Published online Nov 6, 2015. doi: 10.5527/wjn.v4.i5.500
Peer-review started: May 29, 2015
First decision: August 4, 2015
Revised: August 30, 2015
Accepted: October 1, 2015
Article in press: October 8, 2015
Published online: November 6, 2015
Processing time: 169 Days and 8.8 Hours
Hypertension (HTN) develops very early in childhood chronic kidney disease (CKD). It is linked with rapid progression of kidney disease, increased morbidity and mortality hence the imperative to start anti-hypertensive medication when blood pressure (BP) is persistently > 90th percentile for age, gender, and height in non-dialyzing hypertensive children with CKD. HTN pathomechanism in CKD is multifactorial and complexly interwoven. The patient with CKD-associated HTN needs to be carefully evaluated for co-morbidities that frequently alter the course of the disease as successful treatment of HTN in CKD goes beyond life style modification and anti-hypertensive therapy alone. Chronic anaemia, volume overload, endothelial dysfunction, arterial media calcification, and metabolic derangements like secondary hyperparathyroidism, hyperphosphataemia, and calcitriol deficiency are a few co-morbidities that may cause or worsen HTN in CKD. It is important to know if the HTN is caused or made worse by the toxic effects of medications like erythropoietin, cyclosporine, tacrolimus, corticosteroids and non-steroidal anti-inflammatory drugs. Poor treatment response may be due to any of these co-morbidities and medications. A satisfactory hypertensive CKD outcome, therefore, depends very much on identifying and managing these co-morbid conditions and HTN promoting medications promptly and appropriately. This review attempts to point attention to factors that may affect successful treatment of the hypertensive CKD child and how to attain the desired therapeutic BP target.
Core tip: Hypertension (HTN) is often difficult to control in chronic kidney disease (CKD). Failure to achieve the desired therapeutic BP target in the hypertensive CKD child could be due to comorbidities and toxic effects of HTN promoting medications. So, before starting or altering anti-hypertensive medications, it is important that patients are evaluated for the roles that HTN promoting medications and co-morbidities like chronic anaemia, hyperphosphataemia, progressive tunica media calcifications, and serum parathyroid hormone levels that are well above the acceptable limits for CKD stage could be playing in the entire process. Ways of solving this important clinical problem are the focus of this article.
- Citation: Olowu WA. Pre-treatment considerations in childhood hypertension due to chronic kidney disease. World J Nephrol 2015; 4(5): 500-510
- URL: https://www.wjgnet.com/2220-6124/full/v4/i5/500.htm
- DOI: https://dx.doi.org/10.5527/wjn.v4.i5.500
In the non chronic kidney disease (CKD) paediatric population, hypertension (HTN) is a significant cause of morbidities[1,2] that are further escalated when it co-exists with CKD[3]. HTN develops very early in childhood CKD[3,4]. It is linked with rapid progression of kidney disease hence the Kidney Disease: Improving Global Outcomes recommendation that non-dialyzing hypertensive CKD children should commence antihypertensives when blood pressure (BP) is consistently > 90th percentile and not wait until it is ≥ 95th percentile for age, gender, and height[5]. Therapeutic BP target in such children, particularly those with proteinuria, should be < 50th percentile for age, gender and height except hypotension is a limitation[5].
Pathophysiology of HTN in CKD is multifactorial and complex. In as much as this is so, the management should not be expected to be simple. An individual with CKD-associated HTN (CKD/HTN) needs to be carefully evaluated for co-morbidities that frequently alter the course of the disease as successful treatment of hypertensive CKD goes beyond life style modification and anti-hypertensive therapy alone. Chronic anaemia, volume overload, endothelial dysfunction, and metabolic derangements like hyperparathyroidism, hyperphosphataemia, 1, 25 (OH)2 vitamin D3 (calcitriol) deficiency, and tunica media vascular calcification (VC) are some of the co-morbidities that may cause or worsen HTN in CKD. A satisfactory hypertensive CKD outcome, therefore, depends very much on identifying and managing these co-morbid conditions promptly and appropriately. Before initiating a life style modifying plan or any form of antihypertensive treatment, it is important to know if the index patient has: Hyperphosphataemia, secondary hyperparathyroidism (SHPT), endothelial dysfunction, VC, anaemia, volume overload, and an estimated glomerular filtration rate (eGFR) that is < 15 mL/min per 1.73 m2. Questions should be asked. Will the patient require dialysis? If so, is the patient on calcium-containing phosphate binder? Can the patient be dialyzed with a dialysis fluid that contains the standard concentration of calcium ions (1.75 mmol/L)? The doctor needs to know if the patient is regularly dialyzed or has received a kidney transplant. It is important to know if the patient is on HTN promoting medications like erythropoietin, cyclosporine, tacrolimus, corticosteroids and non-steroidal anti-inflammatory drugs (NSAID). Successful answers to these questions should guide the physician to further steps in tackling the HTN and achieving the therapeutic BP target for the patient.
This review attempts to point attention to factors that may affect successful treatment of the hypertensive CKD child and how to attain the desired therapeutic BP target.
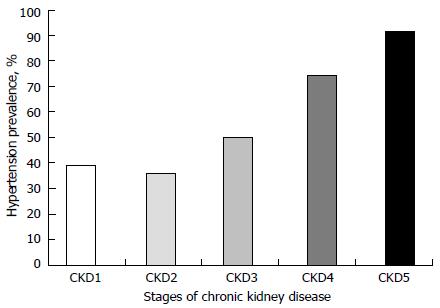
High CKD and co-morbidities, including HTN, prevalence have been reported in many studies. Severe CKDs are most commonly associated with the worst co-morbidities. The frequencies of co-morbidities, including HTN, rise with increasing severity of CKD stage[3,4]. Figure 1, generated from data from reference[3], shows the prevalence pattern of HTN by CKD stage in a population of children. Data on CKD incidence and prevalence from different countries vary widely, depending on whether they are hospital-based or obtained from national renal registries. A hospital-based study from Nigeria showed that the overall CKD incidence in children increased from 6.0 in year 2000 to 20.0 per million children population (pmcp) per year in 2009 while the prevalence increased from 8 to 101 pmcp; the incidence and prevalence of severe CKD (eGFR < 30 mL/min per 1.73 m2) were, however, 3 pmcp/year and 22 pmcp, respectively[3]. Also from Nigeria, another hospital-based study puts the median annual incidence of severe CKD (creatinine clearance, CrCL: < 30 mL/min per 1.73 m2) at 3.0/million age-related population (MARP) per year with a prevalence of 15 patients per MARP[6]. From a hospital-based study in Jordan, the estimated annual incidence and prevalence of severe CKD were reported to be 10.7/MARP per year and 51/MARP, respectively[7]. An Italian national survey reported a median annual incidence and prevalence of 7.7/MARP per year and 21/MARP, respectively for severe CKD[8]. However, in a French study severe CKD incidence was estimated at 7.5/MARP per year in children younger than 16 years while the prevalence was between 29.4 and 54/MARP[9]. Clearly from the above, the burden of CKD is very high and expectedly, the burden of co-morbidities is also high. The prevalence of HTN in childhood CKD is frequently high; it is reported to range between 20.0% and 80.0%[3,10-13]. This contrasts sharply with the 3.2%-3.6% HTN prevalence in the normal paediatric and adolescents’ population[14-16]. Commonly, children with CKD are associated with high nocturnal[17] and masked HTN prevalence[12,13].
Target-organ abnormalities are common features of HTN in children and adolescents. Curiously, CKD children with mild HTN have been reported to have target-organ damage[18-20]. Left ventricular hypertrophy (LVH) is common target-organ damage in HTN[2]. About 34%-38% of paediatric patients with mild and untreated HTN have LVH[21-23]. When associated with proteinuria, HTN has been found to escalate CKD progression and mortality in children and adults[24-26]. In a report, mortality was escalated from 55.5% in non-hypertensive CKD children with heart failure to 84.0% in hypertensive CKD patients with heart failure[3].
It is often difficult to control HTN in CKD. Irrespective of anti-hypertensive medications used, HTN cannot be controlled in more than 50% of children with end-stage renal disease (ESRD)[27-29]. Following treatment with combination antihypertensive medications, only 56% of hypertensive CKD children were able to achieve a BP target of < 50th percentile for age, gender and height[3]. But why is it so difficult to achieve good BP control in hypertensive CKDs? This might be due to failure to critically appraise some of the CKD co-morbidities highlighted above, before starting antihypertensive medications. In a cohort of ESRD children, poor BP control was associated with very young age, post dialysis fluid overload, and hyperphosphataemia. In that report, only 23.5% of treated patients were able to achieve a KDOQI BP target of < 90th percentile[29].
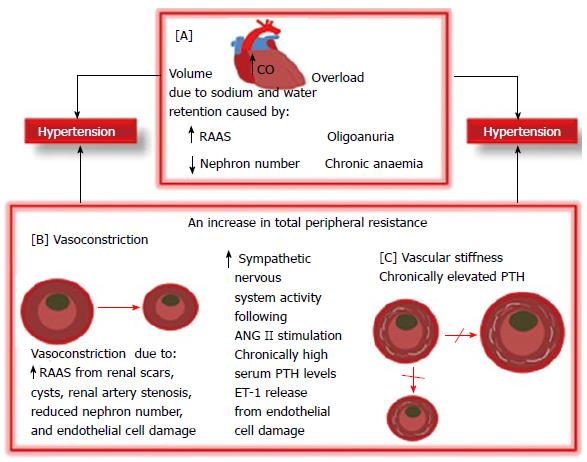
BP regulation is a complex coordination of physiological functions namely cardiac output, fluid volumes, and peripheral resistance among organ systems in the human body. These organ systems encompass the central nervous system, cardiovascular system, kidneys, and adrenal glands[30]. CKD/HTN develops through a number of complexly interwoven pathomechanisms (Figure 2). Fluid overload and renin-angiotensin-aldosterone-system (RAAS) activation are long recognized important HTN pathophysiological pathways. More recently, increased parathyroid and sympathetic activity and endothelial dysfunction have been reported as contributing to CKD/HTN[31]. HTN may possibly be due to angiotensin II (ANG II)-related vascular constriction and aldosterone-related sodium retention due to renin hyper secretion by under perfused renal scars/cysts and/or severe renal tissue damage from microangiopathy or tubulointerstitial inflammation[32,33]. Furthermore, high circulating levels of ANG II contribute to HTN and end organ injury by promoting mesangial cell proliferation, endothelial cell damage, cardiac enlargement, inflammation, and fibrosis[34]. A further mechanism for CKD/HTN which may be in line with Brenner hypothesis is that reduced nephron number following progressive kidney damage may result in reduced salt and water excretion which may predispose to HTN. The Brenner[35] hypothesis which has since been confirmed in other studies[36,37] is that low sodium excretion with attendant HTN may result from congenital nephron number deficit in the low birth weight infant[35]. While sodium retention and volume overload are established aetiological factors in CKD/HTN, sympathetic hyperactivity remains an important volume-independent cause of HTN whose pathomechanism is unclear[38,39]. Renal afferent signals, dopaminergic abnormalities and leptin accumulation in CKD may be contributory[38,39]. Renal sympathetic nerves in renal tubular epithelial cells and blood vessels are stimulated by ANG II to cause an increase in the local release of norepinephrine which then causes renovascular constriction leading to decreased renal blood flow and GFR and HTN[40]. This excessive sympathetic activity is blocked by an ANG II receptor blocker (ARB)[41]. Hyperparathyroidism is a common disorder in CKD that interferes with cardiovascular structural geometry and functions. Chronic hyperparathyroidism increases vascular smooth muscle cells’ (VSMC) sensitivity to calcium and norepinephrine by promoting calcium ions accumulation within the VSMCs[42,43]. The consequence of this is vasoconstriction and HTN. This action may be countered with calcium channel blockers (CCB)[42,43]. Furthermore, chronic hyperparathyroidism promotes VSMC transformation to osteoblasts and vascular wall mineralization or calcification leading to vascular stiffening, increased peripheral resistance to blood flow with consequent HTN.
Children on maintenance dialysis are reported to have significant incidence of HTN that is as high as 53%-65% and 45%-58% in haemodialysis and peritoneal dialysis patients, respectively[44]. Haemodialysis substantially contributes to HTN by increasing both plasma renin activity and catecholamines[45].
Nitric oxide (NO) is a major vasodilator factor that vascular endothelia secrete, and lack of it causes severe HTN[46]. Endothelium-dependent vasodilatation is impaired in uraemia due to deficient NO synthesis[47]. A circulating endothelium-derived NO synthase inhibitor, presumably asymmetric dimthylarginine, which accumulates in uraemia is possibly responsible. Endothelin-1 (ET-1), the most potent vasoconstrictor known, is secreted by the vascular endothelium. Plasma ET-1 concentrations rise directly with BP increase in ESRD, suggesting a role for ET-1 in the causation of CKD/HTN[48]. By preventing the breakdown of vasodilatory kinins, angiotensin converting enzyme inhibitors (ACEi) are able to reduce ET-1 expression and suppress ET-1 induced HTN[49,50].
For a number of reasons, CKD patients receive medications like erythropoietin, NSAID, cyclosporine, tacrolimus, and corticosteroids that could predispose to or make HTN worse. These agents cause HTN through a variety of mechanisms that involve interference with arachidonic acid metabolism, ET-1 and NO syntheses. The ultimate result of this interference is HTN through increased TPR due to vasoconstriction with reduced renal perfusion and GFR, increased sodium and water reabsorption as a consequence of RAAS activation. In a review by Krapf et al[51], post erythropoietin therapy HTN occurs through increased syntheses of vasoconstrictors like ET-1 and thromboxane (TXB2) but decreased productions of vasodilators like prostacyclin (PGI2) and NO. Reduced production of NO is secondary to decreased expression of endothelium-derived nitric oxide synthase (NOS), an enzyme that catalysis the production of NO. NSAID associated HTN occurs through cyclooxygenase inhibition by preventing arachidonic acid conversion to vasodilator prostanoids like prostaglandin E2 (PGE2) and PGI2[52]. This action leads to increased TPR and volume overload and HTN through increased production of ET-1, increased Na+ and Cl- ions reabsorption in the loop of Henle (thick ascending segment) and anti-diuretic hormone-mediated increased water reabsorption. Through another metabolic pathway, NSAID may cause HTN by promoting the release of cytochrome P450-mediated vasoconstricting metabolites of arachidonic acid such as epoxyeicosatrienoic and hydroxyeicosatetraenoic acid[52]. Calcineurin inhibitors, namely cyclosporine and tacrolimus cause HTN through reduced productions of PGI2 and NO but increased productions of ET-1 and TXB2[53] with consequent vasoconstriction, reduced renal blood flow and GFR, sodium and water retention. The mechanism by which corticosteroids causes HTN is not yet clear; however, one of the mechanisms known currently is inhibition of the release of arachidonic acid from phospholipids thereby preventing prostaglandins formation leading to decreased production of vasodilator prostanoids[54]. In-vitro, cortisol has been demonstrated to potentiate vascular smooth muscles cells pressor responsiveness to epinephrine and norepinephrine by inhibiting catechol-o-methyl transferase, an enzyme that degrades catecholamines neurotransmitters such as dopamine, epinephrine, and norepinephrine[54]. While, for obvious reasons, these drugs cannot be stopped the dosages can be lowered, in order to achieve good BP control, to levels that will not compromise the primary indications for their prescription.
HTN in the transplanted CKD patient is often caused by volume overload, corticosteroids, and calcineurin inhibitors. ACEi and ARB are avoided in the first few weeks post-transplant to avoid renal insufficiency in the setting of diminished effective arterial blood volume[55]. To prevent calcineurin inhibitor-induced graft dysfunction, therefore, HTN is treated with CCB in the immediate post-operative period[55]. CCBs like nifedipine and amilodipine have been used with satisfactory outcomes in paediatric transplant patients[56].
It is inconceivable that in the setting of chronic anaemia, progressive arteriosclerosis, uncontrolled SHPT, and HTN promoting medications, the therapeutic BP target can be attained in CKD/HTN. Successful HTN treatment outcome demands that these factors be carefully evaluated and managed accordingly.
Anaemia is a frequent comorbidity in childhood CKD[3,4,57]. Failure to attain the target therapeutic BP goal in CKD/HTN may be due to untreated or poorly treated anaemia. Anemia-associated tissue hypoxia causes peripheral vasodilatation. Reduced BP caused by vasodilatation stimulates increased sympathetic activity with attendant tachycardia and increased stroke volume. This is accompanied by increased cardiac output and vasoconstriction. The latter causes reduced renal blood flow, increased RAAS activity and anti-diuretic hormone production leading to salt and water retention[58]. The long term effect of this is HTN or worsening of existing HTN. All hypertensive CKD patients should be carefully assessed for anaemia and volume overload and managed accordingly. Anaemia in childhood CKD is defined as haemoglobin (Hb) concentration < 11.0, < 11.5, and < 12.0 g/dL in children aged 0.5-5, 5-12, and 12-15 years, respectively[57]. It is suggested that when correcting anaemia, the target Hb concentration in all paediatric CKD patients receiving erythrocytes stimulating agent therapy should be maintained within 11.0 to 12.0 g/dL range[57]. Excess volume can be removed with a low ceiling diuretic, like a thiazide, when eGFR is ≥ 60 mL/min per 1.73 m2 or with frusemide, a high ceiling diuretic, when eGFR is < 60 mL/min per 1.73 m2. eGFR ≤ 15 mL/min per 1.73 m2 will rarely respond to diuretics. Fluid removal will have to be by dialytic ultrafiltration. It is important to note that when treating anaemia with erythropoietin, HTN may occur following weeks of therapy; this is partly due to increase in the red blood cell mass, increased blood viscosity and resistance to blood flow. Other mechanisms include vascular wall remodeling with resultant rise in vascular resistance[59]. It is also possible that due to direct action of erythropoietin on voltage-independent Ca2+ channels in the VMSCs, the sensitivity of the latter to the vasodilatory action of NO may be diminished[60]. Erythropoietin has been reported to exacerbate HTN in both non-dialyzing and dialyzing CKD children[61,62]. This complication can be ameliorated by reducing the dose of erythropoietin.
It is a well-known fact that tunica media VC, a form of CKD-mineral and bone disorder, is associated vascular wall rigidity with attendant progressive vascular pulse wave deceleration and abnormal vascular wall geometry. Increasing vascular rigidity ultimately leads to cardiac damage from long standing cardiomyocytes ischaemia, from high oxygen consumption, and diminished coronary blood flow[63]. Dialysis history, consumption of high doses of active vitamin D, deficiencies of inhibitors of calcification, hypercalcaemia and hyperphosphataemia are risk factors for VC in CKD[64]. Hyperphosphataemia is an important and possibly a principal promoter of VC because it has been clearly linked with increased VC and mortality[65,66]. Fibroblast growth factor-23 (FGF23) together with its anti-ageing cofactor, Klotho have been recognized as major regulators of phosphate homeostasis, in addition to inhibiting production and release of parathyroid hormone (PTH) and suppressing renal production of 1, 25 (OH)2 vitamin D. In an experiment by Sitara et al[67], FGF23 null mice and Klotho null mice developed similar phenotypes, characterized by very high serum concentrations of phosphate and 1, 25-dihydroxyvitamin D3 with disordered bone mineralization including multiple soft-tissue calcifications. A 13-year-old child with a Klotho gene mutation suffered severe vascular and soft-tissue calcifications, despite markedly elevated serum FGF23. Thus, deficiency of Klotho in this patient prevented FGF23 from exerting its phosphate-lowering effects and its protection against soft tissue calcification[68]. This shows that without Klotho, FGF23 cannot correctly exert its normal physiological functions. Klotho prevents soft tissue calcification by three main mechanisms namely, phosphaturia, kidney function preservation and directly inhibiting phosphate uptake and dedifferentiation by the VSMCs[69]. In CKD, serum levels of FGF23 increase in proportion to the decrease of GFR[70]. This increase can be considered as an appropriate compensatory mechanism in the defense against phosphate retention, in concert with PTH, although it also leads to an inhibition of renal calcitriol synthesis, in contrast to PTH which promotes renal calcitriol synthesis[71]. Of note, chronic dialysis patients and uremic animals have been shown to exhibit a relative resistance to the inhibitory action of FGF23 on parathyroid gland function[72-74]. This is probably due to down regulation of Klotho and FGF23 receptor expression in CKD. Increased systolic BP, resulting in elevated cardiac afterload and LVH and decreased diastolic BP and impaired coronary perfusion are initial major consequences of arterial stiffening[75]. Cardiovascular calcification (CVC) is not only a progressive disorder; it is also severer among CKD patients, with poorer cardiovascular outcome, compared with other populations[76]. VC must, therefore, be recognized very early in CKD and aborted as progression will worsen both the kidney disease and HTN thereby making BP therapeutic goal unattainable with dire consequences for the patient. High pulse pressure suggests arterial stiffening/rigidity and therefore, should be an indication for anyone or combination of the following investigations: flow mediated dilation for endothelial dysfunction, carotid intimal medial thickness (cIMT), pulse wave velocity (PWV) and echocardiography for valvular calcification; plain X-rays of the hands including the wrists can also detect VC in the radial and digital arteries[76,77]. Similarly, lateral lumbar spine (lateral abdominal X-ray) and pelvic radiographs can detect VC in the abdominal aorta and femoral and iliac arteries[64]. However, in detecting and quantifying CVC, including the coronary arteries, the electron-beam computed tomography (EBCT) and multislice CT (MSCT) are the most sensitive radiologic techniques available[78-82]. cIMT, PWV, EBCT and MSCT are established indicators of structural and functional anomalies of blood vessels, including calcification in children and adults[78-82]. cIMT in paediatric CKD patients was adversely affected by high plasma phosphate[78-80]. In 85 dialyzing children, the cIMT increased by 0.15 mm for each mmol/L rise in the serum phosphate concentration[78].
Currently, there is no definitive treatment for VC reversal but the process leading to it can be halted through preventive measures. The most important preventive measure is to ensure that serum phosphorous level is kept within the normal age-specific range. The approaches to reducing high plasma phosphate level should include reducing dietary phosphate intake[83], and gastrointestinal absorption with phosphate binders[84], and giving more dialysis to increase clearance in those with 5D-CKD[85,86]. Stages 3-5 CKD patients can have their serum phosphorous kept within acceptable limits of 0.81-1.45 mmol/L (2.5-4.5 mg/dL); high values should, however, be brought down to the normal limits in CKD-5D. On the other hand, serum calcium should be kept within the normal limits of 2.1-2.6 mmol/L (8.8-10.5 mg/dL) in individuals with 3-5D CKD[85,86]. However, a dialysate fluid having low calcium concentration of 1.25-1.50 mmol/L (2.5-3.0 mEq/L) is advised for use in order to avoid hypercalcaemia, adynamic bone disease and rapid VC progression that may occur with the standard dialysate fluid, containing 1.75 mmol/L of calcium, when used in CKD-5D[76]. It is recommended that serum concentrations of calcium, phosphorus, PTH, and alkaline phosphatase should be determined starting from CKD-2 in paediatric patients[76]. Furthermore, it is suggested that serum calcium and phosphorous be measured in CKD 3, CKD 4, and CKD 5/5D at 6-12, 3-6, and 1-3 mo intervals, respectively[76]. In hyperphosphataemic individuals with 3-5D CKD, calcium-based phosphate binders are best avoided when there is evidence for arterial calcification and/or adynamic bone disease and/or persistently low serum concentrations of PTH. Calcium-based phosphate binders and/or calcitriol or vitamin D analog are similarly contraindicated when such patients have hypercalcaemia that is persistent or recurrent[76]. Increased dialytic phosphate removal is suggested for CKD stage 5D if hyperphosphataemia is persistent. Effective alternatives to calcium-based phosphate binders include non calcium-based phosphate binders like sevelamer, and lanthanum salts. Although Sevelamer hydrochloride possesses the additional benefit of reducing total cholesterol and low density lipoprotein cholesterol concentrations in the plasma, patients may need to be on calcium supplement when there is overt hypocalcaemia[76]. Sevelamer hydrochloride has been reported in some studies to attenuate arterial calcification progression in stages 3-5 and 5D CKD patients when compared to similar patients treated with calcium-based phosphate binders[87-91]. Zhang et al[92] have shown in their systematic review of literature on adult patients that lanthanum carbonate efficaciously reduces serum phosphorus and intact PTH levels without raising the serum calcium concentration. The author is currently not aware of any published study on lanthanum carbonate use in children.
The use of pyrophosphate, bisphosphonate and thiosulfate in the prevention of VC is largely experimental. With current level of information available from various experimental studies, they show a lot of future promise for the prevention of VC in humans when they become clinically available. Schibler et al[93] were able to demonstrate that high dose pyrophosphate could inhibit tunica media calcification in rats that were intoxicated with vitamin D. High dose pyrophosphate was used to prevent its rapid hydrolysis to orthophosphate. However, to obviate the need for high dose pyrophosphate, bisphosphonate a non hydrolysable analogue of the former was developed. Medial calcification has been effectively inhibited with bisphosphonate in uraemic rats[94]. Pasch et al[95] demonstrated that tunica media calcification developed within four weeks in a Wister rat model of uraemic renal failure caused by adenine diet-induced severe interstitial nephritis. Using thiosulfate at doses and frequencies that were similar to that used in patients with calcific uraemic arteriolopathy, Pasch et al were able to completely prevent VC in their animal model. However, the drawbacks with the thiosulfate study of Pasch et al[95] are that: (1) the mode of action is unknown; (2)thiosulfate prevents but does not reverse VC; (3) its safety limits in man are unknown; and (4) there is the possibility of reduced bone mineralization.
VC is a common complication of high doses of vitamin D receptor agonists (VDRAs) especially when associated with hypercalcaemia[96-99]. However, using lower doses of VDRAs that are currently in use in clinical practice, Lau et al[100], were able to demonstrate that active vitamin D (calcitriol, 30 ng/kg) and its analog (100 ng/kg paricalcitol) prevented arterial medial VC in CKD mice given high phosphate diet (1.5%). Independently of serum calcium and PTH both VDRAs reduced the degree of VC via: (1) elevated serum Klotho, increased phosphaturia as well as normalized serum phosphate and FGF23 levels; and (2) up regulation of VSMC osteopontin but reduced circulating osteopontin that is associated with VC reduction. Using much lower (physiological) dosages, Mathew et al[97] had earlier noted that both calcitriol and paricalcitol prevent VC. The clinical benefit of both studies with regard to VC needs to be determined by further studies.
As discussed above, hyperparathyroidism causes HTN through vasoconstriction and vascular medial wall calcification[42,43]. PTH level should be determined early in the course of managing CKD/HTN as this may be elevated beyond the expected level for the CKD stage in the patient. Appropriate management of the inappropriately elevated PTH for CKD stage may impact significantly on HTN outcome. In children with CKD, 25 (OH) vitamin D (calcidiol) is a common deficiency; it is one of the factors that may be responsible for SHPT in CKD. The serum level of calcidiol (normal: 8-50 ng/mL) should be determined at baseline in every CKD patient. The ways by which active vitamin D sterols suppress PTH levels include: Increased intestinal calcium absorption, and PTH gene transcription suppression. Given either in daily or intermittent doses, calcitriol and alfacalcidol effectively suppress PTH and improve growth in childhood CKD[101,102]. Hypercalcaemia is, however, a serious side effect especially when ingested with phosphate binders containing calcium. The newer vitamin D analogues namely 22-oxacalcitriol, 19-nor-1, 25-dihydroxy vitamin D2 (paricalcitol) and 1α-hydroxyvitamin D2 (doxercalciferol) are associated with minimal intestinal calcium and phosphorus absorption. PTH levels are effectively reduced by doxercalciferol and paricalcitol; both have the ability to reduce serum calcium levels better than calcitriol in CKD children and adults[103,104]. Where SHPT is due to hyperphosphataemia, appropriate use of phosphate binders may just be sufficient. Cinacalcet is a type II calcimimetic that allosterically modulates the calcium sensing receptor, CaSR thus making it more sensitive to circulating calcium ions with resultant reduction in PTH release[105]. Studies have shown that calcimimetics effectively act on the parathyroid gland of CKD-5 patients to promote reasonable decreases in circulating serum phosphorus and calcium ions[106,107]. Calcimimetics have on the other hand been associated with unwanted increases in serum phosphorus, through unknown pathways, in CKD-3/4. They should, therefore, be avoided in such patients[108,109]. Calcimimetics have been found useful in the few paediatric CKD-5 patients studied so far[110,111]. Six CKD 5D children aged between 11 mo and 14 years who had uncontrolled SHPT and treated with cinacalcet (doses: 0.4-1.4 mg/kg) showed satisfactory and sustained correction of the hyperparathyroidism[112]. Whatever medication that is chosen for the hyperparathyroidism, it is suggested that the target serum PTH in CKD 3, CKD 4, and CKD 5/5D should, respectively be in the 35-70, 70-110, and 200-300 pg/mL range to avoid adynamic bone disease from too low serum PTH[113]. In CKD, the serum PTH should be maintained within 2-9 times the upper limits of the normal laboratory range[76]. It is important that serum PTH and alkaline phosphatase are determined at baseline, every 6-12, and 3-6 mo, respectively in patients with progressive CKD 3, CKD 4, and CKD 5/5D[76].
The pathomechanism of HTN in CKD is multifactorial and complexly interwoven. Successful treatment of HTN in CKD, therefore, goes beyond life style modification and anti-hypertensive therapy alone. The patient with CKD/HTN needs to be carefully evaluated for co-morbidities that frequently alter the course of the disease. It is also important to know if the HTN is caused or made worse by the toxic effects of medications like erythropoietin, cyclosporine, tacrolimus, corticosteroids and NSAID. A satisfactory therapeutic outcome in the hypertensive CKD, therefore, depends very much on identifying and managing these co-morbid conditions promptly and appropriately.
P- Reviewer: Watanabe T S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Stabouli S, Kotsis V, Rizos Z, Toumanidis S, Karagianni C, Constantopoulos A, Zakopoulos N. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. 2009;24:1545-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555-576. [PubMed] |
| 3. | Olowu WA, Adefehinti O, Aladekomo TA. Epidemiology and clinicopathologic outcome of pediatric chronic kidney disease in Nigeria, a single cenetr study. Arab J Nephrol Transplant. 2013;6:105-113. [PubMed] |
| 4. | Wong H, Mylrea K, Feber J, Drukker A, Filler G. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int. 2006;70:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2012;2 Suppl 5:S372-376. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Anochie I, Eke F. Chronic renal failure in children: a report from Port Harcourt, Nigeria (1985-2000). Pediatr Nephrol. 2003;18:692-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hamed RM. The spectrum of chronic renal failure among Jordanian children. J Nephrol. 2002;15:130-135. [PubMed] |
| 8. | Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382-e387. [PubMed] |
| 9. | Deleau J, Andre JL, Briancon S, Musse JP. Chronic renal failure in children: an epidemiological survey in Lorraine (France) 1975-1990. Pediatr Nephrol. 1994;8:472-476. [PubMed] |
| 10. | Seeman T, Simková E, Kreisinger J, Vondrák K, Dusek J, Gilík J, Feber J, Dvorák P, Janda J. Control of hypertension in children after renal transplantation. Pediatr Transplant. 2006;10:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Lingens N, Dobos E, Witte K, Busch C, Lemmer B, Klaus G, Schärer K. Twenty-four-hour ambulatory blood pressure profiles in pediatric patients after renal transplantation. Pediatr Nephrol. 1997;11:23-26. [PubMed] |
| 12. | Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Adegoke SA, Elusiyan JBE, Olowu WA, Adeodu OO. Relationship between body mass index and blood pressure among Nigerian children aged 6-18years. Niger Endocrine Pract. 2009;3:35-43. |
| 15. | McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-664, 644.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 371] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 552] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 17. | Mitsnefes MM, Kimball TR, Daniels SR. Office and ambulatory blood pressure elevation in children with chronic renal failure. Pediatr Nephrol. 2003;18:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR. Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int. 1996;50:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF. Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol. 2001;16:318-323. [PubMed] |
| 20. | Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Left ventricular mass and systolic performance in pediatric patients with chronic renal failure. Circulation. 2003;107:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Belsha CW, Wells TG, McNiece KL, Seib PM, Plummer JK, Berry PL. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens. 1998;11:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 705] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 25. | Wingen AM, Fabian-Bach C, Schaefer F, Mehls O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet. 1997;349:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Ardissino G, Testa S, Daccò V, Viganò S, Taioli E, Claris-Appiani A, Procaccio M, Avolio L, Ciofani A, Dello Strologo L. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project. Pediatr Nephrol. 2004;19:172-177. [PubMed] |
| 27. | Tkaczyk M, Nowicki M, Bałasz-Chmielewska I, Boguszewska-Baçzkowska H, Drozdz D, Kołłataj B, Jarmoliński T, Jobs K, Kiliś-Pstrusińska K, Leszczyńska B. Hypertension in dialysed children: the prevalence and therapeutic approach in Poland--a nationwide survey. Nephrol Dial Transplant. 2006;21:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Mitsnefes M, Stablein D. Hypertension in pediatric patients on long-term dialysis: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Am J Kidney Dis. 2005;45:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | VanDeVoorde RG, Barletta GM, Chand DH, Dresner IG, Lane J, Leiser J, Lin JJ, Pan CG, Patel H, Valentini RP. Blood pressure control in pediatric hemodialysis: the Midwest Pediatric Nephrology Consortium Study. Pediatr Nephrol. 2007;22:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Hadtstein C, Schaefer F. Hypertension in children with chronic kidney disease: pathophysiology and management. Pediatr Nephrol. 2008;23:363-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Loghman-Adham M, Soto CE, Inagami T, Cassis L. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2004;287:F775-F788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Ibrahim HN, Hostetter TH. The renin-aldosterone axis in two models of reduced renal mass in the rat. J Am Soc Nephrol. 1998;9:72-76. [PubMed] |
| 34. | Wolf G, Butzmann U, Wenzel UO. The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol. 2003;93:P3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 895] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 36. | Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 382] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Hughson M, Farris AB, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 546] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 38. | Kuchel OG, Shigetomi S. Dopaminergic abnormalities in hypertension associated with moderate renal insufficiency. Hypertension. 1994;23:I240-I245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Wolf G, Chen S, Han DC, Ziyadeh FN. Leptin and renal disease. Am J Kidney Dis. 2002;39:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 445] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 40. | Böke T, Malik KU. Enhancement by locally generated angiotensin II of release of the adrenergic transmitter in the isolated rat kidney. J Pharmacol Exp Ther. 1983;226:900-907. [PubMed] |
| 41. | Wong PC, Bernard R, Timmermans PB. Effect of blocking angiotensin II receptor subtype on rat sympathetic nerve function. Hypertension. 1992;19:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Iseki K, Massry SG, Campese VM. Effects of hypercalcemia and parathyroid hormone on blood pressure in normal and renal-failure rats. Am J Physiol. 1986;250:F924-F929. [PubMed] |
| 43. | Schiffl H, Fricke H, Sitter T. Hypertension secondary to early-stage kidney disease: the pathogenetic role of altered cytosolic calcium (Ca2+) homeostasis of vascular smooth muscle cells. Am J Kidney Dis. 1993;21:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) 2002 annual report. 2007. Available from: http://www.naprtcs.org. |
| 45. | Rauh W, Hund E, Sohl G, Rascher W, Mehls O, Schärer K. Vasoactive hormones in children with chronic renal failure. Kidney Int Suppl. 1983;15:S27-S33. [PubMed] |
| 46. | Baylis C, Vallance P. Nitric oxide and blood pressure: effects of nitric oxide deficiency. Curr Opin Nephrol Hypertens. 1996;5:80-88. [PubMed] |
| 47. | Morris ST, McMurray JJ, Rodger RS, Jardine AG. Impaired endothelium-dependent vasodilatation in uraemia. Nephrol Dial Transplant. 2000;15:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Larivière R, Lebel M. Endothelin-1 in chronic renal failure and hypertension. Can J Physiol Pharmacol. 2003;81:607-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Largo R, Gómez-Garre D, Liu XH, Alonso J, Blanco J, Plaza JJ, Egido J. Endothelin-1 upregulation in the kidney of uninephrectomized spontaneously hypertensive rats and its modification by the angiotensin-converting enzyme inhibitor quinapril. Hypertension. 1997;29:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Elmarakby AA, Morsing P, Pollock DM. Enalapril attenuates endothelin-1-induced hypertension via increased kinin survival. Am J Physiol Heart Circ Physiol. 2003;284:H1899-H1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol. 2009;4:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Frishman WH. Effects of nonsteroidal anti-inflammatory drug therapy on blood pressure and peripheral edema. Am J Cardiol. 2002;89:18D-25D. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Textor SC, Taler SJ, Canzanello VJ, Schwartz L, Augustine JE. Posttransplantation hypertension related to calcineurin inhibitors. Liver Transpl. 2000;6:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Whitworth JA. Mechanisms of glucocorticoid-induced hypertension. Kidney Int. 1987;31:1213-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Kiberd BA. Cyclosporine-induced renal dysfunction in human renal allograft recipients. Transplantation. 1989;48:965-969. [PubMed] |
| 56. | Silverstein DM, Palmer J, Baluarte HJ, Brass C, Conley SB, Polinsky MS. Use of calcium-channel blockers in pediatric renal transplant recipients. Pediatr Transplant. 1999;3:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. 2012;2 Suppl 5:S279–335. |
| 58. | Anand IS, Chandrashekhar Y, Ferrari R, Poole-Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J. 1993;70:357-362. [PubMed] |
| 59. | Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995;47:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Vaziri ND. Mechanism of erythropoietin-induced hypertension. Am J Kidney Dis. 1999;33:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Offner G, Hoyer PF, Latta K, Winkler L, Brodehl J, Scigalla P. One year’s experience with recombinant erythropoietin in children undergoing continuous ambulatory or cycling peritoneal dialysis. Pediatr Nephrol. 1990;4:498-500. [PubMed] |
| 62. | Warady BA, Arar MY, Lerner G, Nakanishi AM, Stehman-Breen C. Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2006;21:1144-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1259] [Cited by in RCA: 1318] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 64. | Disthabanchong S. Vascular calcification in chronic kidney disease: Pathogenesis and clinical implication. World J Nephrol. 2012;1:43-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, Lui SF, Li PK, Sanderson JE. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20:1676-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 66. | Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 67. | Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7:318-319. [PubMed] |
| 69. | Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J Nephrol. 2007;23 Suppl 16:S136-S144. [PubMed] |
| 70. | Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 540] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 71. | Cozzolino M, Mazzaferro S. The fibroblast growth factor 23: a new player in the field of cardiovascular, bone and renal disease. Curr Vasc Pharmacol. 2010;8:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 73. | Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | O’Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 257] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;S1-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 1076] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 77. | Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Gonçalves M, Negrao AP. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 78. | Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J. Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol. 2007;18:2996-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 79. | Mitsnefes MM, Kimball TR, Kartal J, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Cardiac and vascular adaptation in pediatric patients with chronic kidney disease: role of calcium-phosphorus metabolism. J Am Soc Nephrol. 2005;16:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Ziolkowska H, Brzewski M, Roszkowska-Blaim M. Determinants of the intima-media thickness in children and adolescents with chronic kidney disease. Pediatr Nephrol. 2008;23:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Civilibal M, Caliskan S, Adaletli I, Oflaz H, Sever L, Candan C, Canpolat N, Kasapcopur O, Kuruoglu S, Arisoy N. Coronary artery calcifications in children with end-stage renal disease. Pediatr Nephrol. 2006;21:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol. 2005;16:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Takeda E, Yamamoto H, Nishida Y, Sato T, Sawada N, Taketani Y. Phosphate restriction in diet therapy. Contrib Nephrol. 2007;155:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin. 2007;23:3167-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Ayus JC, Achinger SG, Mizani MR, Chertow GM, Furmaga W, Lee S, Rodriguez F. Phosphorus balance and mineral metabolism with 3 h daily hemodialysis. Kidney Int. 2007;71:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 496] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 87. | Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 88. | Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 1019] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 89. | Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 567] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 90. | Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 91. | Barreto DV, Barreto Fde C, de Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M. Phosphate binder impact on bone remodeling and coronary calcification--results from the BRiC study. Nephron Clin Pract. 2008;110:c273-c283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 92. | Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013;14:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Schibler D, Russell RG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35:363-372. [PubMed] |
| 94. | Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 95. | Pasch A, Schaffner T, Huynh-Do U, Frey BM, Frey FJ, Farese S. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int. 2008;74:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Wu-Wong JR, Noonan W, Ma J, Dixon D, Nakane M, Bolin AL, Koch KA, Postl S, Morgan SJ, Reinhart GA. Role of phosphorus and vitamin D analogs in the pathogenesis of vascular calcification. J Pharmacol Exp Ther. 2006;318:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA. Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol. 2008;19:1509-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 98. | Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 99. | Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol. 2000;20:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Lau WL, Leaf EM, Hu MC, Takeno MM, Kuro-o M, Moe OW, Giachelli CM. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012;82:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 101. | Waller SC, Ridout D, Cantor T, Rees L. Parathyroid hormone and growth in children with chronic renal failure. Kidney Int. 2005;67:2338-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Schmitt CP, Ardissino G, Testa S, Claris-Appiani A, Mehls O. Growth in children with chronic renal failure on intermittent versus daily calcitriol. Pediatr Nephrol. 2003;18:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, Elashoff RM, Jüppner H. Sevelamer controls parathyroid hormone-induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. J Am Soc Nephrol. 2005;16:2501-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 104. | Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, Williams ME, Bishop CW. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43:877-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 105. | de Francisco AL. New strategies for the treatment of hyperparathyroidism incorporating calcimimetics. Expert Opin Pharmacother. 2008;9:795-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 735] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 107. | Block GA, Zeig S, Sugihara J, Chertow GM, Chi EM, Turner SA, Bushinsky DA. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2008;23:2311-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Chonchol M, Locatelli F, Abboud HE, Charytan C, de Francisco AL, Jolly S, Kaplan M, Roger SD, Sarkar S, Albizem MB. A randomized, double-blind, placebo-controlled study to assess the efficacy and safety of cinacalcet HCl in participants with CKD not receiving dialysis. Am J Kidney Dis. 2009;53:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Cannata-Andía JB, Fernández-Martín JL. Mineral metabolism: Should cinacalcet be used in patients who are not on dialysis? Nat Rev Nephrol. 2009;5:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Silverstein DM, Kher KK, Moudgil A, Khurana M, Wilcox J, Moylan K. Cinacalcet is efficacious in pediatric dialysis patients. Pediatr Nephrol. 2008;23:1817-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Muscheites J, Wigger M, Drueckler E, Fischer DC, Kundt G, Haffner D. Cinacalcet for secondary hyperparathyroidism in children with end-stage renal disease. Pediatr Nephrol. 2008;23:1823-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Platt C, Inward C, McGraw M, Dudley J, Tizard J, Burren C, Saleem MA. Middle-term use of Cinacalcet in paediatric dialysis patients. Pediatr Nephrol. 2010;25:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53:S11-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |