Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.461
Peer-review started: January 18, 2016
First decision: February 29, 2016
Revised: July 16, 2016
Accepted: August 17, 2016
Article in press: August 19, 2016
Published online: September 6, 2016
Processing time: 227 Days and 17.7 Hours
To describe the technique of immunofluorescence on paraffin embedded tissue sections and discuss the potential pitfalls with an in depth review of literature.
Immunofluorescence is integral to diagnostic renal pathology. Immunofluorescence on paraffin embedded renal biopsies (IF-P) after enzyme treatment has been described in literature, however has not found widespread use in renal pathology laboratories. In our laboratory proteinase K digestion of paraffin embedded renal biopsy material was standardized and applied prospectively in cases where immunofluorescence on fresh frozen tissue was non contributory or not possible. Diagnostic utility was assessed and in a cohort of cases comparison of intensity of staining with routine immunofluorescence was performed.
Over the 5-year study period, of the 3141 renal biopsies received IF-P was performed on 246 cases (7.7%) and was interpretable with optimal digestion in 214 cases (6.8%). It was of diagnostic utility in the majority of cases, which predominantly included glomerular disease. Non-diagnostic IF-P was found in membranous nephropathy (2 of 11 cases), membranoproliferative glomerulonephritis (2 of 32 cases), lupus nephritis (1 of 25 cases), post infectious glomerulonephritis (1 of 11 cases) and chronic glomerulonephritis (3 of 8 cases). Comparing cases with both routine IF and IF-P, 35 of 37 showed either equal intensity or a minor difference in intensity of staining (1+) for the diagnostic immunoglobulin/complement. Technically assessment of immunofluorescence on the paraffin embedded tissue was found to be easier with clearly observed morphology, however a false positive staining pattern was observed in under-digested tissue.
As a “salvage” technique, immunofluorescence on paraffin embedded renal biopsies is of great diagnostic utility, however not without pitfalls.
Core tip: Immunofluorescence on formalin fixed paraffin embedded tissue is a useful “salvage” technique for renal diagnostic pathology, in case of non-availability of representative fresh frozen tissue. This article describes the technique of immunofluorescence on paraffin embedded tissue sections, discusses the potential pitfalls with an in depth review of literature.
- Citation: Singh G, Singh L, Ghosh R, Nath D, Dinda AK. Immunofluorescence on paraffin embedded renal biopsies: Experience of a tertiary care center with review of literature. World J Nephrol 2016; 5(5): 461-470
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/461.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.461
Immunofluorescence (IF) is an indispensible technique for rendering an accurate diagnosis in renal pathology. Diseases such as IgA nephropathy (IgAN), C1q nephropathy (C1qN) and C3 glomerulopathy (C3G) cannot be diagnosed without IF. Direct immunofluorescence (DIF) on fresh frozen tissue (IF-F) is the most widely used IF technique. Not uncommonly, however IF-F is not satisfactory due to non-representative sampling (medulla) or is not possible due to unavailability of fresh unfixed tissue, such as in referral cases and archived tissue. This leads to incomplete diagnosis and suboptimal patient management. To overcome these hurdles a method of enzymatic digestion of formalin fixed paraffin embedded tissue was standardized and introduced in our laboratory in 2011.
Enzymatic digestion breaks the protein cross linkages formed during formalin fixation[1] thereby exposing the antigenic immune complexes to staining with FITC (fluorescein isothiocyanate) labeled antibodies. Though this technique has been described in literature using different enzymes with the earliest report in 1976[2], it is still not in widespread use in laboratories handling renal biopsies.
We discuss our experience with this technique in day-to-day diagnostic renal pathology, its utility in reaching final diagnoses and compare it with usual IF-F where available. Technical and interpretation issues faced are described in detail, and may be helpful to any laboratory planning to introduce this technique.
Standardization: In a case of diffuse proliferative lupus nephritis, proteinase K (Sigma Aldrich, United States) enzymatic digestion was standardized (at concentrations according to manufacturer’s protocol) with variation in timing of exposure at room temperature. Results were compared for the adequacy of digestion and intensity of staining for FITC-IgG.
IF-P was performed prospectively in cases where there was inadequate/non representative fresh frozen tissue, in referral blocks where fresh frozen tissue was not available and in cases where the renal pathologists wanted to confirm the findings of routine IF-F. The FITC labeled antibodies to be applied were dictated by light microscopic differential diagnoses in the case and included both full panel (IgA, IgG, IgM, C3, C1q, kappa and lambda) as well as limited panels.
In cases where there was optimal digestion and adequate material the IF-P results were evaluated by 2 renal pathologists (LS and GS) and semiquantitatively graded on a 0-3+ scale. In cases where IF-F was available for comparison, these were graded independently in a blinded manner and compared to the grading of IF-P results. All immunofluorescence images were digitally captured and archived.
Enzyme digestion with proteinase K was standardized and the protocol followed is described in Table 1. Standardization was performed at room temperature and slight variations in enzyme exposure depending on ambient temperature (ranging from 15 to 20 min) gave optimal digestion results. This obviated the need for maintaining slides at 37 °C in a water bath.
| Cut formalin fixed paraffin embedded tissue at 3-4 μ thickness on poly-L-Lysine coated slides |
| Deparrafinize and rehydrate tissue sections |
| Immerse in Tris EDTA pH 9 for 30 min at room temperature |
| Perform enzymatic digestion with proteinase K 1.25 mg/mL (Sigma Aldrich, United States) at room temperature for 15 min1 |
| Stop digestion by immersing in Tris EDTA at 4 °C |
| Leave in Tris EDTA for 40 min at 4 °C |
| Rinse in PBS for 10 min |
| Apply FITC conjugated polyclonal rabbit antibodies directed against IgG (dilution 1:50), IgM (1:60), IgA (1:60), C3 (1:30), C1q (1:30), kappa (1:25), and lambda (1:40) (BIOSB, Santa Barbara, CA, United States). Incubate for 2 h in a moist chamber in the dark |
| Rinse with PBS |
| Mount in glycerine |
| Examine slides under a dark field immunofluorescence microscope |
In the 5-year study period between March 2011 and May 2015, 3171 biopsies (both native and transplant) were received. IF-P was performed on a total of 246 cases (7.7%). The results could not be interpreted in 32 cases (13%) due to technical issues of under digestion (18 cases) and floating of tissue/inadequate tissue (14 cases).
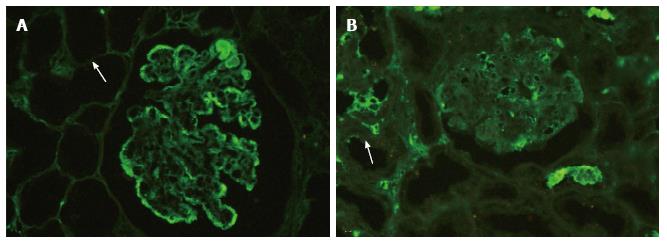
Therefore in 214 cases with adequate tissue, optimal digestion was achieved. Optimal digestion was determined on each individual slide by observing the tubules. From experience, the disappearance of tubular epithelial cell outline with only visible tubular basement membranes correlated with optimal digestion and detection of immune complexes in the tissue (Figure 1A). In under-digested glomeruli, a non-specific staining pattern was observed (Figure 1B) with the antibody appearing to stick to the surface of the capillary walls in a blotchy manner rather than labeling immune complexes/complement with granularity. This staining pattern was recognized as false positive.
The major utility of this technique was in classifying glomerular diseases, with limited utility in tubulo-interstitial diseases. Table 2 demonstrates the range of renal pathologies that were diagnosed.
| Diagnosis | Total number of cases | Number of cases with non diagnostic IF-P (%) | Remarks |
| MPGN | 32 | 21 (6.2%) | Classification into immune complex mediated MPGN ( 18 cases) and complement mediated MPGN ( 12 cases ) was possible 1In two cases C3 was not demonstrated and electron microscopy showed features of dense deposit disease |
| Membranous nephropathy | 11 | 21 (22.2%) | In one case staining intensity of IgG was only 1+, however staining pattern was classical 1In 2 cases significant fine granular immunofluorescence was not noted |
| Lupus nephritis | 25 | 11 (4%) | Classification into Class II ( 2 cases), Class III/IV ( 15 cases) and Class V (5 cases ) was possible Two cases of lupus podocytopathy were diagnosed 1In one case of lupus nephritis only IgM was demonstrated significantly, though electron dense deposits were noted on electron microscopy |
| Diffuse proliferative glomerulonephritis - post infectious glomerulonephritis | 12 | 11 (8.3%) | 1In one case only 1+ IgG and trace C3 deposition noted No tissue for electron microscopy was available |
| Pauciimmunecrescentic glomerulonephritis | 7 | - | - |
| IgAN | 39 | - | In 64 cases (minimal change morphology, mesangial proliferation or FSGS), IgAN was excluded by IF-P In one case of diabetic nephropathy IF-P was used to exclude secondary IgAN |
| C1q nephropathy | 2 | - | - |
| Light chain deposition disease | 1 | - | Tubular basement membrane and vascular deposits were also noted in addition to the glomerular deposits |
| Amyloidosis | 4 | - | 2 cases of AL amyloid (demonstrating light chain restriction) and 2 cases of AA amyloid |
| CGN | 8 | 3 (37.5%) | The immune complexes could not be demonstrated in 3 cases of chronic glomerulonephritis, one of these was a case of biopsy proven MPGN and the other was a case of IgAN. In one case of CGN no immune complexes were seen, however no previous renal biopsy record was available |
| Cast nephropathy | 2 | - | One case also demonstrated light chain restriction |
| Tubulointerstitial nephritis | 9 | - | Associated immune complex mediated glomerular disease was excluded |
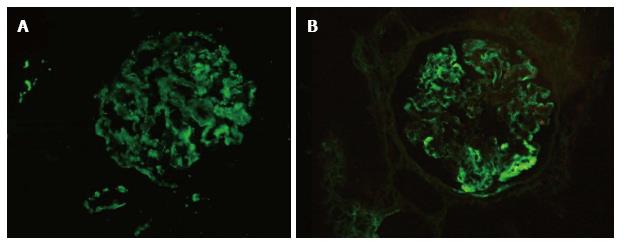
In membranoproliferative glomerulonephritis (MPGN, 32 cases), 30 cases could be adequately diagnosed and sub-classified based on the results of IF-P into immune complex mediated (18 cases) and complement mediated (12 cases) MPGN. In two cases with intramembranous dense transformation of the glomerular basement membrane on electron microscopy (dense deposit disease) no significant C3 deposition was noted on IF-P. One of these cases had a comparative IF-F with the diagnostic C3 dominant pattern and intensity 2+ (0-3+ scale) (Figure 2). One case of IC-MPGN showed 1+ IgG, kappa and lambda with 2+ C3 deposition, but characteristic MPGN type 1 pattern on ultrastructure examination; no comparative IF-F was available. The rest of the cases of MPGN showed at least 2+ intensity of diagnostic immunoglobulin/complement.
Diffuse proliferative glomerulonephritis (12 cases) were diagnosed as post infectious glomerulonephritis (PIGN) in 10 cases based on classical lumpy bumpy deposits of C3 and IgG. In one case with diffuse proliferative exudative pattern of injury and prior episode of febrile illness, a limited IF-P panel of IgA, IgG and C3 was applied to differentiate a PIGN from a proliferative IgA nephropathy. The intensity of IgG and C3 was only 1+, while IgA was negative. The IF-P results were deemed noncontributory in this case. Tissue for electron microscopy was not available.
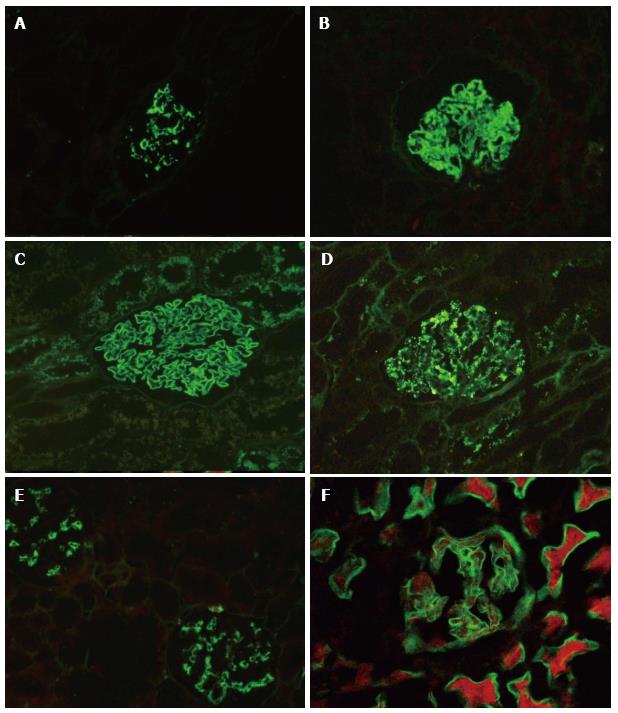
Within the lupus nephritides (LN, 25 cases) localization of the deposits as mesangial and/or capillary wall aided in accurate classification of the glomerulonephritis (Table 2, Figure 3). The lack of deposits was also significant, as demonstrated by two cases of systemic lupus erythematosus (SLE) presenting with proteinuria and nonspecific light microscopic findings. Further electron microscopic examination confirmed a lupus podocytopathy. In one case of class II LN significant full house positivity could not be demonstrated. Only IgM showed 2+ mesangial staining and the rest of the immunoglobulins and complements were focal. The EM of this case however revealed numerous predominantly mesangial electron dense deposits along with few subepithelial and subendothelial deposits.
Of 11 cases of membranous nephropathy (MN, 11 cases) diagnostic immunofluorescence with IgG was noted in 8 cases. One case showed weak (1+) staining with characteristic fine granularity and two cases were negative for IgG.
To make the diagnosis of IgA nephropathy (39 cases, 16%) or to exclude it in cases with minimal change morphology, mesangial proliferation or focal segmental glomerulosclerosis (64 cases, 26.3%) constituted the bulk of indication for IF-P in our routine practice. In two cases with isolated hematuria, suspected IgA nephropathy and nonspecific light microscopy, IF-P was negative for immunoglobulins which prompted ultrastructural examination of the cases. Classical glomerular basement membrane changes of collagenopathy consistent with Alport syndrome and thin basement membrane disease were identified. IF-P was also performed in patients of diabetic nephropathy with hematuria to exclude secondary IgAN.
In this series there were 8 cases which were diagnosed as chronic glomerulonephritis (CGN), the underlying etiology could be established in 5 cases (IgAN = 3, IC-MPGN = 1 and C-MPGN = 1). The immune complexes could not be demonstrated in 3 cases of chronic glomerulonephritis, one of which was a case of biopsy proven MPGN and the other was a case of IgAN. In one case no immune complexes were seen, however no previous renal biopsy record was available.
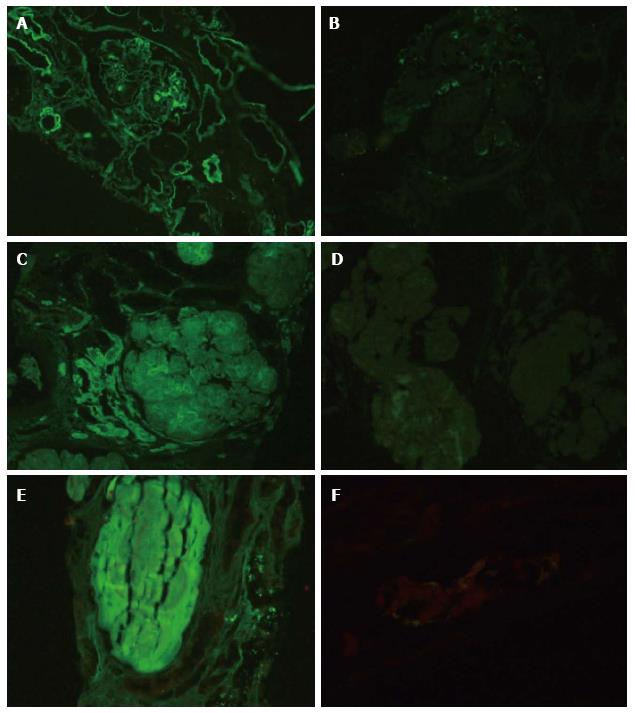
In one case of post transplant recurrence of nodular glomerulosclerosis of undetermined cause, IF-P resulted in confirming the diagnosis of light chain deposition disease (LCDD) with kappa restriction[3]. Deposits were identified in the glomerular nodules, tubular basement membranes, arterioles and arteries (Figure 4A and B). Primary amyloidosis was identified in 2 cases demonstrating light chain restriction (Figure 4C and D). Light chains were also identified in tubular casts, confirming the diagnosis of cast nephropathy in two cases. One of these cases demonstrated light chain restriction (Figure 4E and F). Other than suspected cast nephropathy, IF-P was performed in cases of primary tubulointersitial disease with significant proteinuria or hematuria to exclude concomitant glomerular disease.
Comparative IF-F and IF-P was available in 37 cases. Thirty-five of these cases (93.8%) had either equal intensity or a minor difference in intensity of staining (1+) for the diagnostic immunoglobulin/complement. Significant difference was observed in just 2 cases; a case of C-MPGN and a case of MN (Table 3).
| Disease | Number of cases with no difference in intensity of diagnostic immunoglobulin/complement (%) IF-F = IF-P | Number of cases with difference in intensity of diagnostic immunoglobulin/complement (%) IF-F > IF-P | Total number of cases | |
| Difference of 1+ | Difference of 2+ | |||
| IgA nephropathy | 7 (78%) | 2 (22%) | - | 9 |
| C-MPGN | 1 (25%) | 2 (50%) | 1 (25%) | 4 |
| IC-MPGN | 4 (100%) | - | - | 4 |
| Lupus nephritis | 3 (50%) | 3 (50%) | - | 6 |
| C1q nephropathy | 2 (100%) | - | - | 2 |
| Membranous nephropathy | 3 (43%) | 3 (43%) | 1 (14%) | 7 |
| Post infectious glomerulonephritis | 1 (100%) | - | - | 1 |
Technically assessment of immunofluorescence on the paraffin embedded tissue was found to be less challenging than IF-F with clearly observed morphology and ease of comparison with light microscopic findings.
IF studies are integral to diagnostic renal pathology and renal pathologists are often left frustrated by a lack of representative tissue in material sent for routine IF. The technique of IF-F is well established however requires a separate representative core of kidney tissue, a cryostat and technical expertise for satisfactory results. Descriptions of enzyme treatment of formalin fixed paraffin embedded (FFPE) tissue followed by IF studies (IF-P) can be found in literature from as early as 1976, however the technique has still not found a place in most renal pathology laboratories[2].
The use of a cross linking fixative like formaldehyde leads to masking of antigens. In addition calcium and other divalent ions form complexes with proteins during fixation and these complexes can block the antigenic determinants[1]. It is to unmask these determinants that enzyme treatment of FFPE tissue is necessary before applying antibodies. In our laboratory the technique of IF-P was standardized using proteinase K and it was applied prospectively as a “salvage” technique with good results.
Proteinase K is an enzyme that exhibits broad substrate specificity. It is isolated from a fungus, Engyodontium album (formerly Tritirachium album) and is able to digest keratin hence the name proteinase “K”[4]. Different proteolytic enzymes including pronase, trypsin and pepsin have been tried in various studies as demonstrated in Table 4[5-15].
