Published online Sep 6, 2016. doi: 10.5527/wjn.v5.i5.398
Peer-review started: March 19, 2016
First decision: April 19, 2016
Revised: April 20, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: September 6, 2016
Processing time: 168 Days and 12.4 Hours
Chronic kidney disease (CKD) patients are endangered with the highest mortality rate compared to other chronic diseases. Cardiovascular events account for up to 60% of the fatalities. Cardiovascular calcifications affect most of the CKD patients. Most of this calcification is related to disturbed renal phosphate handling. Fibroblast growth factor 23 and klotho deficiency were incriminated in the pathogenesis of vascular calcification through different mechanisms including their effects on endothelium and arterial wall smooth muscle cells. In addition, deficient klotho gene expression, a constant feature of CKD, promotes vascular pathology and shares in progression of the CKD. The role of gut in the etio-pathogenesis of systemic inflammation and vascular calcification is a newly discovered mechanism. This review will cover the medical history, prevalence, pathogenesis, clinical relevance, different tools used to diagnose, the ideal timing to prevent or to withhold the progression of vascular calcification and the different medications and medical procedures that can help to prolong the survival of CKD patients.
Core tip: The last 2 decades witnessed the failure of all intervention studies targeting different risk factors of vascular calcification in chronic kidney disease (CKD) patients on regular hemodialysis. The main aim of all these studies was to decrease cardiovascular morbidity and mortality among such patients. These disappointing results criticized the value of such interventions in clinical practice. On the other hand, when similar trials were run on patients at an earlier stage of CKD, most of these trials showed a significant impact on patient survival and/or cardiovascular morbidity. Such discrepancy indicates the value of timing of interference. We are trying in this review to develop the ideal strategy that would optimize the management of CKD patients to avoid the devastating vascular calcification, highlighting the value of different medicines used in this plan. Meanwhile we are showing the update in guidelines concerned with this issue.
- Citation: Sharaf El Din UAA, Salem MM, Abdulazim DO. Vascular calcification: When should we interfere in chronic kidney disease patients and how? World J Nephrol 2016; 5(5): 398-417
- URL: https://www.wjgnet.com/2220-6124/full/v5/i5/398.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i5.398
Vascular calcification (VC) affects either the arterial tree or cardiac valves. Deposits of hydroxyapatite in the arterial wall occur within either tunica intima or tunica media. VC is a strong predictor of increased cardiovascular mortality among chronic kidney disease (CKD) patients. However, the different clinical studies that tried to manipulate the different risk factors of VC in dialysis patients failed to show a significant impact on patient survival. On the other hand, when pre-dialysis patients underwent similar studies, there was a significant decrease of cardiovascular and overall mortality rates, beside a comparable effect on vascular calcification progress rate. These results have probably two explanations. Dialysis patients might have advanced VC rendering their arteries permanently and irreversibly damaged [approaching end stage arterial damage (ESAD[1])] or they might have additional pathologic problems exceeding in their survival impact the VC The authors of this review are inclined towards the 1st possibility and will try to outline the best way to tackle this devastating pathology.
In 1855, “metastatic calcification” was described in three patients with renal disease[2]. Eight years later, Virchow[3] reported that this calcification is a definite ossification.
The first reported VC in infants was probably that of Durante[4] describing aortic and pulmonary artery calcification. Calcification of peripheral vessels has been described in a few children with CKD as early as 1942[5,6].
VC affected humans in ancient history. The most ancient calcification so far reported is 5000 years ago, “identified in the recently discovered ice man”[7]. On the other hand, the earliest coronary calcification reported is that of an Egyptian mummy who was living 4000 years ago[8].
A high percentage of CKD patients show VC The prevalence among predialysis CKD G3-5 patients was 79% of cases in one study[9]. It might approach 100% in patients starting dialysis[10]. VC significantly contributes to morbidity and mortality of CKD[11-13]. Up to 3- to 4-fold increase in VC has been reported in the earliest phases of CKD[14].
CKD patients show VC in almost all arteries whether large, medium or small-sized vessels, including the coronary arteries[10,15-17]. VC can affect the tunica intima and/or the tunica media of the arterial wall[18]. Intimal calcification is mainly a feature of atherosclerosis[19]. CKD patients can have intimal and medial calcification. Medial calcification is reported in CKD of any age[20,21]. When epigastric arteries of patients with end-stage renal disease (ESRD) were examined at the time of kidney transplantation, vessel calcification was detected regardless of patient age and/or the presence of other risk factors for atherosclerosis[22]. In one study, hemodialysis (HD) patients have higher calcification scores than either peritoneal dialysis (PD) or CKD G4. More heavily calcified patients were significantly older and mostly male[23]. In HD patients, coronary calcification progresses steadily[24]. High serum phosphate concentration was a strong independent risk factor only in non-diabetic patients. Diabetic patients lack similar association[25].
Fifty percent of CKD patients die out of cardiovascular events[26]. Cardiovascular mortality is 20- to 30-times of controls matching in age, race and gender[27]. Patients starting dialysis at age of 25-29 years have a median life expectancy of 18.5 years. This means that their survival is 33 years less than normal personnel[28]. Arterial calcification is one of the predictors of this increased cardiovascular mortality[28]. Patients with CKD should, therefore, receive aggressive preventive measures to reduce this cardiovascular disaster[29].
Coronary artery calcification (CAC) is common among CKD patients whether adolescents, adults or old aged, starting in early stages of CKD and steadily progressing in HD patients[30-32]. Postmortem study of atheromatous lesions in ESRD patients found more intense calcification of such lesions compared to age- and sex- matched controls[33].
Calcification of the internal iliac arteries in CKD patients was greater compared with controls[34].
Large vessel disease is associated with decreased arterial compliance as detected by ultrasound and accounts for the increased mortality[35,36].
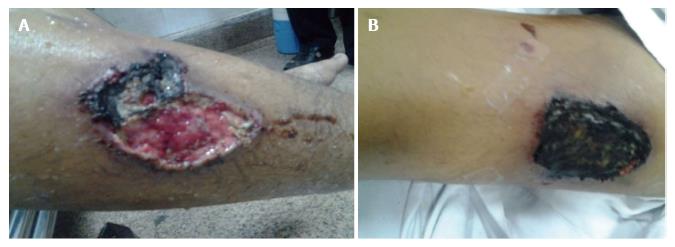
Calcific uremic arteriolopathy (CUA), also called calciphylaxis is an obliterative vasculopathy affecting cutaneous arterioles. It occurs almost exclusively in ESRD patients. Affected arterioles show medial calcification[37]. Ischemia and necrosis of the skin, subcutaneous fat, visceral organs and skeletal muscles eventually ensues. The skin manifests by necrotic foci and painful ulcers (Figure 1)[38].
Death with a functioning graft is one of the major causes of graft loss (accounting for 42% of graft loss) in kidney transplant recipients (KTRs). Cardiovascular events are the first cause of death in this population affecting 36% to 55% of patients. The impact of VC on morbidity and mortality of KTRs is not appreciated enough[39-41]. Three point five percent to five percent of KTRs experience fatal or non-fatal cardiovascular events annually. This rate is much higher than in the general population. The prevalence of coronary artery calcification (CAC) in KTRs is higher (61%-75%) than that assessed in stage 3 CKD[42-44] and lower than that found in HD patients[45]. Moe et al[37] did not observe CAC progression after a successful kidney transplant. On the other hand, Oschatz et al[46] observed a significant progression within the first 6 mo, but no significant change between months 6 and 12 after a kidney transplant. All these trials were short term. When longer-term follow-up trials were performed, kidney transplant was found to favorably affects but does not halt CAC progression, with an annual rate of CAC progression ranging between 11% and 12.5%[47-49]. The risk of progression was higher in Caucasian race, with increased body mass index, higher baseline CAC score, higher diastolic blood pressure and lower glomerular filtration rate 3 mo after transplantation[50]. Other risk factors included inflammation, hyperparathyroidism and dialysis duration[47,51,52]. CAC score was significantly lower in KTR who had a pre-emptive transplant in comparison to those who underwent dialysis before transplantation (3.7 vs 102.9, P < 0.001)[52]. According to these studies, it seems that pre-emptive kidney transplant gives ESRD patients their best chance to avoid progressive VC.
Many factors summate the pathogenesis of VC in CKD. Such factors are either traditional or CKD related. The factors related to CKD include high serum calcium and phosphorus, increased dialysis vintage, increased duration of uremia[53], low serum fetuin-A level[53], and high serum level of fibroblast growth factor 23 (FGF23)[10,54-63]. Dialysis vintage, disturbed mineral metabolism and FGF23 are the most relevant factors having impact in the VC of CKD[37]. There is an association between VC and indices of low bone turnover in dialysis patients[64].
More than 150 years ago, Virchow[2] was the first to report that vascular calcium deposits were real ossification. In CUA, vascular smooth muscle cells express osteopontin, bone sialoprotein, and osteonectin[37,65]. In non-calcified arteries in the same skin biopsy section, osteopontin or other bone proteins were not observed[65]. It seems that the deposition of these proteins predispose calcification[37,66].
Vascular smooth muscle cells and osteoblasts originate from the same mesenchymal cell. Core binding factor α-1 (Cbfa1) turns the mesenchymal cell into osteoblast[37,67]. β-glycerophosphate is a phosphate donor. Vascular smooth muscle cells mineralize in the presence of this phosphate donor and increased Cbfa1 activation[37,68]. Calcific arterial lesions in patients devoid of CKD showed increased expression of Cbfa1 while normal arteries failed to show similar finding[37,69]. The findings of Cbfa1 in both CKD vascular lesions and non-CKD arterial disease might denote a common pathogenesis of VC. A significant relationship between increased serum phosphorus and obstructive atherosclerotic coronary artery disease was observed in non-CKD patients[37,70,71].
When bovine vascular smooth muscle cells (BVSMCs) were incubated in uremic serum and healthy control serum, upregulation of Cbfa1 was significantly higher with uremic serum. When β-glycerophosphate was added to increase the inorganic phosphorus within culture media, Cbfa1 significantly increased in normal control serum culture and the significant difference in Cbfa1 was muffled[72]. This increase in Cbfa1 was completely inhibited after addition of foscarnet (an inhibitor of sodium/phosphate co-transport) to the normal serum. In case of uremic serum, inhibition was partial, denoting other factors might have an action on Cbfa1 beside hyperphosphatemia[37]. Bone morphogenic protein-2 (BMP-2) concentration is doubled in CKD serum. BMP-2 was detected in human calcified arteries[37,73-75] and human uremic serum can induce in vitro calcification that increases as the CKD advances[37,76].
Fibroblast growth factor 23 (FGF23) was isolated 16 years ago[77]. FGF23 is responsible for autosomal dominant hypophosphataemic rickets (ADHR) in humans[78] and is the humoral factor secreted by tumors inducing hypophosphatemia and osteomalacia (TIO)[79]. FGF23 plays an important role in the regulation of serum phosphate level. FGF23 is secreted by osteocytes in bone[80]. Other sites might share in FGF23 synthesis, including bone marrow, thalamus, lymph nodes and thymus[81]. The serum levels of FGF23 are derived mainly from bone[82]. FGF23 exerts its hypophosphatemic effect through inhibition of phosphate reabsorption by proximal tubular epithelial cells. It down-regulates the luminal sodium-phosphate co-transporters. FGF23 also inhibits 1α hydroxylase[83]. It was not clear if FGF23 stimulates secretion of parathyroid hormone (PTH)[82] or PTH stimulates FGF23 secretion. Klotho acts as a co-receptor for FGF23 by markedly increasing the affinity of FGF23 for ubiquitous FGF receptors (FGFR)[84]. Klotho, is highly expressed in the kidney and the parathyroid glands[84,85].
Klotho is an anti-senescence protein[86]. It exists in 2 forms: The transmembrane and the soluble secreted form[87,88]. Klotho is detected as soluble protein in body fluids including blood, urine[89-91] and cerebrospinal fluid[89].
The highest expression of Klotho is in kidney and brain[86,90,91], but it is also expressed in parathyroid gland[92,93] and heart[94] with less abundance.
The similarity of the phenotypes between Kl-/- mice[86] and Fgf23-/- mice is striking[95], which strongly suggests a common signaling pathway shared by these molecules[96,97]. Now it is well documented that membrane Klotho functions as the coreceptor for FGF23, which amplifies and confers specificity of FGF23 action[84,85,98,99].
In contrast, soluble Klotho protein functions independently of FGF23[91] and plays an important role in modulation of ion transporters or channels[91,100], antioxidation[101] and anti senescence[102,103], in addition to simply supporting FGF23 action[104]. The protective effect of Klotho against soft tissue calcification is mediated by at least 3 mechanisms: Increasing urine phosphate excretion, renal protection and inhibition of phosphate uptake by vascular smooth muscle cells (VSMCs) and their dedifferentiation[104].
Klotho and FGF23 are likely responsible for calcium and phosphate homeostasis[105,106]. In vitro PTH secretion and mRNA transcription are inhibited by FGF23[107]. On the contrary, primary hyperparathyroidism in rodents is associated with increased FGF23 levels that are reduced by parathyroidectomy. PTH stimulates osteocytes to secrete FGF23[108]. In physiological settings in which there are normal Klotho and FGFR expression, FGF23 decreases PTH production, increases expression of both the parathyroid Ca-sensing receptor and the vitamin D receptor, and decreases cell proliferation[92].
In Klotho mutant mice, the different pathologic manifestations could be reversed when deficient Klotho is replaced[109-111]. Exogenous klotho was found to ameliorate kidney injury and renal fibrosis in a rat model of CKD[112]. It can also ameliorate endothelial cell senescence and muffles the binding of NFκB to nuclear DNA[113].
Patients with stages 3b-5 CKD and dialysis patients often develop high serum FGF23[114]. This elevation can even occur as early as stage 2 CKD, long before any changes in calcium, phosphate, or PTH are apparent[115]. Elevation in FGF23 stimulates the excretion of phosphorus by surviving nephrons. This would prevent the early onset of hyperphosphatemia in spite of increased bone turnover and the progressive decline in functioning nephrons. Development of CKD is associated with significant decline of Klotho mRNA expression[116]. This deficiency might explain the increased serum FGF23 levels in CKD as a result of end-organ resistance to the action of FGF23. By the time the patients reach ESRD, FGF-23 concentrations are often 100- to 1000- fold above the normal range[117], and moreover, circulating FGF-23 in ESRD patients is mostly intact and biologically active[118]. Three possible explanations could account for such elevation. First, increased secretion into and decreased removal of FGF23 from the circulation. Treatment with corticosteroids could activate osteocytes in pediatric CKD patients, and then significantly stimulate FGF-23 synthesis[119]. FGF-23 levels and estimated glomerular filtration rate (eGFR) were inversely correlating among individuals with CKD stage G4-5[120]. Second, the other cause of increased levels of FGF-23 may be related to decreased klotho and end organ resistance to FGF23 action in CKD[121]. Treatment of CKD patients with vitamin D may be the third cause. In 5/6 nephrectomized rats, intravenous administration of 1,25-(OH)2D, three times a week increased serum FGF-23[122].
The first report of a positive correlation between FGF23 and VC among HD patients was 6 years ago[10]. Similar results were reported in cases with CKD stages 2-5D. Patients with higher aortic and coronary calcification scores had elevated FGF23 levels[62]. Similar results were found in healthy older men irrespective of traditional risk factors[123]. Pediatric studies confirmed the same results in children with CKD[124]. The same association was recorded in patients kept on HD for more than one year[125].
Klotho deficiency in CKD vessels likely potentiates the development of accelerated calcification[126]. Restoration of Klotho and FGFRs by vitamin D receptor activators renders human vascular smooth muscle cells FGF23-responsive, and that may be the mechanism of their anti calcific effects[126].
Increased FGF23 level is associated with increased risk for mortality among incident HD patients, during their first year of treatment[127]. This association was also confirmed in prevalent dialysis patients[128]. Neutralization of FGF23 in CKD rats was found to accelerate VC and increases mortality[129].
Atherosclerosis and VC accelerate in states of chronic inflammation. The later is one of the hallmarks of uremia. Uremic status was incriminated in the pathogenesis of chronic inflammation, however, the exact pathogenesis was not fully understood. Altered gut microbiome might affect the integrity of the intestinal barrier leading to facilitated blood translocation of bacteria and uremic toxins[130]. Inflammation also results from multiple co-morbid conditions activating inflammation (like infections and autoimmune systemic diseases)[131]. Many of the inflammatory markers and mediators are found to promote VC in CKD patients. These factors include interleukin 1 (IL-1), IL-6, C-reactive protein and tumor necrosis factor alpha (TNFα)[132-137].
The association between FGF-23 and vascular calcification was mitigated when corrected for inflammation markers[138]. In spite of this important role of inflammation that might underlie the role of Klotho-FGF23 axis, no intervention studies to target inflammation to prevent or stop VC progression in CKD were done.
All CKD patients are exposed to the uremic environment, however, not all of them will develop VC, suggesting that protective mechanisms also exist[139].
Fetuin-A inhibits precipitation of calcium-phosphate[140]. Fetuin-A synthesis is mainly hepatic. Its serum concentration falls with activation of cell mediated immunity[141]. Fetuin-A calcium phosphate complex is called calciprotein particles (CPP). In comparison to hydroxyapatite, CPP induce significantly less cytokine secretion when macrophages are exposed to equimolar concentrations of hydroxyapatite and CPP[142]. Mice deficient in fetuin-A develop extensive renal, myocardial, pulmonary, lingual and cutaneous calcifications[140]. CKD patients with fetuin-A deficiency develop increased cardiovascular mortality[140].
Matrix GIa protein (MGP) is a vitamin K dependent protein, synthesized in the bone[143]. MGP has an inhibitory role in VC[144,145]. MGP inhibits the formation of calcium crystal[73]. CKD is associated with decreased uncarboxylated MGP level with subsequent increased rate of VC and atherosclerosis[146].
Osteoprotegerin (OPG) is another anti-calcific agent. High OPG level is reported in patients with vascular calcification[147,148]. Increase in OPG level may be a self-defensive mechanism against factors promoting VC[148].
Vitamin K likely prevents post-menopausal fractures[149]. Vitamin K deficiency increases the chance of severe aortic calcification[150]. Treatment of rodent with vitamin K2 reduced VC[151]. Treatment of HD patients with vitamin K increases serum MPG and osteocalcin levels[140]. Dietary menaquinone might be more effective compared to phylloquinone, in prevention of the progression of vascular calcification. Studies linking vitamin K status to calcification outcomes in CKD are needed to determine the therapeutic value in such cases[152].
Pyrophosphate (PPi) directly blocks hydroxyapatite formation. PPi is synthesized in VSMCs[153]. PPi deficiency results in excessive arterial calcification[154]. Plasma PPi is deficient in HD patients, and is negatively correlating with VC[155,156].
Vitamin D deficient mice develop excessive VC[157]. Vitamin D deficiency is frequent among CKD patients. Decreased dietary intake, decreased synthesis in the skin and decreased 1α-hydroxylase activity in the failing kidney are the main causes. Further inhibition of 1α-hydroxylase ensues when serum FGF23 rises[158]. In CKD G 3-4, CAC was elevated in both the mild and severe vitamin D deficient cases[159]. Serum levels of 25(OH)D is negatively associated with VC in CKD G4-5[160]. Low plasma level of 25-hydroxy - vitamin D is associated with increased mortality in different stages of CKD. Progression to ESRD was accelerated in vitamin D deficient patients[161-163]. At therapeutic dosages sufficient to correct secondary hyperparathyroidism, VDR activator (VDRA) treatment of mouse model of CKD protected the vasculature from calcifying, but higher doses stimulated aortic calcification[164]. The latter was probably caused by indirect, endocrine VDRA effects resulting in hyperphosphatemia and hypercalcemia. Organ cultures of human arteries from patients with CKD exhibited significant upregulation of Klotho mRNA levels following 48 h of calcitriol or paricalcitol treatment. This treatment effect was not observed in arteries from healthy individuals. Therapeutic dosages of VDRA were also found to reduce VSMC phenotype transformation in the aorta[124].
To sum up, it seems clear that VC is triggered by different promoting factors that increase in CKD together with the deficiency of different protective factors. In other words, VC in CKD patients is the result of the interaction of this collection of offenders and inhibitors[165].
Sudden cardiac death, arrhythmia, congestive heart failure, or stroke are the major causes of death in patients with VC[166,167]. Most of the data on prognostic value of VC are extrapolated from studies in patients with normal kidney function. CKD patients sill need prospective clinical trials evaluating the prognostic impact of aortic, coronary and carotid calcification in different CKD stages[168]. The European Renal Best Practice (ERBP) work group recommends screening of incident dialysis patients[169], whereas some national guidelines dictated the screening of any CKD patient[170]. KDIGO guidelines, issued during 2009, considered that patients with CKD stages 3-5D and with known VC as highest vascular risk and that this information should guide the management[171]. On the other hand, Zoccali et al[172] denied VC as a risk factor for ongoing vascular disease. Their opinion relies on many studies, one of them is the recent meta-analysis of different clinical trials on the impact of different phosphate binders on mortality in CKD[173], the ADVANCE trial[174] and the EVOLVE trial[175]. In addition, Wanner[176] criticized any effort offered for diagnosis or treatment of VC as long as all the last mentioned trials failed to change the prognosis in HD patients.
In our opinion, the medical practitioners should do their best effort to prevent this devastating pathology in every CKD patient and not to wait to diagnose its end stage in the dialysis population. This means energetic preventive measures should be offered to every CKD patient all through different stages.
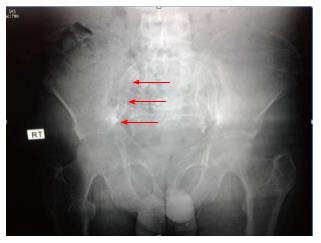
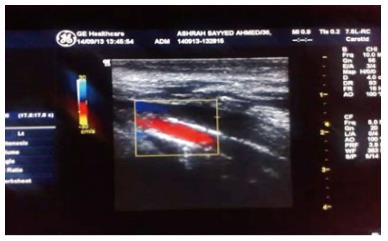
In 2009, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines did not recommend the routine screening of VC as long as there is no clear clinical utility[177]. However, in some cases, imaging of VC might help to guide the treatment plan[178]. The gold standard for the quantification of calcification in different vessels is by computed tomography imaging. Plain X-rays can help to identify aortic and peripheral arterial calcifications (Figure 2); Doppler ultrasound is helpful in imaging the carotid, femoral and popliteal arteries and the aorta (Figure 3); echocardiography is valuable tool for visualizing valvular calcification and mammography for breast arterial calcification (BAC). BAC is a useful radiologic sign. It indicates tunica media calcification commonly encountered in CKD[179].
Quantification of VC is achieved using either the Kauppila score, the Adragao scores, the Agatston score, the volume score or the mass score. The Kauppila scores are used to quantify calcification of abdominal aorta, an indicator of intimal calcification[180,181]. The Adragao score is used to quantify VC in the iliac, femoral, radial, and digital arteries. Adragao score reflects medial calcification[182]. The volume and mass scores are quantitative and more reproducible measurements (mm3 or mg, respectively)[183-185], in addition to being more appropriate for use with modern CT scanners than the Agatston score[186]. However, the Agatston score (semiquantitative) is the most frequently used and reported method in the medical literature so far.
Interference for the traditional risk factors must start very early while the patient is still in stage G1. For nontraditional risk factors, we should start in the very early days of stage G2. The severity of arterial stiffness (as an index of atherosclerosis and VC) was found to increase steadily with more advanced CKD from stages 1 to 5[187]. The different factors concerned with VC begin very early when renal damage is still trivial and before hyperphosphatemia ensues. Therefore, the earlier the intervention the better is the impact on morbidity and mortality[188]. The changes in these factors are sequential. FGF23 is the earliest starting during stage G2. Decline of serum calcitriol follows when GFR falls below 60-70 mL/min per 1.73 m2. PTH elevation follows in the late phase of stage G3a while changes in serum phosphate occur in stage G3b[189]. We would like to emphasize that this sequence of events is triggered by the decreased capability of the injured kidney to manipulate phosphate excretion. FGF23 triggers the increase in the fractional excretion of phosphorus by the surviving nephrons. FGF23 inhibits 1-α hydroxylase enzyme, with subsequent decrease in synthesis of 1α calcidol. Decreased calcitriol synthesis will result in the decline of its serum level, and later in stimulation of parathormone synthesis and secretion. Being the earliest sign of disturbed renal handling of phosphorus, interference should start as soon as FGF23 starts to rise, at a much earlier stage than we do in the time-being[81,190-196].
VC management aims at improvement of survival and morbidity in the CKD population[197]. However, there is a lack of data to guide management strategies in these patients based on CAC scores[198]. KDOQI guidelines recommend special care of CKD patient if VC is detected in more than one site[199]. VC is not reversible, so far. Accordingly, the successful management is based on how to prevent or to stabilize existent lesions[200].
Management of traditional risk factors among dialysis patients still faces concern about its value. Such factors were found correlating with better survival[201]. Initially, treatment of different traditional risk factors in pre-dialysis CKD patients was based on studies mainly done in cohorts without renal disease, many trials tackling many of such factors in CKD patients have evolved but are still limited[202].
The strict control of blood sugar carries little benefit, if any, to CKD G5D patients with or without diabetes mellitus[203]. However, such control has a positive impact on survival of pre-dialysis diabetic CKD patients. Glycemic control might also delay CKD progression and postpones the need for dialysis[204,205].
Blood pressure control muffles the rate of decline in GFR in pre-dialysis CKD patients[206]. Hypertensive CKD patients should be treated according to KDIGO guidelines[207]. The problem is much debatable when discussing hypertension control in dialysis patients[208]. Home BP carries better prognostic impact when compared to recordings in the dialysis unit. Systolic home BP of 115-145 mmHg is associated with the best prognosis in HD patients[209]. Renin-Angiotensin-system (RAS) blockers stimulate Klotho gene expression in CKD patients. This novel mechanism might clarify the vascular, cardiac and renal protective benefits of such agents[210,211]. The RAS mediated renal damage might be through Klotho gene manipulation[212]. Through their manipulation of Klotho gene, RAS blockers can add a new exciting mechanism for their cardiovascular and renal protective effect.
Aldosterone might induce vascular calcification. We are still waiting for clinical studies to evaluate if there is a protective effect of aldosterone antagonists[213].
CKD patients frequently develop dyslipidemia. Treatment with statins to lower LDL cholesterol is recommended by KDOQI and KDIGO in all adult patients with diabetic CKD and in hypercholesrolemic non- diabetic CKD patient. Such treatment can reduce different cardiovascular events complicating atherosclerosis. However, this treatment does not impact overall mortality in these patients[214,215]. Many trials targeting CKD patients were done using different statins or statin-ezetimibe combination. In CKD G3 patients, pravastatin treatment was associated with significant reduction of coronary events[216]. However, another trial using the same statin failed to show any significant impact on 2ry prevention in patients with early CKD[217]. When statins are used for primary prevention, instead, they reduced the risk of cardiovascular events in stages 1-3 CKD by 41%[218]. On the other hand, all trials comparing statins with placebo in HD patients failed to demonstrate any significant impact on clinical outcome or overall mortality. These trials used atorvastatin, 20 mg daily, in the 4D study, rosuvastatin, 10 mg daily, in the AURORA trial and simvastatin, 20 mg plus ezetimibe 10 mg, in the SHARP study[219-221].
Lifestyle modifications including regular muscle exercise, salt restriction, decrease of calorie intake, and smoking cessation carry significant cardiovascular benefits in the general population. However, we lack data supporting such interventions at all CKD stages[202].
The very early elevation of FGF23 during CKD G2 should stimulate the attending physicians to reduce phosphorus intake in CKD patients starting in the early days of stage 2[222]. Phosphate binders, whether calcium containing or calcium-free, should be avoided in this early stage as long as serum phosphorus level is normal or near normal. The very early use of the phosphate binders might be associated with progression of VC while lowering serum phosphorus and attenuating the progression of secondary hyperparathyroidism[223].
Calcium-based phosphate binders are still very useful to control hyperphosphatemia, but can lead to hypercalcemia and/or positive calcium balance and cardiovascular calcification[224].
Sevelamer hydrochloride and carbonate are resin-based binders that appear to have profiles that would prevent or muffle VC[224]. Treatment of non-diabetic stage 3 CKD patients that have normal serum phosphorus with sevelamer did not lower cardiovascular-related outcomes[225]. These findings reinforce the trend to avoid phosphate binders in early stages of CKD where the serum phosphorus is still normal. On the other hand, when sevelamer was used in hyperphosphatemic stage 3-4 CKD patients, a significant impact on all-cause mortality and the need of dialysis was observed in comparison to calcium carbonate[226]. The main drawback of all calcium-containing phosphate binders is the tendency to increase serum calcium level. The higher the dose ingested the greater the extent of VC[227,228]. Thus their use in cases suffering VC, hypercalcemia, low level of parathormone (PTH) and/or adynamic bone disease has to be restricted[229]. In the US Sevelamer is mainly used in dialysis patients to decrease progression of coronary artery and aortic calcifications[230-235]. On the other hand, the European Medicines Agency recommended its use in hyperphosphatemic patients with CKD not yet on dialysis[236-238]. When incident HD patients were assigned to either calcium-based phosphate binders or sevelamer, and were followed for 44 mo, all-cause mortality was lower in subjects assigned to sevelamer compared to patients assigned to calcium-based binders. However, results were of borderline statistical significance. Another important finding in this study is the significant predictive value of baseline CAC score concerning all-cause mortality[239]. In the “Treat to Goal Study”, coronary and aortic calcification progressed in dialysis patients receiving calcium-containing phosphate binders while those receiving sevelamer did not show progression[232]. On the other hand, sevelamer failed to improve mortality rate among prevalent HD patients when compared to calcium-based binders in the multicenter, randomized trial “the DCOR”[240].
We like to emphasize that while the hyperphosphatemic stage 3-4 CKD patients showed benefits in all-cause mortality[226], and the incident HD showed borderline significantly lower mortality after sevelamer use[239], the same agent failed to show a similar benefit in prevalent HD subjects[240]. We should remember that these different groups are in different stages of evolution as regards VC[9,10,160] and that the baseline score of coronary calcification is a strong predictor of all-cause mortality[239]. This confirms that the earlier the approach the better would be the impact on CKD patient survival.
Sevelamer is not just a calcium-free phosphate binder, but also has additional pleiotropic effects such as correcting certain abnormalities of lipid metabolism[241], significant decrease in inflammatory parameters including IL-6, sCD14 and hs-CRP[242,243], reduction of serum uric acid concentration[244], decrease of serum FGF23[123,245,246], increase of serum level of fetuin-A[236,247] and Klotho[246]. Compared to calcium based phosphate binders, sevelamer improves endothelial function in CKD patients[248]. These results suggest that sevelamer has, beside its hypophosphatemic and calcemic actions, important metabolic, and anti-inflammatory actions that help in decreasing uremic vasculopathy. Sevelamer is more expensive compared to calcium-based phosphate binders[249]. The significant reduction in all-cause mortality and the significantly fewer hospitalizations in the sevelamer group can offset the higher acquisition cost for sevelamer[250].
Lanthanum carbonate (LC) is another non-calcium based phosphate binder. It was reported to improve aortic VC progression[251]. There are no trials studying the effect of LC on either coronary or valve calcification[252]. LC had no impact on over all mortality in CKD patients[251,253]. However, the mortality was significantly lower in patients above 65 years in the LC treatment group compared with calcium based phosphate binders. A similar observation was reported in patients receiving sevelamer in the DCOR study[240,254]. In the only trial looking for the impact of LC on the incidence of cardiovascular events, it failed to show any significant difference compared with calcium-based compounds[251].
Contrary to sevelamer, lanthanum carbonate does not have a consistent effect on FGF23. LC failed to cause reductions in iFGF23 in patients with CKD stage G3-4[255,256]. On the other hand, other studies showed that LC was effective in reducing FGF23 levels in CKD G3[257] and CKD G4 - 5 patients[258]. None of the trials on Lanthanum reported any effect on inflammation or inflammatory biomarkers. Although LC is cheaper and more compliant (Table 1) compared to either sevelamer hydrochloride or sevelamer carbonate[259], our target is not just to control phosphorus level. Sevelamer compounds have got more comprehensive trials that showed significant impact on patient mortality during predialysis stages and in incident HD. No similar trials could be encountered for lanthanum. We are still waiting for such studies to assure non-inferiority of Lanthanum in this field.
| CKD stage | Risk factor | Type off interference | Outcome | Ref. |
| Traditional Risk factors | ||||
| G1-G5 | Cigarette smoking | Cessation | No evidence | [202] |
| G1-G5 | Overweight | Decrease calorie intake | No evidence | [202] |
| G1-G5 | Sedentary life | Muscle excercise | No evidence | [202] |
| G1-G5 | Diabetes mellitus | Blood sugar control | Improves survival | [204] |
| Delays CKD progression | [205] | |||
| G1-G5 | Systemic hypertension | Blood pressure control | Delays CKD progression | [206] |
| G1-G5 | Dyslipidemia | Statins | Decreased CV morbidity | [221] |
| CKD Related Risk factors | ||||
| G2-G5 | ↑ | Dietary phosphate restriction | ↓ FGF23 | [222] |
| G3b-G4 | Hyperphosphatemia | Sevelamer | ↓ VC, ↓ mortality | [226] |
| G5 | Preemptive kidney Tx | ↓ VC, ↓ mortality | [52,295] | |
| Incident G5D | Hyperphosphatemia | Sevelamer | ↓ VC, ↓ mortality (borderline) | [231] |
| Prevalent G5D | Hyperphosphatemia | Sevelamer or L.C. | ↓ VC | [232,240,251] |
| Prevalent G5D > 65 yr | Hyperphosphatemia | Sevelamer or L.C. | ↓ VC, ↓ mortality | [240,251] |
The value of nicotinamide (NAM) in phosphate control (as well as its effects on lipid levels) in dialysis patients was explored in some short-term trials[260-262]. However, such trials did not look for either pharmacokinetics or safety. None of these trials studied the impact on VC, FGF23, Klotho or inflammatory mediators.
Iron compounds represent the new class of phosphate binders. Ferric Citrate, Sucroferric oxyhydroxide, and Fermagate (iron-magnesium hydroxycarbonate) were tested in some clinical trials[263]. Most of the clinical studies done so far were using ferric citrate, stressing on phosphate binding and ferrokinetics after short periods of trial. So far, no trials have studied the impact on VC[264-272]. A single study looked for non-inferiority of Sucroferric oxyhydroxide (PA21) compared to sevelamer carbonate concerning phosphate binding[273].
Bixalomer is novel non-calcium, amine-functional polymer that binds phosphate in the gastrointestinal tract and inhibits its absorption. It was approved as hypophosphatemic agent in Japan by June 2012. It proved non-inferiority with much lower adverse effects relative to sevelamer hydrochloride[274].
Salivary phosphorus binding is another approach to reduce serum phosphate level. Chitosan-loaded chewing gum, chewed during fasting periods, may be a valuable add-on to phosphate binders that can lead to a better control of hyperphosphatemia[275].
The possible beneficial effect of bisphosphonates on VC has evolved during the 1970s when their administration was found associated with decreased calcification of soft tissue in animal and clinical trials[276,277]. These observations are probably explained by the paradoxical relation between bone mineral density (BMD) and VC[276-278]. That effect might also be related to the stimulatory action of bisphosphonates on fetuin-matrix Gla protein-mineral complex[279] and their possible inhibitory action on IL-6. Transformation of VSMCs to osteoblasts and calcification of intimal atheromatous lesions might be triggered by IL-6[280]. Bisphosphonates were found to inhibit vascular arterial and cardiac valvular calcifications that develop in rats treated with warfarin[281]. When different members of bisphosphonates were tried in chronic HD patients their anti-calcific effect was favorable in some studies[282-284] and failed in other more recent one[285]. In addition, alendronate failed to withhold the progression of VC in G3-4 CKD patients when compared with placebo for 18 mo[286]. Bisphosphonates are not safe in patients suffering advanced CKD. They can aggravate hyperparathyroidism. They can also lead to adynamic bone disease, osteomalacia or mixed uremic osteodystropy[287]. All the trials of bisphosphonates studied their impact on VC. Only one trial studied the impact of bisphosphonate treatment on cardiovascular outcomes in female CKD patients. This study was retrospective[288].
In the EVOLVE Trial, cinacalcet was tested in chronic HD patients suffering moderate-to-severe 2ry hyperparathyroidism. Inspite of the favorable effects of cinacalcet on serum calcium, it failed to decrease the mortality rate or the major cardiovascular events in such patients[175].
We recommend small dose of vitamin D or vitamin D analogues to be given daily as prophylaxis against VC in spite of the lack of clinical trials favoring the use of either native or active vitamin D analogues to prevent VC progression. The rarity of vitamin D toxicity in general and the privileged survival benefits offered by VDRAs administered in small doses even in cases suffering hyperparathyroidism and/or increased calcium and phosphorus levels supports this concept. Some studies reported the association of low vitamin D serum level with extensive VC[289,290]. Vitamin D inhibits renin activity, inflammation, suppresses stimulators of VC and stimulates inhibitors of VC in the uremic milieu[291].
We are still looking for the possible role of vitamin K supplementation in management of VC[292]. Treatment of CKD rats with vitamin K1 suppressed the development of VC[293]. A prospective trial is going on in RDT patients suffering coronary calcification. The effect of vitamin K1 supplementation on the calcification progression in the thoracic aorta and coronary artery will be addressed. All-cause mortality is a secondary end-point. This study may offer an inexpensive agent to treat or prevent VC[294].
Once the patient proceeds to stage 5, pre-emptive kidney transplantation is the best option to improve patient and graft survival in comparison to patients admitted to dialysis or to patients transplanted after starting dialysis[295-298]. In patients starting dialysis, the shorter the dialysis vintage the better is the post-transplant survival[299]. The survival benefit of transplantation compared to dialysis is most probably related to the decreased rate of VC post-transplant compared to the accelerated progress in VC observed in dialysis. To further decrease the rate of calcification progression after transplantation, perioperative vascular imaging and analysis of serum FGF23 might help in appointing patients more likely to have progression of VC Such patients should continue the anti-calcific measures applied to CKD G3 patients. This advice is based on the previous observation of the strong association between baseline CAC score and CAC progression[39,300] and on the recent finding of high serum level FGF23 in KTR even when they have normal graft function[301]. This disturbance of FGF23 appeared to be related to the endothelial cell injury in KTR[302]. Elevated levels of FGF23 may predict increased risks of death and allograft loss[303].
Since the pathogenesis of CUA is not fully elucidated, its treatment is still not uniform[304]. Cinacalcet appeared to reduce the incidence of CUA in HD recipients who have moderate to severe secondary hyperparathyroidism[305]. Sodium thiosulphate[38,304] is used successfully in treatment. Bisphosphonates may be also used[306,307].
The new definition and staging for CKD suggested by the NKF-KDOQI in 2002 aimed at stimulation and increased awareness of the medical community to early diagnose CKD[308]. Early diagnosis of CKD gives a great chance to delay the progression of such disease, and to have better chance to deal with the different complications. VC has evolved as the most serious complication in CKD patients endangering their life. The only successful treatment for VC is preventive. This treatment should start as early as the early days of stage G1. Control of blood sugar in diabetic pre-dialysis CKD patients is a mandate. Recommended hemoglobin A(1c) level should be around 7%. Hypertensive CKD patients should be treated according to KDIGO guidelines. Statin treatment should be prescribed according to KDIGO guidelines.
Screening for FGF23 would pick up CKD patients requiring phosphorus handling at much earlier stage when they benefit maximally. However, we are still waiting for epidemiologic studies that would determine normal and target levels of FGF23 and the ideal method of assay.
In these early days, moderation of dietary phosphate intake might suffice. If Serum PTH level is high, we should measure serum 25-hydroxy vitamin D level[309]. If such level is below 30 ng/mL the patient should be prescribed either vitamin D2 or D3. We are waiting for prospective clinical trials studying the value of recombinant Klotho treatment in normalization of serum FGF23 level and preventing the development or progression of VC. Regular estimation of serum calcium, phosphorus, Ca x p byproduct and PTH should be performed with the frequency recommended by guidelines[310]. Once serum phosphorus starts to rise above normal, strict restriction of dietary phosphorus and prescription of sevelamer should ensue. Other phosphate binders could be used, however, the lack of clear evidence for their effect on Klotho and on cardiovascular morbidity and mortality would postpone their use in the time being till we have strong evidence for these effects. A small dose of vitamin D analogues should be added to all patients passing to stage 3 and beyond. Vitamin K looks promising in preventing or slowing the progression of VC, however, we are still waiting for the results of the ongoing study looking for its efficacy. Once the patient proceeds to stage 5, pre-emptive kidney transplantation is the best option to improve patient and graft survival in comparison to patients admitted to dialysis or to patients transplanted after starting dialysis. In patients starting dialysis, the shorter the dialysis vintage the better is the post-transplant survival. To further decrease the rate of calcification progression after transplantation, perioperative vascular imaging and analysis of serum FGF23 might help in appointing patients more likely to have progression of VC Such patients should continue the anti-calcific measures applied to CKD G3 patients.
In patients maintained on dialysis, non-calcium phosphate binders still carry the privilege of decreased progression of vascular calcification in spite of their failure to impact either cardiovascular morbidity or mortality. HD patients above 65 years of age showed survival benefit after use of sevelamer or LC, the latter is preferred in this age group based on patient compliance and cost of treatment.
Finally we have to emphasize that huge effort is still needed to support many of the above suggestions by well-designed prospective controlled studies to evaluate either efficacy, safety of such interventions beside the precise definition of optimum dosage and frequency of every individual therapeutic modality.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Elisaf MS, Stavroulopoulos A S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 2. | Virchow R. Kall metastasen. Virchows Arch Pathol Anat. 1855;8:103-113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 89] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Virchow R. Cellular Pathology: As Based upon Physiological and Pathological Histology (translated by Frank Chance, 1971). An unabridged and unaltered republication of the English translation originally published in Dover. New York: Cornell University Library, 1863: 404-408. |
| 4. | Durante G. Atherome congenital de l’aorte et de l’artere pulmonaire. Paris: Bull. Soc. anat 1899; 74-97. |
| 5. | Andersen DH, Schlesinger ER. Renal hyperparathyroidism with calcification of the arteries in infancy. Amer J Dis Child. 1942;63:102-1942. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Stryker WA. Arterial calcification in infancy with special reference to the coronary arteries. Amer J Path. 1946;22:1007. |
| 7. | Murphy WA, Nedden Dz Dz, Gostner P, Knapp R, Recheis W, Seidler H. The iceman: discovery and imaging. Radiology. 2003;226:614-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Allam AH, Thompson RC, Wann LS, Miyamoto MI, Nur El-Din Ael-H, El-Maksoud GA, Al-Tohamy Soliman M, Badr I, El-Rahman Amer HA, Sutherland ML, Sutherland JD, Thomas GS. Atherosclerosis in ancient Egyptian mummies: the Horus study. JACC Cardiovasc Imaging. 2011;4:315-327. [PubMed] |
| 9. | Górriz JL, Molina P, Cerverón MJ, Vila R, Bover J, Nieto J, Barril G, Martínez-Castelao A, Fernández E, Escudero V. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1002] [Cited by in RCA: 1000] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 12. | Rodriguez Garcia M, Naves Diaz M, Cannata Andia JB. Bone metabolism, vascular calcifications and mortality: associations beyond mere coincidence. J Nephrol. 2005;18:458-463. [PubMed] |
| 13. | Qunibi WY, Abouzahr F, Mizani MR, Nolan CR, Arya R, Hunt KJ. Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int. 2005;68:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Adeseun GA, Xie D, Wang X, Joffe MM, Mohler ER, Townsend RR, Budoff M, Rosas SE. Carotid plaque, carotid intima-media thickness, and coronary calcification equally discriminate prevalent cardiovascular disease in kidney disease. Am J Nephrol. 2012;36:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Civilibal M, Caliskan S, Kurugoglu S, Candan C, Canpolat N, Sever L, Kasapcopur O, Arisoy N. Progression of coronary calcification in pediatric chronic kidney disease stage 5. Pediatr Nephrol. 2009;24:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Chen W, Melamed ML. Vascular calcification in predialysis CKD: common and deadly. Clin J Am Soc Nephrol. 2015;10:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 800] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 20. | de Oliveira RB, Okazaki H, Stinghen AE, Drüeke TB, Massy ZA, Jorgetti V. Vascular calcification in chronic kidney disease: a review. J Bras Nefrol. 2013;35:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Mönckeberg JG. Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virchows Arch Pathol Anat Physiol Klin Med. 1903;171:141-167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 139] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Shroff R, Long DA, Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 23. | Sigrist M, Bungay P, Taal MW, McIntyre CW. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Moe SM, O’Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA. Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant. 2004;19:2387-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Taniwaki H, Ishimura E, Tabata T, Tsujimoto Y, Shioi A, Shoji T, Inaba M, Inoue T, Nishizawa Y. Aortic calcification in haemodialysis patients with diabetes mellitus. Nephrol Dial Transplant. 2005;20:2472-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Renal Data System: Causes of death in USRDS Annual Data Report. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 2007. Available from: http// www.usrds.org/2007/pdf/00_intro_07.pdf. |
| 27. | Qunibi WY, Nolan CA, Ayus JC. Cardiovascular calcification in patients with end-stage renal disease: a century-old phenomenon. Kidney Int Suppl. 2002;S73-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Kumar S, Bogle R, Banerjee D. Why do young people with chronic kidney disease die early? World J Nephrol. 2014;3:143-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2429] [Cited by in RCA: 2584] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 30. | Hujairi NM, Afzali B, Goldsmith DJ. Cardiac calcification in renal patients: what we do and don’t know. Am J Kidney Dis. 2004;43:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 1944] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 32. | Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 567] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 33. | Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218-223. [PubMed] |
| 34. | Ibels LS, Alfrey AC, Huffer WE, Craswell PW, Anderson JT, Weil R. Arterial calcification and pathology in uremic patients undergoing dialysis. Am J Med. 1979;66:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Guérin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 687] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 36. | Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63:1852-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 380] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 37. | Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560-567. [PubMed] |
| 38. | Yu Z, Gu L, Pang H, Fang Y, Yan H, Fang W. Sodium thiosulfate: an emerging treatment for calciphylaxis in dialysis patients. Case Rep Nephrol Dial. 2015;5:77-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Cianciolo G, Capelli I, Angelini ML, Valentini C, Baraldi O, Scolari MP, Stefoni S. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol. 2014;39:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Ojo AO, Morales JM, González-Molina M, Steffick DE, Luan FL, Merion RM, Ojo T, Moreso F, Arias M, Campistol JM. Comparison of the long-term outcomes of kidney transplantation: USA versus Spain. Nephrol Dial Transplant. 2013;28:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Kahwaji J, Bunnapradist S, Hsu JW, Idroos ML, Dudek R. Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation. 2011;91:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Seyahi N, Kahveci A, Cebi D, Altiparmak MR, Akman C, Uslu I, Ataman R, Tasci H, Serdengecti K. Coronary artery calcification and coronary ischaemia in renal transplant recipients. Nephrol Dial Transplant. 2011;26:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Maréchal C, Schlieper G, Nguyen P, Krüger T, Coche E, Robert A, Floege J, Goffin E, Jadoul M, Devuyst O. Serum fetuin-A levels are associated with vascular calcifications and predict cardiovascular events in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:974-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Russo D, Morrone L, Russo L. Coronary artery calcification and cardiovascular mortality in predialysis patients. Kidney Int. 2011;79:258; author reply 258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Matsuoka M, Iseki K, Tamashiro M, Fujimoto N, Higa N, Touma T, Takishita S. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin Exp Nephrol. 2004;8:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Oschatz E, Benesch T, Kodras K, Hoffmann U, Haas M. Changes of coronary calcification after kidney transplantation. Am J Kidney Dis. 2006;48:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Mazzaferro S, Pasquali M, Taggi F, Baldinelli M, Conte C, Muci ML, Pirozzi N, Carbone I, Francone M, Pugliese F. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin J Am Soc Nephrol. 2009;4:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Seyahi N, Cebi D, Altiparmak MR, Akman C, Ataman R, Pekmezci S, Serdengecti K. Progression of coronary artery calcification in renal transplant recipients. Nephrol Dial Transplant. 2012;27:2101-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Maréchal C, Coche E, Goffin E, Dragean A, Schlieper G, Nguyen P, Floege J, Kanaan N, Devuyst O, Jadoul M. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Schankel K, Robinson J, Bloom RD, Guerra C, Rader D, Joffe M, Rosas SE. Determinants of coronary artery calcification progression in renal transplant recipients. Am J Transplant. 2007;7:2158-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Mehrotra R, Budoff M, Hokanson JE, Ipp E, Takasu J, Adler S. Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int. 2005;68:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Rosas SE, Mensah K, Weinstein RB, Bellamy SL, Rader DJ. Coronary artery calcification in renal transplant recipients. Am J Transplant. 2005;5:1942-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 624] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 54. | Moe OW, Kuro-o M. Fibroblast growth factor 23 and uremic vascular calcification: is it time to escalate from biomarker status to pathogenic agent? Kidney Int. 2014;85:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Zhang M, Yan J, Zhu M, Ni Z. Fibroblast growth factor 23 predicts coronary calcification and poor prognosis in patients with chronic kidney disease stages 3-5D. Ann Clin Lab Sci. 2015;45:17-22. [PubMed] |
| 56. | Xiao Y, Peng C, Huang W, Zhang J, Xia M, Zhang Y, Ling W. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. PLoS One. 2013;8:e72545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Chen YC, Lin FY, Lin RH, Chuang CL, Chang CC, Tsai CS. Relation between fetuin-A levels and fibroblast growth factor 23 with the severity of coronary artery disease measured by SYNTAX scores. Am J Cardiol. 2013;112:950-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Six I, Okazaki H, Gross P, Cagnard J, Boudot C, Maizel J, Drueke TB, Massy ZA. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One. 2014;9:e93423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 59. | Craver L, Dusso A, Martinez-Alonso M, Sarro F, Valdivielso JM, Fernández E. A low fractional excretion of Phosphate/Fgf23 ratio is associated with severe abdominal Aortic calcification in stage 3 and 4 kidney disease patients. BMC Nephrol. 2013;14:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T, Shimosawa T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014;85:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Chen Z, Chen X, Xie J, Ma X, Zhong F, Hou L, Ling H, Li X, Ren H, Chen N. Fibroblast growth factor 23 is a predictor of aortic artery calcification in maintenance hemodialysis patients. Ren Fail. 2013;35:660-666. [PubMed] |
| 62. | Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23:2017-2025. [PubMed] |
| 63. | Nakayama M, Kaizu Y, Nagata M, Ura Y, Ikeda H, Shimamoto S, Kuma K. Fibroblast growth factor 23 is associated with carotid artery calcification in chronic kidney disease patients not undergoing dialysis: a cross-sectional study. BMC Nephrol. 2013;14:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Ahmed S, O’Neill KD, Hood AF, Evan AP, Moe SM. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Moe SM, O’Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 67. | Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3326] [Cited by in RCA: 3215] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 68. | Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10-E17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1003] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 69. | Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Chen NX, O’Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 242] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 71. | Narang R, Ridout D, Nonis C, Kooner JS. Serum calcium, phosphorus and albumin levels in relation to the angiographic severity of coronary artery disease. Int J Cardiol. 1997;60:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Boström K, Tsao D, Shen S, Wang Y, Demer LL. Matrix Gla protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044-14052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 489] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 75. | Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 760] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 76. | Patidar A, Singh DK, Winocour P, Farrington K, Baydoun AR. Human uraemic serum displays calcific potential in vitro that increases with advancing chronic kidney disease. Clin Sci (Lond). 2013;125:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 389] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 78. | The ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1066] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 79. | Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500-6505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1033] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 80. | Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38-E49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 81. | Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 82. | Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 289] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 83. | Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N. Human fibroblast growth factor-23 mutants suppress Na+ dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 84. | Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1372] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 85. | Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120-6123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1123] [Cited by in RCA: 1036] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 86. | Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2556] [Cited by in RCA: 2845] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 87. | Shiraki-Iida T, Aizawa H, Matsumura Y, Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M, Nabeshima Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 255] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 88. | Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 517] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 89. | Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 419] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 90. | Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 91. | Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 727] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 92. | Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 417] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 93. | Hofman-Bang J, Martuseviciene G, Santini MA, Olgaard K, Lewin E. Increased parathyroid expression of klotho in uremic rats. Kidney Int. 2010;78:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, Yasui K, Lee JK, Kamiya K, Kitaichi K, Yamamoto K. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 96. | Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 97. | Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med. 2006;12:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 99. | Razzaque MS, Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J Endocrinol. 2007;194:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 100. | Huang CL. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2010;77:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, Maekawa Y, Kida I, Miyazaki J, Ogihara T. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82-87. [PubMed] |
| 102. | Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 103. | Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 104. | Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J Nephrol. 2010;23 Suppl 16:S136-S144. [PubMed] |
| 105. | Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Östman Wernerson A, Lanske B, Olauson H, Larsson TE. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol. 2014;25:2169-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 106. | Nitta K, Nagano N, Tsuchiya K. Fibroblast growth factor 23/klotho axis in chronic kidney disease. Nephron Clin Pract. 2014;128:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 107. | Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 108. | Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 109. | Maltese G, Karalliedde J. The putative role of the antiageing protein klotho in cardiovascular and renal disease. Int J Hypertens. 2012;2012:757469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens. 2012;21:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 111. | Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 112. | Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y. Klotho Ameliorates Kidney Injury and Fibrosis and Normalizes Blood Pressure by Targeting the Renin-Angiotensin System. Am J Pathol. 2015;185:3211-3223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 113. | Buendía P, Carracedo J, Soriano S, Madueño JA, Ortiz A, Martín-Malo A, Aljama P, Ramírez R. Klotho Prevents NFκB Translocation and Protects Endothelial Cell From Senescence Induced by Uremia. J Gerontol A Biol Sci Med Sci. 2015;70:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Stubbs JR, He N, Idiculla A, Gillihan R, Liu S, David V, Hong Y, Quarles LD. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2012;27:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 115. | Rotondi S, Pasquali M, Tartaglione L, Muci ML, Mandanici G, Leonangeli C, Sales S, Farcomeni A, Mazzaferro S. Soluble α -Klotho Serum Levels in Chronic Kidney Disease. Int J Endocrinol. 2015;2015:872193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 116. | Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 357] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 117. | Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 118. | Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 119. | Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 120. | Westerberg PA, Linde T, Wikström B, Ljunggren O, Stridsberg M, Larsson TE. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant. 2007;22:3202-3207. [PubMed] |
| 121. | Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358-5364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 122. | Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 357] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 123. | Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, Hawa G, Chapurlat R, Szulc P. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012;97:E575-E583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 124. | Paoli S, Mitsnefes MM. Coronary artery calcification and cardiovascular disease in children with chronic kidney disease. Curr Opin Pediatr. 2014;26:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Zayed BM, Fishawy H, Al-Shihaby AR, Salem MA3, Sharaf El Din UA. Salem MM efficacy of sevelamer hydrochloride and calcium carbonate as phosphate binders on FGF23 and coronary calcification in hemodialysis patients. Available from: http// www.abstracts2view.com/ wcn/lookup_view.php?word=Zayed&where=authors&return=/wcn/authorindex.php?num=25. |
| 126. | Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 127. | Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1420] [Cited by in RCA: 1306] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 128. | Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 129. | Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122:2543-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 130. | Lau WL, Kalantar-Zadeh K, Vaziri ND. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron. 2015;130:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 131. | Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial. 2002;15:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 286] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 132. | Stompór TP, Pasowicz M, Sułowicz W, Dembińska-Kieć A, Janda K, Wójcik K, Tracz W, Zdzienicka A, Konieczyńska M, Klimeczek P. Trends and dynamics of changes in calcification score over the 1-year observation period in patients on peritoneal dialysis. Am J Kidney Dis. 2004;44:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Stompór T, Rajzer M, Kawecka-Jaszcz K, Dembińska-Kieć A, Janda K, Wójcik K, Tabor B, Zdzienicka A, Grzybowska EJ, Sulowicz W. Renal transplantation ameliorates the progression of arterial stiffness in patients treated with peritoneal dialysis. Perit Dial Int. 2005;25:492-496. [PubMed] |
| 134. | Haydar AA, Covic A, Colhoun H, Rubens M, Goldsmith DJ. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients. Kidney Int. 2004;65:1790-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 135. | Stompór T, Pasowicz M, Sulłowicz W, Dembińska-Kieć A, Janda K, Wójcik K, Tracz W, Zdzienicka A, Klimeczek P, Janusz-Grzybowska E. An association between coronary artery calcification score, lipid profile, and selected markers of chronic inflammation in ESRD patients treated with peritoneal dialysis. Am J Kidney Dis. 2003;41:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 136. | Stompór T. An overview of the pathophysiology of vascular calcification in chronic kidney disease. Perit Dial Int. 2007;27 Suppl 2:S215-S222. [PubMed] |
| 137. | Jung HH, Kim SW, Han H. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant. 2006;21:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 138. | Nasrallah MM, El-Shehaby AR, Osman NA, Fayad T, Nassef A, Salem MM, Sharaf El Din UA. The Association between Fibroblast Growth Factor-23 and Vascular Calcification Is Mitigated by Inflammation Markers. Nephron Extra. 2013;3:106-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, Goldsmith DJ. Vascular calcification in chronic kidney disease. Clin Sci (Lond). 2010;119:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 140. | Ketteler M, Wanner C, Metzger T, Bongartz P, Westenfeld R, Gladziwa U, Schurgers LJ, Vermeer C, Jahnen-Dechent W, Floege J. Deficiencies of calcium-regulatory proteins in dialysis patients: a novel concept of cardiovascular calcification in uremia. Kidney Int Suppl. 2003;S84-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 141. | Ketteler M, Vermeer C, Wanner C, Westenfeld R, Jahnen-Dechent W, Floege J. Novel insights into uremic vascular calcification: role of matrix Gla protein and alpha-2-Heremans Schmid glycoprotein/fetuin. Blood Purif. 2002;20:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 142. | Smith ER, Hanssen E, McMahon LP, Holt SG. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS One. 2013;8:e60904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 143. | Price PA, Williamson MK. Primary structure of bovine matrix Gla protein, a new vitamin K-dependent bone protein. J Biol Chem. 1985;260:14971-14975. [PubMed] |
| 144. | Cancela L, Hsieh CL, Francke U, Price PA. Molecular structure, chromosome assignment, and promoter organization of the human matrix Gla protein gene. J Biol Chem. 1990;265:15040-15048. [PubMed] |
| 145. | Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1548] [Cited by in RCA: 1489] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 146. | Parker BD, Ix JH, Cranenburg EC, Vermeer C, Whooley MA, Schurgers LJ. Association of kidney function and uncarboxylated matrix Gla protein: data from the Heart and Soul Study. Nephrol Dial Transplant. 2009;24:2095-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 147. | Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2006;26:2117-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 148. | Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 149. | Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 269] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 150. | Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134:3100-3105. [PubMed] |
| 151. | Schurgers LJ, Teunissen KJ, Hamulyák K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279-3283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 152. | Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 153. | Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 155. | Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 156. | O'Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant. 2010;25:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 157. | Schmidt N, Brandsch C, Kühne H, Thiele A, Hirche F, Stangl GI. Vitamin D receptor deficiency and low vitamin D diet stimulate aortic calcification and osteogenic key factor expression in mice. PLoS One. 2012;7:e35316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 158. | Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukroun G, Fournier A, Massy ZA. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 159. | Pillar R, G Lopes MG, Rocha LA, Cuppari L, Carvalho AB, Draibe SA, Canziani ME. Severe hypovitaminosis D in chronic kidney disease: association with blood pressure and coronary artery calcification. Hypertens Res. 2013;36:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 160. | García-Canton C, Bosch E, Ramírez A, Gonzalez Y, Auyanet I, Guerra R, Perez MA, Fernández E, Toledo A, Lago M. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant. 2011;26:2250-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 161. | Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M. Associations of plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. Am J Kidney Dis. 2012;60:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 162. | Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 163. | Drechsler C, Verduijn M, Pilz S, Krediet RT, Dekker FW, Wanner C, Ketteler M, Boeschoten EW, Brandenburg V. Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Soc Nephrol. 2011;6:1752-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 164. | Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 165. | Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 166. | London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1318] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 167. | Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63:A7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 168. | Dellegrottaglie S, Saran R, Rajagopalan S. Vascular calcification in patients with renal failure: culprit or innocent bystander? Cardiol Clin. 2005;23:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 169. | Goldsmith DJ, Covic A, Fouque D, Locatelli F, Olgaard K, Rodriguez M, Spasovski G, Urena P, Zoccali C, London GM. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guidelines: a European Renal Best Practice (ERBP) commentary statement. Nephrol Dial Transplant. 2010;25:3823-3831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 170. | Torregrosa JV, Bover J, Cannata Andía J, Lorenzo V, de Francisco AL, Martínez I, Rodríguez Portillo M, Arenas L, González Parra E, Caravaca F. Spanish Society of Nephrology recommendations for controlling mineral and bone disorder in chronic kidney disease patients (S.E.N.-M.B.D.). Nefrologia. 2011;31 Suppl 1:3-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 171. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1076] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 172. | Zoccali C, London G. Con: vascular calcification is a surrogate marker, but not the cause of ongoing vascular disease, and it is not a treatment target in chronic kidney disease. Nephrol Dial Transplant. 2015;30:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 173. | Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 174. | Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, Moustafa M, Goodman WG, Lopez N, Downey G. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 387] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 175. | Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 634] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 176. | Wanner C. Moderator’s view: treatment of vascular calcification is a physical impossibility, so far. Nephrol Dial Transplant. 2015;30:358-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 177. | Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, Sprague SM, Goldfarb S. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2010;55:773-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 178. | Bellasi A, Raggi P. Vascular imaging in chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 179. | Duhn V, D’Orsi ET, Johnson S, D’Orsi CJ, Adams AL, O’Neill WC. Breast arterial calcification: a marker of medial vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 180. | Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 508] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 181. | Wilson PW, Kauppila LI, O’Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 466] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 182. | Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Gonçalves M, Negrao AP. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 183. | Miralles M, Merino J, Busto M, Perich X, Barranco C, Vidal-Barraquer F. Quantification and characterization of carotid calcium with multi-detector CT-angiography. Eur J Vasc Endovasc Surg. 2006;32:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 184. | Raman R, Raman B, Napel S, Rubin GD. Semiautomated quantification of the mass and distribution of vascular calcification with multidetector CT: method and evaluation. Radiology. 2008;247:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 185. | Molloi S, Xu T, Ducote J, Iribarren C. Quantification of breast arterial calcification using full field digital mammography. Med Phys. 2008;35:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 186. | Hoffmann U, Brady TJ, Muller J. Cardiology patient page. Use of new imaging techniques to screen for coronary artery disease. Circulation. 2003;108:e50-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 187. | Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 188. | Hruska KA, Mathew S, Davies MR, Lund RJ. Connections between vascular calcification and progression of chronic kidney disease: therapeutic alternatives. Kidney Int Suppl. 2005;S142-S151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 189. | Isakova T, Wolf MS. FGF23 or PTH: which comes first in CKD? Kidney Int. 2010;78:947-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 190. | Prié D, Ureña Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 191. | Ketteler M, Petermann AT. Phosphate and FGF23 in early CKD: on how to tackle an invisible foe. Nephrol Dial Transplant. 2011;26:2430-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 192. | Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 840] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 193. | Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 627] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 194. | Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 195. | Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 668] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 196. | Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1012] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 197. | Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1215] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 198. | Bashir A, Moody WE, Edwards NC, Ferro CJ, Townend JN, Steeds RP. Coronary Artery Calcium Assessment in CKD: Utility in Cardiovascular Disease Risk Assessment and Treatment? Am J Kidney Dis. 2015;65:937-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 199. | K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 200. | London GM. Arterial calcification: cardiovascular function and clinical outcome. Nefrologia. 2011;31:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 201. | Kovesdy CP, Anderson JE. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin Dial. 2007;20:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 202. | Choi HY, Park HC, Ha SK. How do We Manage Coronary Artery Disease in Patients with CKD and ESRD? Electrolyte Blood Press. 2014;12:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 203. | Shurraw S, Majumdar SR, Thadhani R, Wiebe N, Tonelli M. Glycemic control and the risk of death in 1,484 patients receiving maintenance hemodialysis. Am J Kidney Dis. 2010;55:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 204. | Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171:1920-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 205. | Lee CL, Li TC, Lin SY, Wang JS, Lee IT, Tseng LN, Song YM, Tsai SF, Sheu WH. Dynamic and dual effects of glycated hemoglobin on estimated glomerular filtration rate in type 2 diabetic outpatients. Am J Nephrol. 2013;38:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 206. | Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 892] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 207. | KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2012;2:337-414. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 208. | Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 209. | Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 210. | Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 211. | Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol. 2013;8:1899-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 212. | Yoon HE, Choi BS. The renin-angiotensin system and aging in the kidney. Korean J Intern Med. 2014;29:291-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 213. | Lang F, Ritz E, Voelkl J, Alesutan I. Vascular calcification--is aldosterone a culprit? Nephrol Dial Transplant. 2013;28:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 214. | National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 215. | Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 216. | Tonelli M, Isles C, Curhan GC, Tonkin A, Pfeffer MA, Shepherd J, Sacks FM, Furberg C, Cobbe SM, Simes J. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 217. | Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, de Zeeuw D, de Jong PE, van Veldhuisen DJ, van Gilst WH. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 218. | Major RW, Cheung CK, Gray LJ, Brunskill NJ. Statins and Cardiovascular Primary Prevention in CKD: A Meta-Analysis. Clin J Am Soc Nephrol. 2015;10:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 219. | Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1926] [Cited by in RCA: 1862] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 220. | Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1474] [Cited by in RCA: 1476] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 221. | Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1946] [Cited by in RCA: 1762] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 222. | Adema AY, de Borst MH, Ter Wee PM, Vervloet MG. Dietary and pharmacological modification of fibroblast growth factor-23 in chronic kidney disease. J Ren Nutr. 2014;24:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 223. | Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 224. | Locatelli F, Del Vecchio L, Violo L, Pontoriero G. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profiles. Expert Opin Drug Saf. 2014;13:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 225. | Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, Edwards NC, Steeds RP, Ferro CJ. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol. 2013;24:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 226. | Di Iorio B, Bellasi A, Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 227. | Floege J, Ketteler M. Vascular calcification in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;19 Suppl 5:V59-V66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 228. | London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol. 2008;19:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 229. | Cozzolino M, Rizzo MA, Stucchi A, Cusi D, Gallieni M. Sevelamer for hyperphosphataemia in kidney failure: controversy and perspective. Ther Adv Chronic Dis. 2012;3:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 230. | Chertow GM, Burke SK, Raggi P. Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1019] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 231. | Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 567] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 232. | Asmus HG, Braun J, Krause R, Brunkhorst R, Holzer H, Schulz W, Neumayer HH, Raggi P, Bommer J. Two year comparison of sevelamer and calcium carbonate effects on cardiovascular calcification and bone density. Nephrol Dial Transplant. 2005;20:1653-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 233. | Braun J, Asmus HG, Holzer H, Brunkhorst R, Krause R, Schulz W, Neumayer HH, Raggi P, Bommer J. Long-term comparison of a calcium-free phosphate binder and calcium carbonate--phosphorus metabolism and cardiovascular calcification. Clin Nephrol. 2004;62:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 234. | Kakuta T, Tanaka R, Hyodo T, Suzuki H, Kanai G, Nagaoka M, Takahashi H, Hirawa N, Oogushi Y, Miyata T. Effect of sevelamer and calcium-based phosphate binders on coronary artery calcification and accumulation of circulating advanced glycation end products in hemodialysis patients. Am J Kidney Dis. 2011;57:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 235. | Shantouf R, Ahmadi N, Flores F, Tiano J, Gopal A, Kalantar-Zadeh K, Budoff MJ. Impact of phosphate binder type on coronary artery calcification in hemodialysis patients. Clin Nephrol. 2010;74:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 236. | Caglar K, Yilmaz MI, Saglam M, Cakir E, Acikel C, Eyileten T, Yenicesu M, Oguz Y, Vural A, Carrero JJ. Short-term treatment with sevelamer increases serum fetuin-a concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin J Am Soc Nephrol. 2008;3:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 237. | Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 238. | Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis. 2012;59:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 239. | Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 240. | Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 241. | Massy ZA, Maizel J. Pleiotropic effects of sevelamer: a model of intestinal tract chelating agent. Nephrol Ther. 2014;10:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 242. | Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P. Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J. 2005;149:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 243. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Donate-Correa J, Cazaña-Pérez V, García-Pérez J. Effect of phosphate binders on serum inflammatory profile, soluble CD14, and endotoxin levels in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2272-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 244. | Garg JP, Chasan-Taber S, Blair A, Plone M, Bommer J, Raggi P, Chertow GM. Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: a randomized clinical trial. Arthritis Rheum. 2005;52:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 245. | Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moysés RM. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 246. | Lin HH, Liou HH, Wu MS, Lin CY, Huang CC. Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum Klotho levels in chronic haemodialysis patients. Nephrology (Carlton). 2014;19:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 247. | Brandenburg VM, Schlieper G, Heussen N, Holzmann S, Busch B, Evenepoel P, Vanholder R, Meijers B, Meert N, Fassbender WJ. Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant. 2010;25:2672-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 248. | Rastogi A. Sevelamer revisited: pleiotropic effects on endothelial and cardiovascular risk factors in chronic kidney disease and end-stage renal disease. Ther Adv Cardiovasc Dis. 2013;7:322-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 249. | Ossareh S. Clinical and economic aspects of sevelamer therapy in end-stage renal disease patients. Int J Nephrol Renovasc Dis. 2014;7:161-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 250. | Ruggeri M, Cipriani F, Bellasi A, Russo D, Di Iorio B. Sevelamer is cost-saving vs. calcium carbonate in non-dialysis-dependent CKD patients in italy: a patient-level cost-effectiveness analysis of the INDEPENDENT study. Blood Purif. 2014;37:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 251. | Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: A pilot randomized controlled trial. Nephrology (Carlton). 2011;16:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 252. | Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013;14:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 253. | Finn WF. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol. 2006;65:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 254. | Wilson R, Zhang P, Smyth M, Pratt R. Assessment of survival in a 2-year comparative study of lanthanum carbonate versus standard therapy. Curr Med Res Opin. 2009;25:3021-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 255. | Ureña-Torres P, Prié D, Keddad K, Preston P, Wilde P, Wan H, Copley JB. Changes in fibroblast growth factor 23 levels in normophosphatemic patients with chronic kidney disease stage 3 treated with lanthanum carbonate: results of the PREFECT study, a phase 2a, double blind, randomized, placebo-controlled trial. BMC Nephrol. 2014;15:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 256. | Isakova T, Barchi-Chung A, Enfield G, Smith K, Vargas G, Houston J, Xie H, Wahl P, Schiavenato E, Dosch A. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol. 2013;8:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 257. | Gonzalez-Parra E, Gonzalez-Casaus ML, Galán A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant. 2011;26:2567-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 258. | Soriano S, Ojeda R, Rodríguez M, Almadén Y, Rodríguez M, Martín-Malo A, Aljama P. The effect of phosphate binders, calcium and lanthanum carbonate on FGF23 levels in chronic kidney disease patients. Clin Nephrol. 2013;80:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 259. | Keith MS, Wilson RJ, Preston P, Copley JB. Cost-minimization analysis of lanthanum carbonate versus sevelamer hydrochloride in US patients with end-stage renal disease. Clin Ther. 2014;36:1276-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 260. | Lenglet A, Liabeuf S, Guffroy P, Fournier A, Brazier M, Massy ZA. Use of nicotinamide to treat hyperphosphatemia in dialysis patients. Drugs R D. 2013;13:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 261. | Takahashi Y, Tanaka A, Nakamura T, Fukuwatari T, Shibata K, Shimada N, Ebihara I, Koide H. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 262. | Cheng SC, Young DO, Huang Y, Delmez JA, Coyne DW. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 263. | Negri AL, Ureña Torres PA. Iron-based phosphate binders: do they offer advantages over currently available phosphate binders? Clin Kidney J. 2015;8:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 264. | Deger SM, Erten Y, Pasaoglu OT, Derici UB, Reis KA, Onec K, Pasaoglu H. The effects of iron on FGF23-mediated Ca-P metabolism in CKD patients. Clin Exp Nephrol. 2013;17:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 265. | Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y. Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol. 2012;36:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 266. | Dwyer JP, Sika M, Schulman G, Chang IJ, Anger M, Smith M, Kaplan M, Zeig S, Koury MJ, Blumenthal SS. Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis. 2013;61:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 267. | Yokoyama K, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Chertow GM, Hirakata H. A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant. 2014;29:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 268. | Lee CT, Wu IW, Chiang SS, Peng YS, Shu KH, Wu MJ, Wu MS. Effect of oral ferric citrate on serum phosphorus in hemodialysis patients: multicenter, randomized, double-blind, placebo-controlled study. J Nephrol. 2015;28:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 269. | Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, Whittier FC, Linfert DR, Galphin CM, Athreya BP. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 270. | Iida A, Kemmochi Y, Kakimoto K, Tanimoto M, Mimura T, Shinozaki Y, Uemura A, Matsuo A, Matsushita M, Miyamoto K. Ferric citrate hydrate, a new phosphate binder, prevents the complications of secondary hyperparathyroidism and vascular calcification. Am J Nephrol. 2013;37:346-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 271. | Sinsakul M, Sika M, Koury M, Shapiro W, Greene T, Dwyer J, Smith M, Korbet S, Lewis J. The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract. 2012;121:c25-c29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 272. | Mutell R, Rubin JL, Bond TC, Mayne T. Reduced use of erythropoiesis-stimulating agents and intravenous iron with ferric citrate: a managed care cost-offset model. Int J Nephrol Renovasc Dis. 2013;6:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 273. | Floege J, Covic AC, Ketteler M, Rastogi A, Chong EM, Gaillard S, Lisk LJ, Sprague SM. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86:638-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 274. | Hatakeyama S, Murasawa H, Narita T, Oikawa M, Fujita N, Iwamura H, Mikami J, Kojima Y, Sato T, Fukushi K. Switching hemodialysis patients from sevelamer hydrochloride to bixalomer: a single-center, non-randomized analysis of efficacy and effects on gastrointestinal symptoms and metabolic acidosis. BMC Nephrol. 2013;14:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 275. | Savica V, Calò LA, Santoro D, Monardo P, Santoro G, Muraca U, Davis PA, Bellinghieri G. Salivary glands: a new player in phosphorus metabolism. J Ren Nutr. 2011;21:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 276. | Fleisch HA, Russell RG, Bisaz S, Mühlbauer RC, Williams DA. The inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivo. Eur J Clin Invest. 1970;1:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 215] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 277. | Russell RG, Smith R, Bishop MC, Price DA. Treatment of myositis ossificans progressiva with a diphosphonate. Lancet. 1972;1:10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 278. | Toussaint ND, Elder GJ, Kerr PG. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 279. | Price PA, Caputo JM, Williamson MK. Bone origin of the serum complex of calcium, phosphate, fetuin, and matrix Gla protein: biochemical evidence for the cancellous bone-remodeling compartment. J Bone Miner Res. 2002;17:1171-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 280. | Omoigui S. The Interleukin-6 inflammation pathway from cholesterol to aging--role of statins, bisphosphonates and plant polyphenols in aging and age-related diseases. Immun Ageing. 2007;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 281. | Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 282. | Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K, Aoki T, Nihei H. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 283. | Hashiba H, Aizawa S, Tamura K, Shigematsu T, Kogo H. Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Ther Apher Dial. 2004;8:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 284. | Hashiba H, Aizawa S, Tamura K, Kogo H. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Ther Apher Dial. 2006;10:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 285. | Ariyoshi T, Eishi K, Sakamoto I, Matsukuma S, Odate T. Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Investig. 2006;26:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 286. | Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis. 2010;56:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 287. | Liu WC, Yen JF, Lang CL, Yan MT, Lu KC. Bisphophonates in CKD patients with low bone mineral density. Scientific World Journal. 2013;2013:837573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 288. | Hartle JE, Tang X, Kirchner HL, Bucaloiu ID, Sartorius JA, Pogrebnaya ZV, Akers GA, Carnero GE, Perkins RM. Bisphosphonate therapy, death, and cardiovascular events among female patients with CKD: a retrospective cohort study. Am J Kidney Dis. 2012;59:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 289. | Razzaque MS. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011;79:708-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 290. | Valdivielso JM, Cannata-Andía J, Coll B, Fernández E. A new role for vitamin D receptor activation in chronic kidney disease. Am J Physiol Renal Physiol. 2009;297:F1502-F1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 291. | Heaf JG, Joffe P, Marckmann P. Vitamin d and stage 5 chronic kidney disease: a new paradigm? Semin Dial. 2012;25:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 292. | Schurgers LJ. Vitamin K: key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013;83:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 293. | McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, Holden RM. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 294. | Krueger T, Schlieper G, Schurgers L, Cornelis T, Cozzolino M, Jacobi J, Jadoul M, Ketteler M, Rump LC, Stenvinkel P. Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): a rationale and study protocol. Nephrol Dial Transplant. 2014;29:1633-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 295. | Gill JS, Tonelli M, Johnson N, Pereira BJ. Why do preemptive kidney transplant recipients have an allograft survival advantage? Transplantation. 2004;78:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 296. | Liem YS, Weimar W. Early living-donor kidney transplantation: a review of the associated survival benefit. Transplantation. 2009;87:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 297. | Grochowiecki T, Szmidt J, Gałazka Z, Nazarewski S, Madej K, Meszaros J, Paczek L, Durlik M, Wyzgał J, Grygiel K. Comparison of 1-year patient and graft survival rates between preemptive and dialysed simultaneous pancreas and kidney transplant recipients. Transplant Proc. 2006;38:261-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 298. | Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 299. | Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 300. | McCullough PA, Chinnaiyan KM. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch Intern Med. 2009;169:2064-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 301. | Bleskestad IH, Thorsen IS, Jonsson G, Skadberg Ø, Bergrem H, Gøransson LG. Soluble Klotho and intact fibroblast growth factor 23 in long-term kidney transplant patients. Eur J Endocrinol. 2015;172:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 302. | Malyszko J, Koc-Zorawska E, Matuszkiewicz-Rowinska J, Malyszko J. FGF23 and Klotho in relation to markers of endothelial dysfunction in kidney transplant recipients. Transplant Proc. 2014;46:2647-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 303. | Ponticelli C, Sala G. Vitamin D: a new player in kidney transplantation? Expert Rev Clin Immunol. 2014;10:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 304. | Tamayo-Isla RA, Cuba de la Cruz M. Calciphylaxis in end-stage renal disease prior to dialytic treatment: a case report and literature review. Int J Nephrol Renovasc Dis. 2015;8:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 305. | Floege J, Kubo Y, Floege A, Chertow GM, Parfrey PS. The Effect of Cinacalcet on Calcific Uremic Arteriolopathy Events in Patients Receiving Hemodialysis: The EVOLVE Trial. Clin J Am Soc Nephrol. 2015;10:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 306. | Sprague SM. Painful skin ulcers in a hemodialysis patient. Clin J Am Soc Nephrol. 2014;9:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 307. | Vo TM, Disthabanchong S. Are there ways to attenuate arterial calcification and improve cardiovascular outcomes in chronic kidney disease? World J Cardiol. 2014;6:216-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 308. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 309. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Guideline 8. Prevention and Treatment of Vitamin D Insufficiency and Vitamin D Deficiency in CKD Patients. USA: National Kidney Foundation, Inc 2005; Available from: http//www.kidney.org/ sites/default/files/docs/boneguidelines.pdf. |
| 310. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Guideline 1. Evaluation of Calcium and Phosphorus Metabolism. USA: National Kidney Foundation, Inc 2005; Available from: http//www.kidney.org/sites/ default/ files/ docs/boneguidelines.pdf. |