Published online Nov 6, 2014. doi: 10.5527/wjn.v3.i4.295
Revised: September 5, 2014
Accepted: October 1, 2014
Published online: November 6, 2014
Processing time: 132 Days and 3 Hours
Renal tubules regulate blood pressure and humoral homeostasis. Mediators that play a significant role in regulating the transport of solutes and water include angiotensin II (AngII) and nitric oxide (NO). AngIIcan significantly raise blood pressure via effects on the heart, vasculature, and renal tubules. AngII generally stimulates sodium reabsorption by triggering sodium and fluid retention in almost all segments of renal tubules. Stimulation of renal proximal tubule (PT) transport is thought to be essential for AngII-mediated hypertension. However, AngII has a biphasic effect on in vitro PT transport in mice, rats, and rabbits: stimulation at low concentrations and inhibition at high concentrations. On the other hand, NO is generally thought to inhibit renal tubular transport. In PTs, NO seems to be involved in the inhibitory effect of AngII. A recent study reports a surprising finding: AngII has a monophasic stimulatory effect on human PT transport. Detailed analysis of signalling mechanisms indicates that in contrast to other species, the human NO/guanosine 3’,5’-cyclic monophosphate/extracellular signal-regulated kinase pathway seems to mediate this effect of Ang II on PT transport. In this review we will discuss recent progress in understanding the effects of AngII and NO on renal tubular transport.
Core tip: Angiotensin II (AngII) and nitric oxide (NO) play important roles in the regulation of renal tubular transport. AngII has a biphasic effect on renal proximal tubule (PTs) transport, and NO seems to inhibit the effect of AngII. In human PTs, however, AngII seems to have an NO-dependent monophasic stimulatory effect. We will discuss the recent findings in this field.
- Citation: Horita S, Nakamura M, Shirai A, Yamazaki O, Satoh N, Suzuki M, Seki G. Regulatory roles of nitric oxide and angiotensin II on renal tubular transport. World J Nephrol 2014; 3(4): 295-301
- URL: https://www.wjgnet.com/2220-6124/full/v3/i4/295.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i4.295
Angiotensin II (AngII) is a strong pressor, acting on various organs and systems, including the kidney. It binds to angiotensin receptors, of which the main subtypes are angiotensin receptor type 1 (AT1R) and type 2 (AT2R)[1]. Although other classes of angiotensin and their receptors, such as AT7R[2], occur, the receptor with the dominant effect in the kidney seems to be AT1R. Recently, Coffman et al[3] demonstrated that renal AT1R is the essential target of AngII-induced hypertension[3]. By showing the importance of renal AT1R in the emergence of hypertension, their study suggests that renal AT1R will be the target for therapy and the prevention of hypertension.
Nitric oxide (NO) is a gaseous vasoactive substance produced by nitric oxide synthase (NOS). NO has been shown to play important roles in the regulation of renal tubular transport. However, its role seems to be pleiotropic and varies according to circumstances.
NOS has three isoforms, NOS1, NOS2 and NOS3, previously referred to as neuronal NOS (nNOS), inducible NOS (iNOS), and epithelial NOS (eNOS), respectively. Renal tubules have each of these NOS isoforms[4,5]; however, the details of their actions in the tubules are still unclear.
NO seems to inhibit NaCl reabsorption in the renal tubules and induces natriuresis. Inhibiting NOS decreased urine volume and NaCl excretion, without changing renal blood flow and the glomerular filtration rate[6-10]. Overall, NO is thought to inhibit the reabsorption of NaCl and fluid by tubules.
AngII in proximal tubules
AngII has been widely known as a strong pressor and regulator of cardiovascular and renal function[11]. In the classical pathway, AT1R mediates the effects of AngII[1]. Proximal tubules (PTs) reabsorb approximately 60% to 70% of the sodium filtered in the glomeruli. Therefore, the regulation of sodium reabsorption in this segment is important for the maintenance of blood pressure and humoral homeostasis[12,13]. In the PTs, AngII is known to stimulate sodium and water transport. Although AngII affects transport processes in several nephron segments, as discussed below, its effect on PT transport may be its most important effect. In particular, the stimulatory effect of AngII in the PTs has significant importance for the emergence and progression of hypertension[14].
AngII acts mainly via type 1 and type 2 angiotensin receptors. The type 1 receptor has 1A and 1B subtypes and is thought to raise blood pressure[1]. AT2R is also thought to be located in the PTs[15-17]. Some investigators argue that AT2R may mediate the inhibitory effect of AngII[18]. However, most data, including our own obtained from AT1R knockout mice[13,19-21], indicate that AT1R is the dominant receptor mediating the biphasic effects of AngII in the PTs.
In PTs, the basolateral electrogenic sodium-bicarbonate cotransporter type 1 (NBCe1) and the apical sodium-proton exchanger type 3 (NHE3) mainly regulate sodium reabsorption[22]. In addition, sodium is reabsorbed and coupled with amino acids[23], glucose[24], phosphate[25], and other solutes from the apical side[14]. Sodium is also reabsorbed via Na+-K+-ATPase (NKA) from the basolateral side[26], which offers the driving forces for NBCe1 and NHE3.
AngII is known to have biphasic effect on the PTs of rats, mice and rabbits. Low concentrations (picomolar to nanomolar) of AngII stimulate PT transport, while high concentrations (nanomolar to micromolar) inhibit PT transport[27,28]. In PTs, AngII regulates major sodium transporters, such as NHE3, NBCe1, and NKA, in a biphasic manner[19,29-32]. The activation of protein kinase C and/or a decrease in cAMP concentration, followed by the activation of the extracellular signal-regulated kinase (ERK) pathway, may be responsible for the stimulatory effect of AngII[33-35]. On the other hand, the activation of the phospholipase A2/arachidonic acid/5,6-epoxyeicosatrienoic acid (EET) and/or the NO/guanosine 3’,5’-cyclic monophosphate (cGMP) pathways[29,36,37] may be responsible for the inhibitory effect of AngII. The concentration of AngII is known to be much higher in kidney than plasma[38,39], suggesting that the inhibitory effect of AngII may also have some physiological significance in the regulation of renal tubular function and blood pressure.
There are some reports that AngII stimulates net NaCl absorption in the thick ascending limb (TAL). Wang and colleagues showed that AngII stimulates basolateral Cl- channels by activating the protein kinase C-dependent NADPH oxidase pathway, inducing net NaCl absorption[40]. Garvin et al[41] investigated the regulation of NKA activity in AngII-induced hypertension[41]. They showed that AngII-induced hypertension is accompanied by increased NKA activity in rat TAL, which may be at least partially due to AngII-stimulated superoxide production[42] via NADPH oxidase[43]. Moreover, AngII binding to AT1R was shown to inhibit ADH-stimulated transport in the rat TAL suspension cells[44]. Overall, AngII seems to stimulate Na+ reabsorption in the TAL via AT1R.
In the distal tubules, approximately 10% to 20% of the filtered Na+ is reabsorbed. Na+ enters the tubule cells via the sodium-chloride cotransporter (NCC) and exits from the basolateral side via NKA, while Cl- exits via chloride channels (ClC-Kb)[14].
Recent studies indicate that With-No-Lysine Kinase (WNK), Oxidative stress-responsive kinase (OSR) 1, and STE20/SPS1-related proline alanine-rich kinase (SPAK) importantly regulate transport in distal tubules.
WNKs are atypical protein kinases, as their name “With No Lysine (K)” implies[45]. They are expressed in various organs and tissues, including renal distal tubules, and modulate several biological processes, such as solute transport, cell growth, and neurotransmission[46]. WNKs have subtypes, such as WNK1, WNK2, WNK3, WNK4 and kidney-specific (ks-) WNK1. The kidney expresses WNK1, WNK3, WNK4 and ks-WNK1, where they modulate the function of NCC in the distal tubules.
In distal tubules, AngII seems to activate NCC via phosphorylation. Hoorn et al[47] showed that AngII induces the phosphorylation of NCC, enhancing sodium retention in rat kidneys, independent of aldosterone[47]. On the other hand, Uchida and colleagues showed that, although AngII increases NCC phosphorylation via the WNK-OSR1/SPAK pathway, the effect of aldosterone in this pathway is predominant[48]. Using WNK4 knockout mice, Gamba et al[49] showed that AngII stimulates NCC via a WNK4-SPAK dependent pathway and that WNK4 is involved in AngII-stimulated aldosterone secretion[49]. The detailed mechanisms by which AngII, WNK-SPAK/OSR1 and aldosterone regulate transport in the distal tubules transport remain to be clarified.
In the last portion of the tubules, the connecting tubules (CNT) and the collecting tubules (CD), Na+ is mainly reabsorbed via an epithelial Na+ channel (ENaC) on the luminal side and NKA on the basolateral side. The amount of Na+ reabsorbed from these segments represents only a small fraction of the total Na+ absorption by the kidney, but its regulation contributes to the fine-tuning of sodium and fluid homeostasis.
ENaC is a heteromultimeric channel, with three homologous subunits (α, β, γ)[50,51]. Loss-of-function mutations of ENaC cause pseudohypoaldosteronism type I (PHA-I), while gain-of-function mutations cause Liddle’s syndrome[52,53]. PHA-I features renal salt wasting associated with hyperkalaemia, while Liddle’s syndrome shows arterial hypertension with hypokalaemia. Pharmacologically, amiloride directly and reversely blocks the ENaC.
In the CNT and CD segments, aldosterone has been thought to play a principal role in regulating basal and long-term ENaC activity[54]. Recently, however, Korbmacher et al[55] demonstrated that, in the distal convoluted tubules (DCT2) and CNT, ENaC function is largely independent of aldosterone[55]. They suggested that glucocorticoids and/or AngII may be responsible for the aldosterone-independent ENaC activity. AngII itself may directly stimulate amiloride-sensitive Na+ reabsorption in CNT and CD, independent of aldosterone[56,57]. Indeed, several studies have reported that the AngII/AT1R pathway can regulate ENaC expression[58-61]. This effect of AngII is thought to be mediated via AT1R[62,63]. In obese Zucker rats, moreover, enhanced AT1R activity may result in the ENaC activation, suggesting a role for AngII in Na retention in diabetes and obesity[60].
As described above, NO has been thought to inhibit net NaCl and fluid absorption through renal tubules. However, Wang and colleagues argued that NO has a biphasic effect on the PTs. Low concentrations of an NO donor, sodium nitroprusside (SNP; 10-6 mol), stimulated PT fluid (Jv) and bicarbonate absorption (JHCO3) by 30%-50%, while high concentration of SNP (10-3 mol) inhibited Jv and JHCO3 by 50%-70%[64]. However, most other studies report that NO inhibits PT transport[65-67]. In particular, NO has been shown to decrease NHE3 and NKA activities[67,68]. Overall, NO is generally thought to inhibit NaCl, HCO3-, and volume reabsorption in the PTs.
In the TAL, approximately 30% of filtered Na+ is reabsorbed[14]. The major Na+ transporters here are the Na+/K+/2Cl- cotransporter (NKCC2) and NHE3 on the apical side as well as NKA on the basolateral side.
Garvin and colleagues found that NO donors inhibit Cl- and HCO3- reabsorption[69,70]. They found that NO inhibits NKCC2 and NHE3 activity, but not NKA activity[71,72]. Using NOS3-/- mice, they also showed that NOS3 is responsible for NO production in the TAL[73,74]. HCO3- reabsorption in the TAL is accomplished by H+ secretion via apical NHE3[75]. In the rat TAL, NO increases cGMP levels[76,77], and cGMP analogues inhibit JHCO3[70] and JCl[78,79]. The inhibition of cGMP-dependent kinase (cGK) blocked the inhibitory effect of NO on JHCO3, but not on JCl[70,80]. On the other hand, the inhibition of cGMP-stimulated phosphodiesterase (PDEII) blocked the inhibitory effect of NO on JCl[80]. Thus, the NO/cGK pathway seems to mediate the inhibitory effect on JHCO3, while the NO/PDEII pathway seems to mediate the inhibitory effect on JCl[80].
Recently, Wall and colleagues have showed that NO reduces Cl- absorption through ENaC in mouse CD[81]. In the cultured Xenopus laevis distal nephron cell line 2F3, Bao and colleagues showed that the activity of ENaC was reduced by a cyclic GMP analogue or by an atrial natriuretic peptide[82]. Moreover, in cGKII knockout mice, ENaC inhibition induced a much greater increase in UNa+V (2.6-fold) than in wild-type mice (1.9-fold), suggesting that ENaC activity is upregulated in the knockouts[83]. Integrating these results, NO and its signal transduction system appear to inhibit ENaC in CD and to induce natriuresis, therefore preventing sodium retention and hypertension.
As previously described, the AngII effect on Na+ reabsorption in the proximal tubule is biphasic in rodents and rabbits[27,28]. The inhibitory effect of AngII is mediated by the PLA2/arachidonic acid/EET and/or NOS/NO/cGMP pathways. In rat PTs, for example, the regulation of NKA by AngII seems to be dependent on the NO/cGMP pathway[35,36].
On the other hand, the effects of AngII on PT sodium transport in humans have not yet been clarified. To this end, we analysed the effects of AngII on human PTs isolated from the cortex of kidneys removed for renal carcinoma. Surprisingly, AngII, in contrast to other species, was found to induce a monophasic stimulation of human PT transport[84]. Specifically, AngII induced a dose-dependent stimulation of NBCe1, NHE3, and JHCO3 that was apparently mediated by both luminal and basolateral AT1Rs.
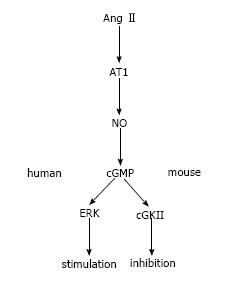
In contrast to other animals, both arachidonic acid and 5,6-EET failed to inhibit NBCe1 stimulation, which may partly account for the lack of an inhibitory effect of AngII in human PTs. Notably, however, we found that the contrasting responses to the NO/cGMP pathway could largely explain the different actions of AngII on PT transport in humans and other species. Thus, inhibition of the NOS/cGMP/cGKII pathway converted the inhibitory effect of 10-6 mol AngII on mouse PT transport into a stimulatory effect. SNP dose-dependently inhibited PT transport in wild-type but not in cGKII mice. By contrast, the inhibition of NOS/cGMP/ERK pathway completely suppressed the stimulatory effect of AngII on human PT transport. While the inhibition of cGKII did not affect the AngII effects, SNP dose-dependently stimulated transport in human PT. Western blotting with phosphor-specific antibodies revealed that AngII induced a dose-dependent cGKII activation in mouse but not in human kidney cortex samples. On the other hand, SNP induced a dose-dependent ERK activation in human but not in mouse samples. Collectively, these results indicate that while the NO/cGMP/cGKII pathway mediates the inhibitory effect of AngII in mouse PTs, the NO/cGMP/ERK pathway mediates the stimulatory effect in human PTs as shown in Figure 1.
We confirmed that human PTs do express cGKII. On the other hand, NO/cGMP failed to activate ERK in PTs from cGKII KO mice, indicating that the simple removal of cGKII from mouse PTs cannot reproduce the dose-dependent stimulatory effect of AngII in human PTs. Therefore, the reason why the NO/cGMP pathway, acting as the down-stream mediator of AngII, has contrasting effects on PT transport in humans and in other species is currently unknown. However, it is interesting to note that while the role of intrarenal NO in the adaptive natriuretic response to sodium loading has been well established in rodents, a similar role for NO has not been established in humans[85-90]. In any case, the human-specific stimulatory effect of the NO/cGMP pathway on PT transport may offer a novel therapeutic target for human hypertension.
The absorption of Na+ in renal tubules is regulated by various factors, among which AngII and NO play significant roles. In general, AngII stimulates sodium reabsorption and triggers fluid retention, leading to hypertension, while NO seems to induce natriuresis. However, our in vitro data suggest that NO may have distinct effects on PT transport in human and other species. Tables 1 and 2 summarize the effects of AngII and NO on renal tubular transport.
| Nephron segment | Potential targets | Effects | Ref. |
| PT | NHE3, NBCe1 | biphasic in rats, mice, rabbits | [19,27-32] |
| JHCO3 | monophasic stimulation in humans | [84] | |
| TAL | NKA, NKCC2, Cl channel | stimulation | [40-43] |
| NADPH oxidase | |||
| DCT | NCC | stimulation | [47,49] |
| WNK4? | |||
| CNT/CD | ENaC | stimulation | [55,59-61] |
| Nephron segment | Potential targets | Effects | Ref. |
| PT | NHE3, NBCe1 | inhibition in rats, mice, rabbits | [65-68,84] |
| JHCO3 | biphasic in rats? | [64] | |
| monophasic stimulation in humans | [84] | ||
| TAL | NKA, NKCC2 | inhibition | [69-72] |
| JCl, JHCO3 | |||
| CNT/CD | ENaC | inhibition | [81,83] |
P- Reviewer: Lymperopoulos A, Pedersen EB, Wu S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258-8263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1368] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 3. | Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985-17990. [PubMed] |
| 4. | Herrera M, Garvin JL. Recent advances in the regulation of nitric oxide in the kidney. Hypertension. 2005;45:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol. 2002;282:F777-F784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Lahera V, Salom MG, Miranda-Guardiola F, Moncada S, Romero JC. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol. 1991;261:F1033-F1037. [PubMed] |
| 7. | Lahera V, Salom MG, Fiksen-Olsen MJ, Raij L, Romero JC. Effects of NG-monomethyl-L-arginine and L-arginine on acetylcholine renal response. Hypertension. 1990;15:659-663. [PubMed] |
| 8. | Lahera V, Navarro J, Biondi ML, Ruilope LM, Romero JC. Exogenous cGMP prevents decrease in diuresis and natriuresis induced by inhibition of NO synthesis. Am J Physiol. 1993;264:F344-F347. [PubMed] |
| 9. | Majid DS, Williams A, Kadowitz PJ, Navar LG. Renal responses to intra-arterial administration of nitric oxide donor in dogs. Hypertension. 1993;22:535-541. [PubMed] |
| 10. | Majid DS, Omoro SA, Chin SY, Navar LG. Intrarenal nitric oxide activity and pressure natriuresis in anesthetized dogs. Hypertension. 1998;32:266-272. [PubMed] |
| 11. | Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990;15:451-458. [PubMed] |
| 13. | Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Garvin JL, Herrera M, Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol. 2011;73:359-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503-F508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238-1246. [PubMed] |
| 18. | Haithcock D, Jiao H, Cui XL, Hopfer U, Douglas JG. Renal proximal tubular AT2 receptor: signaling and transport. J Am Soc Nephrol. 1999;10 Suppl 11:S69-S74. [PubMed] |
| 19. | Horita S, Zheng Y, Hara C, Yamada H, Kunimi M, Taniguchi S, Uwatoko S, Sugaya T, Goto A, Fujita T. Biphasic regulation of Na+-HCO3- cotransporter by angiotensin II type 1A receptor. Hypertension. 2002;40:707-712. [PubMed] |
| 20. | Zheng Y, Horita S, Hara C, Kunimi M, Yamada H, Sugaya T, Goto A, Fujita T, Seki G. Biphasic regulation of renal proximal bicarbonate absorption by luminal AT(1A) receptor. J Am Soc Nephrol. 2003;14:1116-1122. [PubMed] |
| 21. | Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD. Renal proximal tubule angiotensin AT1A receptors regulate blood pressure. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1067-R1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol. 2006;17:2368-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Pinto V, Pinho MJ, Soares-da-Silva P. Renal amino acid transport systems and essential hypertension. FASEB J. 2013;27:2927-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011;S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 25. | Murer H, Biber J. Phosphate transport in the kidney. J Nephrol. 2010;23 Suppl 16:S145-S151. [PubMed] |
| 26. | Jaitovich A, Bertorello AM. Salt, Na+,K+-ATPase and hypertension. Life Sci. 2010;86:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch. 1977;367:295-297. [PubMed] |
| 28. | Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest. 1984;73:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M. Signaling pathways in the biphasic effect of angiotensin II on apical Na/H antiport activity in proximal tubule. Kidney Int. 1996;50:1496-1505. [PubMed] |
| 30. | Banday AA, Lokhandwala MF. Angiotensin II-mediated biphasic regulation of proximal tubular Na+/H+ exchanger 3 is impaired during oxidative stress. Am J Physiol Renal Physiol. 2011;301:F364-F370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Coppola S, Frömter E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. I. Effect of picomolar concentrations. Pflugers Arch. 1994;427:143-150. [PubMed] |
| 32. | Coppola S, Frömter E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. II. Effect of micromolar concentrations. Pflugers Arch. 1994;427:151-156. [PubMed] |
| 33. | Liu FY, Cogan MG. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest. 1989;84:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Li Y, Yamada H, Kita Y, Kunimi M, Horita S, Suzuki M, Endo Y, Shimizu T, Seki G, Fujita T. Roles of ERK and cPLA2 in the angiotensin II-mediated biphasic regulation of Na+-HCO3(-) transport. J Am Soc Nephrol. 2008;19:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Banday AA, Lokhandwala MF. Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling. Hypertension. 2008;52:1099-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Zhang C, Mayeux PR. NO/cGMP signaling modulates regulation of Na+-K+-ATPase activity by angiotensin II in rat proximal tubules. Am J Physiol Renal Physiol. 2001;280:F474-F479. [PubMed] |
| 37. | Li Y, Yamada H, Kita Y, Suzuki M, Endo Y, Horita S, Yamazaki O, Shimizu T, Seki G, Fujita T. Arachidonic acid metabolites inhibit the stimulatory effect of angiotensin II in renal proximal tubules. Hypertens Res. 2008;31:2155-2164. [PubMed] |
| 38. | Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10 Suppl 11:S189-S195. [PubMed] |
| 39. | Fukuchi S. Estimation of urinary angiotensin II by radioimmunoassay. Tohoku J Exp Med. 1974;114:205-213. [PubMed] |
| 40. | Wu P, Wang M, Luan H, Li L, Wang L, Wang WH, Gu R. Angiotensin II stimulates basolateral 10-pS Cl channels in the thick ascending limb. Hypertension. 2013;61:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Gonzalez-Vicente A, Garvin JL. Angiotensin II-induced hypertension increases plasma membrane Na pump activity by enhancing Na entry in rat thick ascending limbs. Am J Physiol Renal Physiol. 2013;305:F1306-F1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol. 2002;283:F957-F962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol Cell Physiol. 2012;303:C781-C789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Silva GB, Juncos LI, Baigorria ST, Garcia NH. Angiotensin II inhibits ADH-stimulated cAMP: role on O2- and transport-related oxygen consumption in the loop of Henle. J Biol Regul Homeost Agents. 2012;27:569-578. [PubMed] |
| 45. | Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1083] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 46. | Hoorn EJ, Ellison DH. WNK kinases and the kidney. Exp Cell Res. 2012;318:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Talati G, Ohta A, Rai T, Sohara E, Naito S, Vandewalle A, Sasaki S, Uchida S. Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem Biophys Res Commun. 2010;393:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+: Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109:7929-7934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 50. | Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1535] [Cited by in RCA: 1509] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 51. | Riazi S, Khan O, Hu X, Ecelbarger CA. Aldosterone infusion with high-NaCl diet increases blood pressure in obese but not lean Zucker rats. Am J Physiol Renal Physiol. 2006;291:F597-F605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 807] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 53. | Rossier BC, Schild L. Epithelial sodium channel: mendelian versus essential hypertension. Hypertension. 2008;52:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Barbry P, Hofman P. Molecular biology of Na+ absorption. Am J Physiol. 1997;273:G571-G585. [PubMed] |
| 55. | Nesterov V, Dahlmann A, Krueger B, Bertog M, Loffing J, Korbmacher C. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol. 2012;303:F1289-F1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131-1135. [PubMed] |
| 57. | Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143-F149. [PubMed] |
| 58. | Ohsawa M, Tamura K, Wakui H, Maeda A, Dejima T, Kanaoka T, Azushima K, Uneda K, Tsurumi-Ikeya Y, Kobayashi R. Deletion of the angiotensin II type 1 receptor-associated protein enhances renal sodium reabsorption and exacerbates angiotensin II-mediated hypertension. Kidney Int. 2014;86:570-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 59. | Lütken SC, Kim SW, Jonassen T, Marples D, Knepper MA, Kwon TH, Frøkiaer J, Nielsen S. Changes of renal AQP2, ENaC, and NHE3 in experimentally induced heart failure: response to angiotensin II AT1 receptor blockade. Am J Physiol Renal Physiol. 2009;297:F1678-F1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Madala Halagappa VK, Tiwari S, Riazi S, Hu X, Ecelbarger CM. Chronic candesartan alters expression and activity of NKCC2, NCC, and ENaC in the obese Zucker rat. Am J Physiol Renal Physiol. 2008;294:F1222-F1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Mujais SK, Kauffman S, Katz AI. Angiotensin II binding sites in individual segments of the rat nephron. J Clin Invest. 1986;77:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273:F170-F177. [PubMed] |
| 64. | Wang T. Nitric oxide regulates HCO3- and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. Am J Physiol. 1997;272:F242-F248. [PubMed] |
| 65. | Wu XC, Harris PJ, Johns EJ. Nitric oxide and renal nerve-mediated proximal tubular reabsorption in normotensive and hypertensive rats. Am J Physiol. 1999;277:F560-F566. [PubMed] |
| 66. | Eitle E, Hiranyachattada S, Wang H, Harris PJ. Inhibition of proximal tubular fluid absorption by nitric oxide and atrial natriuretic peptide in rat kidney. Am J Physiol. 1998;274:C1075-C1080. [PubMed] |
| 67. | Roczniak A, Burns KD. Nitric oxide stimulates guanylate cyclase and regulates sodium transport in rabbit proximal tubule. Am J Physiol. 1996;270:F106-F115. [PubMed] |
| 68. | Guzman NJ, Fang MZ, Tang SS, Ingelfinger JR, Garg LC. Autocrine inhibition of Na+/K(+)-ATPase by nitric oxide in mouse proximal tubule epithelial cells. J Clin Invest. 1995;95:2083-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol. 1999;276:F159-F163. [PubMed] |
| 70. | Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO(3)(-) transport by rat thick ascending limb. Kidney Int. 2000;58:2069-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na(+)-K(+)-2Cl(-) cotransporter activity. Am J Physiol Renal Physiol. 2001;281:F819-F825. [PubMed] |
| 72. | Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol. 1999;277:F377-F382. [PubMed] |
| 73. | Plato CF, Shesely EG, Garvin JL. eNOS mediates L-arginine-induced inhibition of thick ascending limb chloride flux. Hypertension. 2000;35:319-323. [PubMed] |
| 74. | Ortiz PA, Hong NJ, Wang D, Garvin JL. Gene transfer of eNOS to the thick ascending limb of eNOS-KO mice restores the effects of L-arginine on NaCl absorption. Hypertension. 2003;42:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Good DW, Watts BA. Functional roles of apical membrane Na+/H+ exchange in rat medullary thick ascending limb. Am J Physiol. 1996;270:F691-F699. [PubMed] |
| 76. | García NH, Plato CF, Stoos BA, Garvin JL. Nitric oxide-induced inhibition of transport by thick ascending limbs from Dahl salt-sensitive rats. Hypertension. 1999;34:508-513. [PubMed] |
| 77. | Lu M, Wang X, Wang W. Nitric oxide increases the activity of the apical 70-pS K+ channel in TAL of rat kidney. Am J Physiol. 1998;274:F946-F950. [PubMed] |
| 78. | Neant F, Bailly C. Luminal and intracellular cGMP inhibit the mTAL reabsorptive capacity through different pathways. Kidney Int. 1993;44:741-746. [PubMed] |
| 79. | Néant F, Imbert-Teboul M, Bailly C. Cyclic guanosine monophosphate is the mediator of platelet-activating factor inhibition on transport by the mouse kidney thick ascending limb. J Clin Invest. 1994;94:1156-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Ortiz PA, Garvin JL. NO Inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension. 2001;37:467-471. [PubMed] |
| 81. | Pech V, Thumova M, Dikalov SI, Hummler E, Rossier BC, Harrison DG, Wall SM. Nitric oxide reduces Cl⁻ absorption in the mouse cortical collecting duct through an ENaC-dependent mechanism. Am J Physiol Renal Physiol. 2013;304:F1390-F1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Guo LJ, Alli AA, Eaton DC, Bao HF. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol. 2013;304:F930-F937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Schramm A, Schinner E, Huettner JP, Kees F, Tauber P, Hofmann F, Schlossmann J. Function of cGMP-dependent protein kinase II in volume load-induced diuresis. Pflugers Arch. 2014;466:2009-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Shirai A, Yamazaki O, Horita S, Nakamura M, Satoh N, Yamada H, Suzuki M, Kudo A, Kawakami H, Hofmann F. Angiotensin II dose-dependently stimulates human renal proximal tubule transport by the nitric oxide/guanosine 3’,5’-cyclic monophosphate pathway. J Am Soc Nephrol. 2014;25:1523-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 86. | Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230-236. [PubMed] |
| 87. | Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res. 2006;99:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 88. | Kone BC, Baylis C. Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol. 1997;272:F561-F578. [PubMed] |
| 89. | Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension. 1999;33:1008-1012. [PubMed] |
| 90. | Schmidt RJ, Beierwaltes WH, Baylis C. Effects of aging and alterations in dietary sodium intake on total nitric oxide production. Am J Kidney Dis. 2001;37:900-908. [PubMed] |