| Ref. | Year | Enzyme used | Cases (n) | IF panel applied | Significant results |
| [2] | 1976 | Trypsin for 120 min | NA | Immunoglobulins and complement | Feasible to demonstrate immunoglobulins but not complement Reduced background immunofluorescence |
| [5] | 1979 | Trypsin | 52 renal biopsies | IgG, IgA, IgM, C3, Fibrinogen | Accurate detection of immunoglobulins (90%) and complement (75%) in comparison with IF on frozen |
| [6] | 1980 | Trypsin | 21 (LN, MN, IgAN) | IgG, IgM, IgA | IF on trypsin-digested tissue was as sensitive as IF-F for immunoglobulins but less sensitive for complement |
| [7] | 1980 | Pepsin (0.4%) and trypsin | Experimental mice model of anti GBM disease | IgG | Pepsin +/- trypsin digestion better than trypsin alone Enzyme digested tissue showed trivial decrease in sensitivity but good preservation in comparison with IF on frozen |
| [8] | 1989 | Pronase (0.75 g/L for 60 min at 37 °C) | IgAN (10), MN (8), Proliferative LN (10) | IgG, IgA, IgM, C3, C1q | Correct diagnosis possible in all cases Better structural details and less fading of IF Lower intensity staining for C3 Retrospectively performed digestion on 1 and 2 yr old blocks, satisfactory in 86% cases |
| [9] | 2005 | Microwave treatment (10 min) followed by Protease VII (0.05% for 30/60 min) Trypsin (0.25% for 120 min) | IgAN (7), LN (7), MN (7), MPGN (3) | IgG, IgA, IgM, C3 | Microwave treatment followed by protease digestion better than trypsin digestion Diagnostic immunoglobulin found in more than 80% cases |
| [10] | 2006 | Pronase (0.75 g/L for 60 min at 37 °C) | MN (8), MPGN (5), LN (5), PIGN (5), IgAN (8), Cryo GN (5), Fibrillary GN (5), Anti GBM (5), Cast nephropathy (5), Amyloid (5), LCDD (5), LCFS (10) | IgG, IgA, IgM, C3, C1q, kappa and lambda | Diagnostic utility in 83% cases Useful in dysproteinemia related renal disease particularly LCFS Less sensitive for staining with C3 in MPGN type I, Cryo GN, PIGN Less sensitive for IgG in MGN and anti-GBM disease |
| [11] | 2007 | Proteinase XXIV | LN (5), antiGBM (5), MN (9) | NA | IF-P on proteinase XXIV is more sensitive than IF-P with pronase In LN, better intensity staining for C1q and IgG In anti GBM, 80% sensitivity for detection of IgG In MGN, 55% sensitivity for detection of IgG |
| [12] | 2009 | Microwave treatment and/or Proteinase K – (30 or 60 min) | IgAN (24), MN (22), LN (24) | IgG, IgA, IgM, C3 | Rate of agreement between immunofluorescence on paraffin sections and immunofluorescence on frozen sections with respect to the presence of IgA was 56.5%, IgM - 44.4%, IgG - 73.9%, and C3 - 51.5% IF-P may be used as a salvage technique when frozen tissue is not available |
| [13] | 2011* | Trypsin (30 min), Pepsin | IgAN (20), MN (25) | IgA, IgG, HBsAg, HbcAg | Trypsin digestion better than pepsin digestion IF-P slightly weaker signal than IF-F |
| [14] | 2012 | Heat - Tris/Citrate buffer Pronase RTU ( 60 min at 37 °C) | LN (15), MN (11), IgMN (10), MPGN (2), IgAN (2) | IgG, IgA, IgM, C3, C1q | Heat based retrieval using Tris buffer showed superior results Pronase digestion shows less sensitivity for detection of immunoglobulins and complement |
| [15] | 2015 | Proteinase K for 20 min | 304 cases (207 cases as salvage and 97 cases for antigen unmasking ) | IgG, IgA, IgM, C3, C4, C1q, fibrinogen, kappa and lambda | Not only a good salvage technique but prevents misdiagnosis due to masked immune complex or light chain deposition |
As evident from Table 4 multiple studies comparing IF-P and IF-F have clearly established that IF-P is a feasible and valuable “salvage” technique. Comparable staining intensities have been demonstrated for immunoglobulins, with albeit lower sensitivity for detection of complement.
In an early study by Fogazzi et al[8] paraffin embedded sections were treated with pronase (0.75 g/L for 60 min) and the fluorescence intensity and location was compared with frozen sections in cases of IgAN (n = 10), membranous nephropathy (n = 8) and proliferative lupus nephritis (n = 10). The diagnostic immunoglobulins were detected with equal or increased intensity in 100% cases with a slightly reduced immunoreactivity for C3 in enzyme treated tissue. Structural details were better assessed in terms of location and morphology of deposits. On retrospective digestion of 1 and 2 year old blocks identical staining patterns were obtained in approximately 86% of cases.
Using a similar protocol as Fogazzi et al[8], Nasr et al[10] compared IF-F and IF-P in 71 renal biopsies including a spectrum of renal diseases. In glomerular diseases diagnostic findings were obtained in 100% of cases of lupus nephritis, acute post-infectious glomerulonephritis, cryoglobulinemic glomerulonephritis, fibrillary glomerulonephritis, primary amyloidosis, 88% of cases of IgAN, 80% cases of LCDD, 60% of cases of MPGN type 1, 50% cases of idiopathic MN and 20% of cases of anti-glomerular basement membrane (anti-GBM) disease. In all disease categories studied IF-P was less sensitive than IF-F for the detection of C3 similar to Fogazzi et al[8]. In addition they found reduced sensitivity for the detection of IgG in cases of MN (50%) and anti-GBM (20%) disease. They also demonstrated utility of the technique in tubulointerstitial diseases such as myeloma cast nephropathy and light chain proximal tubulopathy and found IF-P satisfactory in demonstrating light chain restriction.
More recently Messias et al[15] studied paraffin immunofluorescence in 304 native renal biopsies. The false positive staining on the surface and within capillary lumina attributed to sera adsorption secondary to fixation by the authors was also recognized in our cases and was more pronounced in under-digested tissue. They described a novel utility of the technique in evaluating masked paraprotein and immune complex deposits. The light chain crystals in light chain proximal tubulopathy were only demonstrated after enzyme digestion. Out of 61 cases where IF-P was performed to unmask immunoglobulins, it was helpful in 20 cases which included 9 cases of membranous like glomerulopathy with masked IgG-kappa deposits (MGMIDK) a novel entity first described by Larsen et al[16], 4 cases of MPGN with light chain restriction and 7 cases of MPGN with mixed essential cryoglobulinemia, which would have been misdiagnosed as C3 glomerulopathy. They recommended that all cases of C3 glomerulopathy based on routine immunofluorescence should be subjected to paraffin immunofluorescence to reach the correct diagnosis and avoid unnecessary investigations into complement abnormalities. In addition any case where the routine immunofluorescence findings do not match the ultrastructural findings should undergo paraffin immunofluorescence. However the authors reiterated, and we concur that IF on paraffin embedded tissue cannot supplant routine IF-F in renal biopsy interpretation.
In the present series we found comparable results for staining with IF-F and IF-P. As described in other studies in a few cases expected immunofluorescence results were not obtained by IF-P, including two cases of membranous nephropathy, two cases of dense deposit disease, one case of lupus nephritis, one case of suspected PIGN and three cases of chronic glomerulonephritis; even in the presence of optimal enzyme digestion. Most of these cases (except PIGN and CGN) had electron microscopic confirmation of presence of electron dense deposits, thus they were truly false negative results. We opine that this variability may be a result of differences in time of exposure of the renal biopsy to formalin, making the unmasking of antigenic determinants more difficult. This of course becomes a limitation of IF-P in a “salvage” scenario, as a negative result in the presence of optimal digestion would always be questionable; however a positive result will always aid in the diagnosis[3,17].
Nonetheless in the majority of cases undergoing routine fixation and processing, IF-P was successful in providing immunofluorescence results which added to the final diagnosis. Based on our results, we also now offer this technique for skin biopsies and for amyloid characterization in extra renal sites.
Based on the experience in our laboratory, we conclude that immunofluorescence on formalin fixed paraffin embedded tissue is a useful “salvage” technique in case of non-availability of representative fresh frozen tissue; however it is not without pitfalls. Technically assessment of enzyme digestion on each slide is mandatory for accurate interpretation of staining. Antibody staining of under digested tissue can result in both false positive as well as false negative results. Even with optimal digestion expected immunofluorescence results are sometimes not obtained and there is a yet unexplained reduced sensitivity for complement as demonstrated in multiple studies; all of which may result in a misdiagnosis. The extra slices of the renal biopsy taken for IF-P from the paraffin block apart from the routine stains result in insufficient tissue remaining in the block for any further staining or review. Within these limitations, we have demonstrated a significant diagnostic utility of this technique particularly in glomerular diseases and continue to offer it as a “salvage” option.
We would like to acknowledge Madhusudhan Bhatt, PhD student in our laboratory for his technical assistance and our laboratory attendant Mr. Harish for his sincere work.
Immunofluorescence (IF) is an indispensible technique for rendering an accurate diagnosis in renal pathology. Diseases such as IgA nephropathy, C1q nephropathy and C3 glomerulopathy cannot be diagnosed without IF. Direct IF on fresh frozen tissue (IF-F) is the most widely used IF technique.
IF on paraffin embedded renal biopsies after enzyme treatment has not found widespread use in renal pathology laboratories. This leads to incomplete diagnosis and suboptimal patient management.
To overcome the hurdles above, a method of enzymatic digestion of formalin fixed paraffin embedded tissue was standardized and introduced in the authors’ laboratory in 2011.
The authors discussed their experience with this technique in day-to-day diagnostic renal pathology, its utility in reaching final diagnoses and comparing it with usual IF-F where available. Technical and interpretation issues faced are described in detail, and may be helpful to any laboratory planning to introduce this technique.
It is an interesting paper that could be very useful for pathologists or nephrologists involved in renal pathology.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Farris AB, Mubarak M, Santoro D S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Hed J, Eneström S. Detection of immune deposits in glomeruli: the masking effect on antigenicity of formalin in the presence of proteins. J Immunol Methods. 1981;41:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Huang SN, Minassian H, More JD. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976;35:383-390. [PubMed] |
| 3. | Nambirajan A, Bhowmik D, Singh G, Agarwal SK, Dinda AK. Monoclonal gammopathy of renal significance with light-chain deposition disease diagnosed postrenal transplant: a diagnostic and therapeutic challenge. Transpl Int. 2015;28:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Rheinhardt JM, Finkbeiner WE. Protease XXIV increases detection of mucin gene expression during in situ hybridization in archival tissue. J Histochem Cytochem. 2001;49:923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Qualman SJ, Keren DF. Immunofluorescence of deparaffinized, trypsin-treated renal tissues. Preservation of antigens as an adjunct to diagnosis of disease. Lab Invest. 1979;41:483-489. [PubMed] |
| 6. | Choi YJ, Reiner L. Immunofluorescence of renal lesions in paraffin-embedded and fresh-frozen sections. Am J Clin Pathol. 1980;73:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Eneström S, Hed J, Hultman P. Detection of immune deposits in glomeruli: a comparative study of paraffin-embedded, enzyme-treated sections and cryostat sections as substrates in immunofluorescence. J Immunol Methods. 1980;37:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Fogazzi GB, Bajetta M, Banfi G, Mihatsch M. Comparison of immunofluorescent findings in kidney after snap-freezing and formalin fixation. Pathol Res Pract. 1989;185:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Chowdhury AR, Ehara T, Higuchi M, Hora K, Shigematsu H. Immunohistochemical detection of immunoglobulins and complements in formaldehyde-fixed and paraffin-embedded renal biopsy tissues; an adjunct for diagnosis of glomerulonephritis. Nephrology (Carlton). 2005;10:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Nasr SH, Galgano SJ, Markowitz GS, Stokes MB, D'Agati VD. Immunofluorescence on pronase-digested paraffin sections: a valuable salvage technique for renal biopsies. Kidney Int. 2006;70:2148-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | van der Ven K, Nguyen TQ, Goldschmeding R. Immunofluorescence on proteinase XXIV-digested paraffin sections. Kidney Int. 2007;72:896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Wagrowska-Danilewicz M, Danilewicz M. Immunofluorescence on paraffin-embedded sections in evaluation of immune complex deposits in renal biopsy specimens. Pol J Pathol. 2009;60:3-9. [PubMed] |
| 13. | Zhang Y, Chen J, Liu HJ, Lu M. [Use of paraffin sections for immunofluorescence staining in renal biopsy]. Beijing Daxue Xuebao. 2011;43:900-902. [PubMed] |
| 14. | Mubarak M, Kazi Javed I, Kulsoom U, Ishaque M. Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. J Nephropathol. 2012;1:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Messias NC, Walker PD, Larsen CP. Paraffin immunofluorescence in the renal pathology laboratory: more than a salvage technique. Mod Pathol. 2015;28:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Larsen CP, Ambuzs JM, Bonsib SM, Boils CL, Cossey LN, Messias NC, Silva FG, Wang YH, Gokden N, Walker PD. Membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int. 2014;86:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Singh L, Singh G, Bhardwaj S, Sinha A, Bagga A, Dinda A. Dense Deposit Disease Mimicking a Renal Small Vessel Vasculitis. J Am Soc Nephrol. 2016;27:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |