Srivali N, Harrison AM, Gunderson TM, Kittanamongkolchai W, Greason KL, Kashani KB. AKI after Transcatheter or Surgical Aortic Valve Replacement. J Am Soc Nephrol. 2016;27:1854-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Najjar M, Salna M, George I. Acute kidney injury after aortic valve replacement: incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther. 2015;13:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67:1112-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth. 2012;109 Suppl 1:i29-i38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Rawn JD. Blood transfusion in cardiac surgery: a silent epidemic revisited. Circulation. 2007;116:2523-2524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Madershahian N, Scherner M, Liakopoulos O, Rahmanian P, Kuhn E, Hellmich M, Mueller-Ehmsen J, Wahlers T. Renal impairment and transapical aortic valve implantation: impact of contrast medium dose on kidney function and survival. Eur J Cardiothorac Surg. 2012;41:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, Masson JB, Gutiérrez MJ, Clavel MA, Bertrand OF. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865-874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 16. | Barbash IM, Ben-Dor I, Dvir D, Maluenda G, Xue Z, Torguson R, Satler LF, Pichard AD, Waksman R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Saia F, Ciuca C, Taglieri N, Marrozzini C, Savini C, Bordoni B, Dall’Ara G, Moretti C, Pilato E, Martìn-Suàrez S. Acute kidney injury following transcatheter aortic valve implantation: incidence, predictors and clinical outcome. Int J Cardiol. 2013;168:1034-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Van Linden A, Kempfert J, Rastan AJ, Holzhey D, Blumenstein J, Schuler G, Mohr FW, Walther T. Risk of acute kidney injury after minimally invasive transapical aortic valve implantation in 270 patients. Eur J Cardiothorac Surg. 2011;39:835-842; discussion 842-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Yamamoto M, Hayashida K, Mouillet G, Chevalier B, Meguro K, Watanabe Y, Dubois-Rande JL, Morice MC, Lefèvre T, Teiger E. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Konigstein M, Ben-Assa E, Abramowitz Y, Steinvil A, Leshem Rubinow E, Havakuk O, Arbel Y, Halkin A, Keren G, Banai S. Usefulness of updated valve academic research consortium-2 criteria for acute kidney injury following transcatheter aortic valve implantation. Am J Cardiol. 2013;112:1807-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Thongprayoon C, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Greason KL, Kashani KB. Incidence and risk factors of acute kidney injury following transcatheter aortic valve replacement. Nephrology (Carlton). 2015; Dec 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Tchetche D, Van der Boon RM, Dumonteil N, Chieffo A, Van Mieghem NM, Farah B, Buchanan GL, Saady R, Marcheix B, Serruys PW. Adverse impact of bleeding and transfusion on the outcome post-transcatheter aortic valve implantation: insights from the Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC Plus) initiative. Am Heart J. 2012;164:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Kong WY, Yong G, Irish A. Incidence, risk factors and prognosis of acute kidney injury after transcatheter aortic valve implantation. Nephrology (Carlton). 2012;17:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Elhmidi Y, Bleiziffer S, Piazza N, Hutter A, Opitz A, Hettich I, Kornek M, Ruge H, Brockmann G, Mazzitelli D. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J. 2011;161:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Sinning JM, Ghanem A, Steinhäuser H, Adenauer V, Hammerstingl C, Nickenig G, Werner N. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 26. | Généreux P, Kodali SK, Green P, Paradis JM, Daneault B, Rene G, Hueter I, Georges I, Kirtane A, Hahn RT. Incidence and effect of acute kidney injury after transcatheter aortic valve replacement using the new valve academic research consortium criteria. Am J Cardiol. 2013;111:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | van Rosendael PJ, Kamperidis V, van der Kley F, Katsanos S, Al Amri I, Regeer MV, Schalij MJ, de Weger A, Marsan NA, Bax JJ. Atherosclerosis burden of the aortic valve and aorta and risk of acute kidney injury after transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr. 2015;9:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Nuis RJ, Rodés-Cabau J, Sinning JM, van Garsse L, Kefer J, Bosmans J, Dager AE, van Mieghem N, Urena M, Nickenig G. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2012;5:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Nuis RJ, Van Mieghem NM, Tzikas A, Piazza N, Otten AM, Cheng J, van Domburg RT, Betjes M, Serruys PW, de Jaegere PP. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2011;77:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17508] [Article Influence: 1094.3] [Reference Citation Analysis (1)] |
| 31. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12615] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 32. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 33. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46435] [Article Influence: 2110.7] [Reference Citation Analysis (3)] |
| 34. | Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867-872. [PubMed] |
| 35. | Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 529] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 36. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4984] [Article Influence: 276.9] [Reference Citation Analysis (0)] |
| 37. | Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1678] [Cited by in RCA: 1923] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 38. | Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 994] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 39. | Comporti M, Signorini C, Buonocore G, Ciccoli L. Iron release, oxidative stress and erythrocyte ageing. Free Radic Biol Med. 2002;32:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 996] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 41. | Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 670] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 42. | Bove T, Calabrò MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:442-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, Dupuis JY, Fremes SE, Kent B, Laflamme C. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 511] [Article Influence: 31.9] [Reference Citation Analysis (0)] |