Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.448
Peer-review started: March 18, 2016
First decision: April 18, 2016
Revised: May 2, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: September 6, 2016
Processing time: 168 Days and 12.2 Hours
To study the relationship between overhydration (OH) in peritoneal dialysis (PD) patients and cardiac mortality.
OH, as measured by body composition monitor (BCM), is associated with increased mortality in dialysis patients. BCM has been used to guide treatment on the assumption that correcting OH will improve cardiac morbidity and mortality although data demonstrating causality that is reversible is limited. We wished to determine if OH in PD patients predicted cardiac mortality, and if there was a correlation between OH and cardiac troponin-T (cTnT) levels. Finally, we wished to determine if improving OH values would lead to a decrement in cTnT. All prevalent PD patients over the study period of 57 mo who had contemporaneous BCM and cTnT measurements were followed irrespective of transplantation or PD technique failure. We also studied a cohort of patients with who had severe OH (> +2L). The Fresenius Body Composition Monitor was used to obtain hydration parameters. cTnT levels were done as part of routine clinical care. Data was analysed using SPSS version 20.0.
There were 48 deaths in the 336 patients. The patients that died from cardiac or non-cardiac causes were similar with respect to their age, incidence of diabetes mellitus, gender, ethnicity and cause of renal failure. However, the patients with cardiac causes of death had significantly shorter dialysis vintage (10.3 mo vs 37.0 mo, P < 0.0001) and were significantly more overhydrated by BCM measurement (2.95 L vs 1.35 L, P < 0.05). The mean (standard error of the means) hydration status of the 336 patients was +1.15 (0.12) L and the median [interquartile range (IQR)] cTnT level was 43.5 (20-90) ng/L. The cTnT results were not normally distributed and were therefore transformed logarithmically. There was a statistically significant correlation between Log (cTnT) with the OH value (Spearman r value 0.425, P < 0.0001). We identified a sub-group of patients that were severely overhydrated; median (IQR) hydration at baseline was +2.7 (2.3 to 3.7) L. They were followed up for a minimum of 6 mo. Reduction in OH values in these patients over 6 mo correlated with lowering of cTnT levels (Spearman r value 0.29, P < 0.02).
Patients that were overhydrated had higher cTnT, and had deaths that were more likely to be cardiac related. Reduction in OH correlated with lowering of cTnT.
Core tip: Overhydration measured by bioimpedance spectroscopy is an independent predictor of death in peritoneal dialysis patients. Most studies on this topic provide only a single baseline bioimpedance assessment. We present longitudinal data showing increased cardiac mortality in overhydrated patients, and significant correlation of overhydration with cardiac troponin-T (cTnT) levels. Over 6 mo, these patients had a mean of 7.4 body composition monitor readings and 3.4 cTnT assessments. Patients whose hydration status improved showed a corresponding improvement in cTnT. While observational studies cannot define causality, our results show overhydration is associated with cardiac mortality, and suggest overhydration may be a reversible risk factor.
- Citation: Oei E, Paudel K, Visser A, Finney H, Fan SL. Is overhydration in peritoneal dialysis patients associated with cardiac mortality that might be reversible? World J Nephrol 2016; 5(5): 448-454
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/448.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.448
Fluid overload measured by bioimpedance spectroscopy (BIS) is an independent predictor of death in peritoneal dialysis (PD) patients[1] and is highly prevalent[2]. This was also shown in haemodialysis (HD)[3]; patients with severe overhydration (OH) had a hazard ratio for all-cause mortality of 2.1, second to only diabetes. However, causality cannot be determined from these retrospective observational studies. Nevertheless, there is circumstantial evidence to support the belief that correcting OH will improve patient outcomes; correcting OH was shown to be associated with improvements in blood pressure, arterial stiffness and left ventricular mass index[4,5]. But very strict attention to fluid restriction was also shown to increase loss of residual renal function[6] and there is an association between low hydration status and intra-dialytic hypotension[7].
While it is often assumed that the observed relationship between OH and mortality is related to cardiovascular damage, an important caveat is that the ratio of extracellular fluid to total body water is also increased in the setting of muscle wasting. Thus, a negative correlation between BIS-OH parameters and malnutrition have been found in several studies[8,9] and it is possible that the increased mortality associated with OH relate to its association with protein energy wasting (PEW)/malnutrition inflammation atherosclerosis (MIA) Syndrome. Thus, in a study of 72 stable HD patients, although N-Terminal pro-Brain Naturetic Peptide (NTpro-BNP) correlated with BIS OH, the authors concluded that NTproBNP was also elevated in malnourished patients[10]. Others have also expressed reservation about the proposed causal relationship between overhydration measure by BIS and mortality[11].
We wished to explore this subject further by determining if PD patients who died from cardiac causes were more severely OH compared to patients that died from other causes. We also wished to determine if there is a relationship between OH and cardiac troponin-T (cTnT) which remains a highly sensitive biomarker for cardiac injury in dialysis patients[12,13]. Thus, we studied patients over a 6-mo period to determine if severe OH improved, did it lead to a corresponding decrements of cTnT. We also examined the changes in cTnT over 6 mo against time-average values of biochemical nutrition parameters (serum albumin and haemoglobin) as well the inflammatory marker of C-reactive protein (CRP).
The study was conducted in accordance with the principles set out by the local ethical committee according to United Kingdom National Health Service audit and clinical service development. We studied a cohort of patients from a single PD unit, consisting of all continuous ambulatory PD (CAPD) and automated PD (APD) patients between 1 January 2008 and 30 March 2012 who had contemporaneous baseline BIS/cTnT readings. All patients with amputations, cardiac pacemakers or defibrillators were excluded as we were unable to perform BIS measurements. Patients were followed up until 15th September 2012 or death. Patients who were transplanted or switched to HD were still followed up. Only patients that recovered renal function or who were transferred to another dialysis unit for geographic relocation reasons were censored at that time point, as their survival follow-up could not be accurately determined. In all cases, baseline characteristics were collated through review of case notes and included primary cause of renal failure, dialysis vintage and presence or absence of diabetes mellitus. To comply with the mandatory United Kingdom Renal Registry submissions, we held weekly multidisciplinary meetings to review and assign causes of death for dialysis patients.
In a subgroup analysis, we identified patients that were severely overhydrated (OH > 2 L). Using the time when their OH was first found to be > 2 L as baseline, we prospectively collected data on their hydration status and their cTnT readings over the subsequent 6 mo.
The Fresenius Body Composition Monitor (BCM - Fresenius Medical Care, Bad Homberg, Germany) was used to obtain hydration parameters such as OH and nutritional indices, namely Fat and Lean Tissue Mass (FTM and LTM respectively). This BIS device employed 50 frequencies between 5 and 1000 kHz, and measurements were performed by placing electrodes on one hand and one foot, with PD dialysate in situ.
Over the duration of the study, cTnT was measured every 3-4 mo as part of routine clinical care. During this study, the cTnT assay (Roche Diagnostics GmbH, Mannheim, Germany) was changed to the high sensitivity assay, though the upper limit of normal was the same (< 14 ng/L, upper 99th percentile). In patients with normal renal function, values > 3 ng/L were suggestive of myocardial injury and values > 14 ng/L were considered diagnostic for myocardial infarction if there were consistent clinical features. Precision of the assays across the range were: 0.5 ng/L (1.9%); 0.0399 (1.6%); 0.133 (1.7%).
Categorical variables are expressed as a number and a percentage. Continuous variables are expressed as means and standard error of the means (SEM) or median with quartile ranges depending on whether the results of the parameters were normally distributed (determined by the D’Agostino and Pearson omnibus normality test). If not normally distributed, these parameters were analyzed on a logarithmic scale. Correlation coefficients and multivariate logistical regression analyses were undertaken with SPSS software for Windows version 20.0 (SPSS Inc., Chicago, IL, United States). The regression model was created based on those clinical variables known to effect survival on PD.
There were 336 APD and CAPD patients who had at least 1 contemporaneous BCM/cTnT assessment during the study period. The median age of patients was 57.9 [interquartile range (IQR): 47.9-69.0] years with a median dialysis vintage of 7.6 (IQR: 0.9-31.4) mo (Table 1). 62% were male, and 37% had diabetes mellitus.
| All | Survivors | Non-cardiac death | Cardiac death | P-value (comparing cardiac vs non cardiac death patients) | |
| No. | 336 | 288 | 35 | 13 | |
| Age1 | 57.9 (48.1-69.0) | 55.4 (46.9-66.6) | 68.9 (61.8-77.0) | 68.9 (62.9-76.5) | NS |
| Male | 207 (62%) | 167 (58%) | 27 (77%) | 13 (100%) | NS |
| Diabetes mellitus | 123 (37%) | 99 (34%) | 15 (43%) | 9 (69%) | NS |
| Assessed as suitable for transplantation | 159 (47%) | 148 (51%) | 10 (29%) | 1 (8%) | NS |
| Dialysis vintage (mo)1 | 7.6 (0.9-31.4) | 6.5 (0.8-24.0) | 37.0 (4.0-57.4) | 10.3 (2.9-23.1) | < 0.00001 |
| Body composition measurements: Mean (SEM) | |||||
| OH (L) | 1.15 (0.12) | 1.04 (0.13) | 1.35 (0.32) | 2.95 (0.78) | < 0.05 |
| Lean tissue index | 13.7 (0.5) | 13.9 (0.5) | 11.9 (0.5) | 12.3 (1.6) | < 0.0001 |
| Fat tissue index | 13.2 (0.3) | 13.3 (0.3) | 11.9 (0.6) | 13.3 (1.0) | < 0.01 |
| Ethnicity | |||||
| Whites | 112 (33%) | 97 (34%) | 14 (40%) | 1 (8%) | NS |
| Blacks | 67 (20%) | 59 (20%) | 5 (14%) | 3 (23%) | |
| Asians | 139 (41%) | 117 (41%) | 15 (43%) | 7 (54%) | |
| Others | 18 (5%) | ||||
| Cause of renal failure: n (%) | |||||
| Unknown | 82 (24%) | 66 (30%) | 8 (23%) | 2 (15%) | NS |
| GN | 51 (15%) | 26 (12%) | 1 (3%) | 0 (0%) | |
| Cancer/trauma | 1 (0%) | 3 (1%) | 0 (0%) | 0 (0%) | |
| Congenital/familial | 8 (2%) | 5 (2%) | 0 (0%) | 0 (0%) | |
| Diabetes | 105 (31%) | 69 (31%) | 14 (40%) | 9 (69%) | |
| Hypertension | 28 (8%) | 16 (7%) | 5 (14%) | 2 (15%) | |
| APKD | 12 (4%) | 11 (5%) | 1 (3%) | 0 (0%) | |
| TIN/chronic pyelonephritis | 49 (15%) | 29 (13%) | 6 (17%) | 0 (0%) | |
| Blood results | |||||
| Baseline log(CRP) (mg/L) | 0.57 (0.03) | 0.52 (0.04) | 0.79 (0.12) | 0.81 (0.20) | NS |
| Time av log (CRP) (mg/L) | 0.67 (0.04) | 0.61 (0.04) | 1.09 (0.11) | 0.98 (0.17) | NS |
| Baseline albumin (g/L) | 38.9 (0.3) | 39.3 (0.3) | 36.1 (1.0) | 36.8 (1.0) | < 0.05 |
| Time av albumin (g/L) | 37.6 (0.2) | 38.2 (0.2) | 33.8 (1.0) | 35.6 (1.1) | < 0.002 |
| Baseline haemoglobin (g/dL) | 11.0 (0.1) | 10.9 (0.1) | 11.8 (0.3) | 11.0 (0.5) | NS |
| Time av haemoglobin (g/dL) | 11.2 (0.1) | 11.2 (0.1) | 11.7 (0.2) | 10.7 (0.3) | NS |
There were 74 patients who had an OH reading > 2 L and a subsequent BCM/cTnT assessment between 6-9 mo later. We excluded 8 patients that had documented acute cardiac events (acute rise in cTnT associated with cardiac pain, pulmonary oedema or haemodynamic instability). For the remaining 66 patients, the median (IQR) “baseline” OH value was 2.7 (2.3-3.7) L. Over the follow-up period, the mean number of BCM measurement per patient was 7.4, whilst the mean number of cTnT measurements per patient was 3.4. Demographic details for this cohort are listed in Table 2.
| Sub-group: Patients with severe OH | |
| Number | 66 |
| Age1 | 60.1 (51.1-71.1) |
| Male (%) | 44 (67%) |
| Diabetes mellitus (%) | 27 (41%) |
| Assessed as suitable for transplantation (%) | 26 (39%) |
| Dialysis vintage (mo)1 | 1.79 (0.5-32.1) |
| Body composition measurements: | |
| Baseline OH (L)1 | 2.7 (2.3-3.7) |
| OH over 6 mo (L/6 mo)1 | -0.7 (-0.3--1.5) |
| Baseline FTM (kg)1 | 23.6 (17.3-28.9) |
| FTM over 6 mo (kg)1 | 2.3 (0.1-3.7)3 |
| Baseline LTM (kg)1 | 37.2 (29.6-44.6) |
| LTM over 6 mo (kg)1 | -1.5 (-4.5-1.7)2 |
| Number of readings | 7.4 (0.4) |
| Cardiac troponin T measurements (ng/L) | |
| Baseline cTnT1 | 60 (37-100) |
| Final cTnT1 | 71 (37-115) |
| Number of readings | 3.4 (0.2) |
| Ethnicity: n (%) | |
| Whites | 26 (39%) |
| Blacks | 14 (21%) |
| Asians | 23 (35%) |
| Others | 3 (5%) |
| Cause of renal failure: n (%) | |
| Unknown | 15 (23%) |
| GN | 9 (14%) |
| Cancer/trauma | 2 (3%) |
| Congenital/familial | 2 (3%) |
| Diabetes | 23 (35%) |
| Hypertension | 6 (9%) |
| APKD | 0 (0%) |
| TIN/chronic pyelonephritis | 9 (14%) |
| Blood results | |
| Baseline log (CRP) (mg/L) | 0.67 (0.08)4 |
| Time average log (CRP) over 6 mo (mg/L) | 0.73 (0.08)4 |
| Baseline albumin (g/L) | 36.4 (0.6)4 |
| Time average albumin over 6 mo (g/L) | 36.0 (0.6)4 |
| Baseline haemoglobin (g/dL) | 10.5 (0.2)4 |
| Time average haemoglobin over 6 mo (g/dL) | 10.6 (0.2)4 |
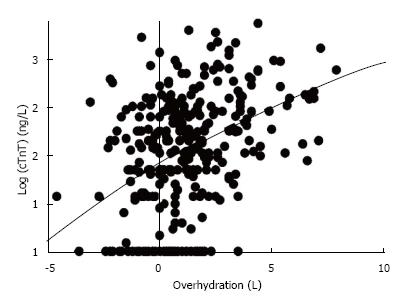
For our cohort of 336 patients, the mean (SEM) hydration status was +1.15 (0.12) L and the median (IQR) cTnT level was 43.5 (20-90) ng/L. The cTnT results were not normally distributed and were therefore transformed logarithmically. There was a statistically significant correlation between Log (cTnT) with the OH value (the Spearman r value was 0.425, P < 0.0001, Figure 1).
Over a median follow-up period of 23.9 mo, 48 patients (14.3%) of the 336 PD patients died. Cardiac causes of death (sudden cardiac death, cardiac failure or myocardial ischaemia) were assigned in 13 (27%) of cases. The patients that died from cardiac or non-cardiac causes were similar with respect to their age, incidence of diabetes mellitus, gender, ethnicity and cause of renal failure (Table 1). However, the patients with cardiac causes of death had significantly shorter dialysis vintage (10.3 mo vs 37.0 mo, P < 0.0001) and were significantly more overhydrated by BCM measurement (2.95 L vs 1.35 L, P < 0.05). The OH status appeared to predict cardiac death that occurred at a mean of 15.5 mo subsequent to the BCM readings. The mean duration between the BCM reading and non-cardiac death was 16.1 mo.
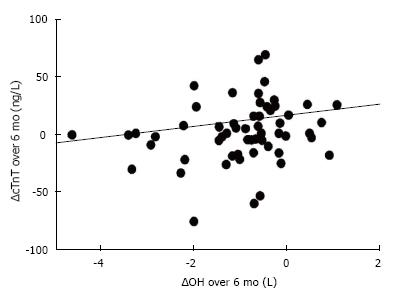
We identified a sub-group of patients that were severely overhydrated; median (IQR) hydration at baseline was +2.7 (2.3 to 3.7) L. They were followed up for a minimum of 6 mo. For each individual, the rate of change of OH (∆OH) was calculated using the “least squares” method to estimate the straight line that best fitted that patient’s data. The median (IQR change of OH over 6 mo was -0.7 (-0.3 to -1.5) L. The median (IQR) baseline cTnT value was 60 (37-100) ng/L (Table 2). We plotted the ∆OH with ∆cTnT and found a statistically significant correlation (Spearman r value = 0.29, P < 0.02; Figure 2). The rates of change of FTM and LTM were also calculated using the “least squares” method. Over a 6-mo period, there was a FTM increase of +2.3 kg (IQR: 0.1-3.7, P < 0.0001, Wilcoxon Signed Rank test). By contrast, patients showed a statistically significant loss of LTM during the follow-up, equivalent to -1.5 kg (IQR: -4.5 to -1.7, P < 0.05; Table 2). There were no significant correlation between ∆OH and any of the biochemical nutrition or inflammatory markers (either baseline or time-average values of serum albumin, haemoglobin and CRP).
In our current study, patients who died from cardiac causes were more severely overhydrated than patients who died from other diseases although this association does not indicate causality or reversibility. This is in keeping with a recent pre-dialysis study that also showed an association between cardiac (as well as all-cause mortality) for patients with chronic kidney disease stages 4 and 5 with fluid overload determined by BIS[14]. We also note that a recent report[15] showed that patients randomized to having their hydration status managed with BCM had lower mortality.
Cardia cTnT has been repeatedly shown to be predictive of cardiac death in patients on dialysis[16,17]. It is therefore significant that we found a direct correlation between OH status and Log cTnT (r = 0.425, P < 0.0001). These results alone do not prove causality and it remains possible that this association is due to PEW/MIA. After all, a large database of haemodialysis patients (MONDO) confirmed that the BIS parameters of FTI and LTI were also associated with mortality[18]. Unfortunately, for a retrospective study, it was difficult to define the exact causes of deaths that were ascribed at the time to be “cardiac/sudden cardiac deaths”.
However, uniquely we also found a statistically significant correlation between the improvement in hydration status and decrement in cTnT levels over 6 mo (r = 0.29, P < 0.02; Figure 2). Patients in this cohort study had a median baseline OH value of +2.7 L and showed a median decrement over 6 mo of -0.7 L. It is unlikely that this magnitude of change is a consequence of correcting malnutrition/PEW. In fact, the BIS data suggested a small but significant loss of LTM over these 6 mo, which may have been due to patients’ salt and fluid restriction with consequent diminished dietary protein intake. We also found that there were no correlation between either baseline or time-averaged biochemical markers of nutrition and inflammation suggesting the improvement in cTnT is likely to be a consequence of fluid status and not nutrition. Of course, it must be acknowledged that using albumin, CRP and presence of anaemia to be indicators of nutrition is imprecise. Nevertheless, we found no signal to suggest changes in nutrition status was a cofounder for the change in cTnT.
It is important to note that our cohort exhibited an increase in FTM that was equivalent to +2.3 kg (IQR: 0.1-3.7) over 6 mo. It is possible that the fat gain was exacerbated through increased use of hypertonic glucose dialysate to improve hydration status.
Although our results do not prove that correction of overhydration will lead to reduced cardiac mortality, it supports the finding by Onofriescu et al[15]. We hope these findings provide added impetus for clinicians to focus on OH particularly as we have previously reported that BCM guided reduction of OH does not cause excessive loss of residual renal function[19,20]. We note that improved hydration status in this study appeared to come at the expense of increasing FTM but the clinical impact of increasing obesity is unclear. In the normal population, obesity is associated with glucose intolerance and cardiovascular risk but in HD, “reverse epidemiology” has been reported (HD patients with high BMI have a survival advantage)[21]. Similarly, studies have shown that PD patients with high FTM have a survival advantage[18,22].
Limitations of this study include the retrospective nature of the data collection and the fact that we included both incident and prevalent patients. BCM measurements were performed with dialysate in situ and this may have reduced the precision of the measurements[23] (although we were consistent in how we performed the measurements). Most critically, our retrospective study can only demonstrate associations and cannot determine causality. The study would have also benefited if we had a formal assessment of cardiac function. Future studies could include simple assessments such as echocardiography but unfortunately contemporaneous echo studies with bioimpedance measurements were not available for all subjects in our study. Equally, it was difficult in a retrospective study to accurately determine “cause” of fluid overload.
In conclusion, we believe our data provides indirect evidence to suggest that overhydration impacts negatively on the heart. Intriguingly, our data also suggests that correcting overhydration is possible and may lead to improved cardiac prognosis but perhaps at the expense of increasing obesity. Randomized controlled studies on this subject will be difficult, but it will be interesting to await the results of two studies that are designed to explore the impact BIA might have on left ventricular mass[24] and survival[25,26].
Despite the importance of euvolaemia, there is still much debate on the most clinically useful method of volume assessment. Overhydration (OH) as measured by body composition monitor (BCM) is associated with increased mortality in dialysis patients. BCM has been used to guide treatment on the assumption that correcting OH will improve cardiac morbidity and mortality, although data demonstrating causality that is reversible is limited.
It has often been assumed that the observed relationship between OH and mortality is related to cardiovascular damage. However, an important caveat is the ratio of extracellular water to total body water is also increased in the setting of muscle wastage. Thus it is possible that the increased mortality associated with OH relate to its association with protein energy wasting (PEW)/malnutrition inflammation atherosclerosis (MIA) Syndrome.
Most research into this area has focused on single time point measurements of OH and cardiac biomarkers. While cardiac troponins-T (cTnT) have been repeatedly shown to be predictive of cardiac death in dialysis patients, the effect of malnutrition on the observed relationship between OH, cardiac biomarkers and outcomes is difficult to establish. More recent trials have shown that targeting a reduction in OH is associated with better survival. However, the temporal relationship of cardiac biomarkers and reduction in OH has not been well described.
In this study, peritoneal dialysis patients who died of cardiac causes had higher OH, compared to patients that died from other causes. Over a 6-mo period, the authors found that reducing OH in severely overhydrated patients was associated with corresponding decrements in cTnT. There was no significant correlation between change in OH and any of the biochemical or nutritional markers studied, suggesting that the improvement in cTnT is likely to be a consequence of fluid status and not nutrition. Although the results do not prove that correction of OH will lead to reduced cardiac mortality, the temporal association observed between OH and cTnT supports the role of fluid status in cardiac risk management of dialysis patients.
OH is a mathematically derived estimate of excess fluid. The BCM expresses the body weight in terms of lean tissue mass (LTM-mainly muscle), adipose tissue mass (ATM-mainly fat) and OH. Each of these compartments has a specific composition and contains a known quantity of water per mass of tissue. The water of LTM and ATM consist of differing proportion of extracellular and intracellular water in addition to solid components. Excess fluid represents an expansion of only the extracellular water, whereas ICW remains unchanged. The excess fluid may reside within adipose tissue or lean tissue raising the hydration of the respective tissue above the “normal” values (e.g., oedema). Alternatively, excess fluid may simply appear as a distinct compartment without altering the hydration of the major tissues (e.g., ascites, pleural effusion). As the extracellular hydration of LTM and ATM is known, the expected “normal” volume of ECW of these tissues can be calculated. The difference between “normal” ECW and measured ECW is the excess fluid, OH.
OH is gaining popularity as an objective measurement of fluid status due to its relatively low cost and ease of measurement. There have been many studies on this topic, but it is uncommon to find studies on repeated measurements of OH and cardiac biomarkers. This study provides indirect evidence to suggest that OH is associated with worse cardiac outcomes, and importantly, that correcting OH may lead to improved cardiac prognosis.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bhimma R, Fujigaki Y, Said SAM S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | O’Lone EL, Visser A, Finney H, Fan SL. Clinical significance of multi-frequency bioimpedance spectroscopy in peritoneal dialysis patients: independent predictor of patient survival. Nephrol Dial Transplant. 2014;29:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, Verger C, Steiger J, Schoder V, Wabel P. Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS One. 2011;6:e17148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 471] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 4. | Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, Kayikcioglu M, Demirci MS, Ozkahya M, Duman S. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 5. | Kwan BC, Szeto CC, Chow KM, Law MC, Cheng MS, Leung CB, Pang WF, Kwong VW, Li PK. Bioimpedance spectroscopy for the detection of fluid overload in Chinese peritoneal dialysis patients. Perit Dial Int. 2014;34:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Hur E, Gungor O, Musayev O, Usta M, Toz H, Asci G, Ozkahya M, Duman S, Ok E. Bioimpedance spectroscopy for the detection of hypervolemia in peritoneal dialysis patients. Adv Perit Dial. 2011;27:65-70. [PubMed] |
| 7. | Machek P, Jirka T, Moissl U, Chamney P, Wabel P. Guided optimization of fluid status in haemodialysis patients. Nephrol Dial Transplant. 2010;25:538-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Ribitsch W, Stockinger J, Schneditz D. Bioimpedance-based volume at clinical target weight is contracted in hemodialysis patients with a high body mass index. Clin Nephrol. 2012;77:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Antlanger M, Hecking M, Haidinger M, Werzowa J, Kovarik JJ, Paul G, Eigner M, Bonderman D, Hörl WH, Säemann MD. Fluid overload in hemodialysis patients: a cross-sectional study to determine its association with cardiac biomarkers and nutritional status. BMC Nephrol. 2013;14:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Booth J, Pinney J, Davenport A. N-terminal proBNP--marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin J Am Soc Nephrol. 2010;5:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Hebert LA, Parikh S. Is fluid overload as measured by bioimpedance spectroscopy harmful in CKD-if so, why? Clin J Am Soc Nephrol. 2015;10:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Bolton K, Beddhu S, Cmpese VM, Chavers BM, Cheung AK, Churchill DN, Goldstein-Fuchs J, Herzog CA, Henrich W, King K. KDOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Available from: http//www.kidney.org/professionals/KDOQI/guidelines_cvd/index.htm. |
| 13. | Ooi DS, Isotalo PA, Veinot JP. Correlation of antemortem serum creatine kinase, creatine kinase-MB, troponin I, and troponin T with cardiac pathology. Clin Chem. 2000;46:338-344. [PubMed] |
| 14. | Tsai YC, Chiu YW, Tsai JC, Kuo HT, Hung CC, Hwang SJ, Chen TH, Kuo MC, Chen HC. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol. 2015;10:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Onofriescu M, Hogas S, Voroneanu L, Apetrii M, Nistor I, Kanbay M, Covic AC. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2014;64:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Ishii J, Nomura M, Okuma T, Minagawa T, Naruse H, Mori Y, Ishikawa T, Kurokawa H, Hirano T, Kondo T. Risk stratification using serum concentrations of cardiac troponin T in patients with end-stage renal disease on chronic maintenance dialysis. Clin Chim Acta. 2001;312:69-79. [PubMed] |
| 17. | Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation. 2002;106:2941-2945. [PubMed] |
| 18. | Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, Gatti E, Grassmann A, Wang Y, Marelli C. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol. 2015;10:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | McCafferty K, Fan S, Davenport A. Extracellular volume expansion, measured by multifrequency bioimpedance, does not help preserve residual renal function in peritoneal dialysis patients. Kidney Int. 2014;85:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Burke SE, Fan SL. Clinical experience using bioimpedance to optimize blood pressure control. Perit Dial Int. 2013;33:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, Kovesdy CP, Greenland S. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am J Epidemiol. 2012;175:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Paudel K, Visser A, Burke S, Samad N, Fan SL. Can Bioimpedance Measurements of Lean and Fat Tissue Mass Replace Subjective Global Assessments in Peritoneal Dialysis Patients? J Ren Nutr. 2015;25:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Davenport A. Does peritoneal dialysate affect body composition assessments using multi-frequency bioimpedance in peritoneal dialysis patients? Eur J Clin Nutr. 2013;67:223-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Su WS, Gangji AS, Margetts PM, Bosch J, Yusuf S, Clase CM, Ganame J, Noseworthy M, Lonn E, Jain AK. The fluid study protocol: a randomized controlled study on the effects of bioimpedance analysis and vitamin D on left ventricular mass in peritoneal dialysis patients. Perit Dial Int. 2011;31:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Liu L, Long G, Ren J, Li J, Xu J, Lei J, Li M, Qiu M, Yuan P, Sun W. A randomized controlled trial of long term effect of BCM guided fluid management in MHD patients (BOCOMO study): rationales and study design. BMC Nephrol. 2012;13:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Baek SH, Oh KH, Kim S, Kim DK, Joo KW, Oh YK, Han BG, Chang JH, Chung W, Kim YS. Control of fluid balance guided by body composition monitoring in patients on peritoneal dialysis (COMPASS): study protocol for a randomized controlled trial. Trials. 2014;15:432. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |