Revised: October 14, 2013
Accepted: November 1, 2013
Published online: February 6, 2014
Processing time: 200 Days and 6.4 Hours
AIM: To examine the risk of renal events in patients with biopsy-proven diabetic nephropathy (DN) and its possible associated factors.
METHODS: Clinical and histological data of 60 patients diagnosed with diabetic nephropathy were retrospectively collected. Patients with evidence or suspicion of other nephropathies were excluded from the study. The final event was defined as renal replacement therapy (RRT) initiation or progression of chronic kidney disease (CKD), according to the KDIGO 2012 definition of a decrease in CKD category and a decrease in GFR of 25% or more.
RESULTS: A total of 45 patients with a follow-up of at least 3 mo were included. Most of the patients presented type 2 DM, with a mean age of 58.3 years old. The time of evolution of DM was 9.6 ± 7.8 years, although in 13 patients, it was less than 5 years. A total of 62% of patients reached the final event in a median period of 3.4 years (95%CI: 2.1-4.7), with 21 of them requiring dialysis. The factors that were independently associated with renal survival were estimated glomerular filtration rate (eGFR) at the time of biopsy, cardiovascular disease (CVD) history and HbA1c less than 7%. Therefore, for each 10 mL/min per 1.73 m2 reduction in eGFR, we obtained a DN progression risk of HR = 2 (1.3-3.0) (P = 0.001); patients with CVD were at greater risk for DN progression (HR = 2.8, 1.1-7.1, P = 0.032), and CKD patients with HbA1c < 7% demonstrated greater renal risk than patients with HbA1c ≥ 7%, with an HR of 2.9 (1.0-8.4) (P = 0.054).
CONCLUSION: A past history of CVD is a risk factor for DN progression. Levels of HbA1c less than 7% could favor an eGFR decrease in these patients.
Core tip: There are other forms of presentation of diabetic nephropathy (DN), in addition to progressive proteinuria, that can result in renal insufficiency. In some cases, DN is diagnosed in advanced stages, without previous suspicion of this diagnosis. The clinical course can be atypical, and the time of evolution of diabetes mellitus can be short. Not all the factors that play a role in the evolution of DN have been elucidated. Our findings suggest that in patients with chronic kidney disease secondary to DN, a previous history of cardiovascular disease and HbA1c less than 7%, are negative prognostic factors for renal function.
- Citation: López-Revuelta K, Galdo PP, Stanescu R, Parejo L, Guerrero C, Pérez-Fernández E. Silent diabetic nephropathy. World J Nephrol 2014; 3(1): 6-15
- URL: https://www.wjgnet.com/2220-6124/full/v3/i1/6.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i1.6
The risk factors for diabetic nephropathy (DN) include genetic predisposition[1,2], poor glycemic control[3], older age[4], male sex[5], duration of diabetes, hypertension[6] and smoking. Classically, the natural history of the disease was considered to be an evolution that began after 5-15 years after the onset of diabetes with albuminuria[7]. Albuminuria increases cardiovascular risk, but it also increases the risk of progression to proteinuria, especially in type 1 diabetes mellitus (T1DM)[8]. It is unclear what predisposes 50% of individuals with albuminuria to progress to proteinuria in a phase that lasts approximately 10 years[9]. After the development of proteinuria, 50% of patients will progress to end-stage renal disease (ESRD) in 7-10 years[10]. High risk of cardiovascular disease (CVD) further increases with deteriorating renal function. Some factors have been implicated in the increased rate of decline in kidney function, especially in type 2 diabetes mellitus (T2DM): higher baseline albuminuria; high systolic blood pressure; higher hemoglobin A1c; estimated glomerular filtration rate (eGFR); age; and coexistence of diabetic retinopathy[10,11]. However, a large inter-individual variation in the rate of decline in glomerular filtration rate (GFR) has been reported in both type 1 and type 2 DM. Recently, a nonalbuminuric renal impairment phenotype was described in T2DM, which has distinct clinical features that are not associated with HbA1c and that are correlated less strongly with retinopathy and hypertension[12]; this phenotype is associated with a higher prevalence of CVD and suggests a predominance of macroangiopathy as the underlying renal pathology, which has yet to be demonstrated. In T1DM, the development of advanced CKD relatively soon after the onset of albuminuria has been described, and this progression was not conditional to the presence of proteinuria[13]. Reduced eGFR also occurs among long-standing normoalbuminuric type 1 diabetic patients and has been associated with more advanced diabetic glomerular lesions and, probably, with increased risk of progression[14].
In patients with DN, there has also been described a nonlinear, abrupt and rapid progression pattern similar to that described by others[15] as rapid-onset end-stage renal disease, which some authors have related to inflammation and episodes of acute renal failure[16].
This spectrum of progression patterns highlights the need for the identification of risk factors for the loss of renal function early in the course of DN, especially in patients with histopathological confirmation of this diagnosis.
The aim of this study was to examine the risk of renal events in patients with biopsy-proven DN and its possible associated factors.
We studied all the patients diagnosed with DN by renal biopsy at a Spanish center between December 1998 and December 2012, who had a minimum 3-mo follow-up after the biopsy. Of a total of 60 patients with histopathological diagnoses of DN at our center, we excluded 3 patients who had less than 6 glomeruli on biopsy, 10 patients with evidence of another associated nephropathy (2 IgA nephropathies, 1 membranous nephropathy, 1 membranoproliferative glomerulonephritis associated with HVC, 2 tubulointerstitial nephritis, 2 acute tubular necrosis, 2 with amyloidosis AA) and 2 patients with acute kidney injury: 1 with a functional etiology and the other with septic shock. A total of 45 patients diagnosed with “pure” DN and a sufficient number of glomeruli for the diagnosis were included.
In all the cases, the nephrologist was the specialist who recommended the biopsy, considering all the available data. We classified the indications for renal biopsy into three groups: nephrotic proteinuria with or without nephrotic syndrome; rapidly progressive kidney injury (RPKI); and CKD. All the renal biopsies were revised by a nephropathologist to confirm the glomerular classification type, the grade of interstitial fibrosis and tubular atrophy (IFTA), interstitial inflammation, arteriolar hyalinosis and the presence of large vessel arteriosclerosis on the basis of the criteria previously described[17]. Four types are described: Glomerular Class I, glomerular basement membrane thickening; Class II, mesangial expansion, mild (IIa) or severe (IIb); Class III, nodular sclerosis (Kimmelstiel-Wilson lesions); and class IV, advanced diabetic glomerulosclerosis.
The demographic, clinical, and laboratory data and comorbid conditions of every patient at the time of biopsy were extracted from clinical records. We recorded the date when the nephrologist began follow-up and whether the patients were receiving treatment with renin-angiotensin aldosterone system inhibitors (RAASIs), statins or antiplatelet drugs.
In 39 patients, we had available information on baseline renal function from 1.1 to 24.1 mo before renal biopsy. We recorded the last follow-up serum creatinine or the starting date of RRT for all the patients.
The glomerular filtration rate was estimated according to the CKD-EPI formula[1] at baseline, at renal biopsy and at the last follow-up visit. The eGFR and albuminuria categories of CKD were classified according to the KDIGO 2012 classification[18]. In this manner, six eGFR categories were recognized (mL/min per 1.73 m2): G1 = eGFR ≥ 90; G2 = 60-89; G3a = 45-59; G3b = 30-44; G4 = 15-29; and G5 < 15. Proteinuria categories were described based on protein-to-creatinine ratio (mg/g) or protein excretion rate (mg/24 h) as follows: A1 ≤ 150; A2 = 150-500; and A3 ≥ 500.
The presentation of RPKI was considered in those cases in which a decrease in eGFR greater than 25% was seen between baseline and biopsy, independent of biopsy indication. The final end-point was defined as RRT initiation or progression of CKD according to the KDIGO 2012 definition as a in CKD category and a decrease in eGFR of 25% or more. The follow-up period was considered from biopsy until endpoint, death or last follow-up.
The silent diabetic nephropathy variable was defined for cases that showed an atypical disease pattern or in which DN was not suspected. This variable grouped patients with RPKI without significant proteinuria (< 0.5 g/d) and/or a duration of DM of less than 5 years and/or the need to start RRT less than 1.5 years from renal biopsy.
The statistical analysis was performed using SPSS, version 17.0 for Windows, and the STATA software, version 12. Quantitative data are described by means ± SDs or medians (interquartile ranges). Qualitative data are described by counts and percentages [n (%)].
Survival median was estimated by the Kaplan-Meier function. The log-rank test was used to compare survival functions. To study factors associated with renal events, univariate analysis was performed, adjusting Cox regression models. The proportionality of hazards assumption was checked graphically. Finally, a multivariate predictive model was adjusted, including statistically significant variables and clinically relevant factors. The model was adjusted by the enter method and including the least number of covariates necessary. Harrell’s c-index[19] was calculated to evaluate the model’s predictive ability. This index measures the ability of a predictor to separate groups with different answers and is still acceptable greater than approximately 0.85. An exploratory descriptive analysis was performed to compare the two samples, defined by the silent DN variable. Association was studied by the χ2 test or Fisher’s exact test and the Mann-Whitney U test. To estimate silent DN’s effects on the risk of renal events, we adjusted the multivariate Cox regression model, including possible confounding factors (complete model). We defined a confusion factor as a difference of more than 10% between the adjusted hazard ratio (HR) and the complete model. HRs are presented with 95%CIs. All the tests were two-tailed, and a significance level ≤ 0.05 was considered statistically significant.
Data from 45 patients were included in this study. The patients’ characteristics at the time of biopsy are detailed in Table 1. Most patients with biopsy-proven DN in our series had type 2 diabetes and were hypertensive, dyslipidemic and smokers. Seventy-one percent were men with a mean age of 58.3 ± 13.3 years old and a DM evolution time of 9.6 ± 7.8 years. Thirty-five percent had cardiovascular disease, 40% had retinopathy, and 40% had microhematuria. Their values of HbA1c were normal, according to international recommendations for these patients, but their cholesterol levels were not normal, although 73% of the patients were on statins. Furthermore, 89% of the subjects were on treatment with RAASIs, as well as 47% on antiplatelet drugs at the time of the biopsy.
| Total | n = 45 |
| Age (yr) (range) | 58.3 ± 13.3 (28-84) |
| Sex (men) | 32 (71.1) |
| Diabetes type 2 | 38 (84.4) |
| Diabetes duration (yr) (range) | 9.6 ± 7.8 (0-35) |
| BMI (kg/m2) (range) | 29.3 ± 5.3 (27.8-47.8) |
| Obesity BMI > 30 kg/m2 | 18 (40.9) |
| Hypertension (yes) | 42 (93.3) |
| Smoker, active or past (yes) | 34 (75.6) |
| Dyslipidemic (yes) | 33 (73.3) |
| Ischemic heart disease (yes) | 7 (15.6) |
| CVA (yes) | 6 (13.3) |
| Peripheral arterial disease (yes) | 8 (17.8) |
| Any CVD | 16 (35.6) |
| Hematuria (yes) | 18 (41.9) |
| Serum albumin (g/dL) (range) | 3.4 ± 0.7 (2-5) |
| HbA1c% (range) | 6.5 ± 1.4 (4.1-9.3) |
| Total cholesterol (mg/dL) | 177.9 ± 58.7 |
| Previous nephrology care (yr) (range) | 1.21 ± 2.4 (0-12) |
| RAASI treatment | 40 (88.9) |
| Statin treatment | 33 (73.3) |
| Antiplatelet drug treatment | 21 (46.7) |
In Table 2, we show the evolution of the renal parameters during follow-up. In 62% of the cases, the biopsy indication was a nephrotic range of proteinuria, with or without nephrotic syndrome. Nine percent of the patients presented proteinuria ≤ 0.5 g/24 h at the time of the biopsy. Although 48.8% of the patients showed baseline creatinine ≤ 1.4 mg/dL, 68% of them showed eGFRs at time of biopsy < 45 mL/min per 1.73 m2, and 15.6% were in the grade 5 eGFR category.
| Previous renal data (n = 39) | Renal biopsy (n = 45) | End of follow-up (n = 24) | ||
| Time prior to biopsy (mo) (range) | 7.3 ± 5.2 | |||
| (1.1-24.1) | ||||
| Follow-up period (yr) (range) | 3.4 ± 2.9 | |||
| (0.2-11.8) | ||||
| Renal biopsy indication | RPKI | 8 (17.8) | ||
| Nephrotic proteinuria | 28 (62.2) | |||
| CKD | 9 (20) | |||
| Serum creatinine (mg/dL) (range) | 1.6 ± 0.8 | 2.3 ± 1.5 | 2.3 ± 1.8 | |
| (0.8-4.5) | (0.8-6) | (0.7-9.1) | ||
| eGFR (mL/min per 1.73 m2) (range) | 51.4 ± 20.9 | 39.1 ± 22.5 | 40.8 ± 25 | |
| (14.7-97.6) | (8.1-101.2) | (5.1-107.1) | ||
| 1eGFR category | G5 < 15 | 7 (15.6) | 24 (53.3) | |
| G4 15-30 | 8 (17.8) | 6 (13.3) | ||
| G3b 30-45 | 15 (33.3) | 5 (11.1) | ||
| G3a 45-60 | 7 (15.6) | 7 (15.6) | ||
| G2 60-90 | 7 (15.6) | 1 (2.2) | ||
| G1 > 90 | 1 (2.2) | 2 (4.4) | ||
| > 25% drop in eGFR prior to biopsy | 13 (33.3) | |||
| 2Proteinuria (range) | 3.7 ± 3.4 | 4.5 ± 2.7 | ||
| (0-12.9) | (1-8.9) | |||
| 3Proteinuria category | A1 < 150 | 3 (8.8) | 0 | |
| A2 150-500 | 2 (5.9) | 4 (8.9) | ||
| A3 > 500 | 29 (85.3) | 41 (91.1) | ||
| RRT | 2 | 5 | 21 (46.7) | |
| 4CKD progression | 7 (15.6) | |||
| eGFR improvement > 25% | 4 (8.9) | |||
| Exitus | 5 (11.1) |
Thirty-three percent of the subjects were classified with RPKI, three of them without significant proteinuria (< 0.5 g). Seven of these patients needed dialysis, two of them only for a mean time of 8 d and the others permanently.
Twenty-eight patients (62%) reached the final event, and 21 of them required RRT. The median renal survival 3.4 years (95%CI: 2.1-4.7).
In Table 3, we describe the clinical and histopathological findings, classified according to the type of glomerular lesions. Most cases (23 patients) presented glomerular class III or nodular sclerosis, and 4 subjects (9%) had advanced diabetic glomerulosclerosis (class IV) that was not suspected when the biopsy has been recommended. The four patients whose diagnoses of DN coincided with the DM diagnosis had advanced forms of DN: 2 cases with class II b, 1 case with class III and 1 case with class IV.
| I-IIa-IIb (n = 18) | III (n = 23) | IV (n = 4) | ||
| Age (yr) | 59.9 ± 12.2 | 58.1 ± 14.1 | 52.3 ± 15.4 | |
| Years of diabetes | 9.1 ± 7.4 | 9.3 ± 6.3 | 15.1 ± 18 | |
| BMI (kg/m2) | 29 ± 4.9 | 30.1 ± 6 | 26.8 ± 2.4 | |
| Serum creatinine (mg/dL) | 2 ± 1.2 | 2.2 ± 1.4 | 4.4 ± 1.9 | |
| eGFR (mL/min per 1.73 m2) | 42.3 ± 21.9 | 40.4 ± 22.9 | 17.6 ± 12.3 | |
| HbA1c (%) | 6.7 ± 1.4 | 6.3 ± 1.3 | 6.7 ± 2 | |
| Proteinuria (g/d) | 3.1 ± 2.6 | 5.2 ± 4 | 4.7 ± 5.6 | |
| Serum albumin (g/dL) | 3.6 ± 0.7 | 3.3 ± 0.7 | 2.9 ± 0.7 | |
| Serum cholesterol (mg/dL) | 165.4 ± 71.6 | 191.5 ± 34.6 | 213 ± 0 | |
| Hypertension | 16 (88.9) | 22 (95.7) | 4 (100) | |
| Diabetic retinopathy | 7 (38.9) | 9 (39.1) | 2 (50) | |
| CVD | 7 (38.9) | 6 (26.1) | 3 (75) | |
| RPKI | 0 | 6 (33.3) | 7 (30.4) | |
| Patients with renal events | 9 (50) | 15 (65.2) | 4 (100) | |
| RRT | 6 (33.3) | 11 (47.8) | 4 (100) | |
| 1Years from biopsy to renal event | 4.2 ± 1.2 | 3.4 ± 1.4 | 0.4 ± 0.4 | |
| % of global glomerulosclerosis | 18.1 ± 12.8 | 18.4 ± 15.1 | 77.3 ± 16.9 | |
| Interstitial fibrosis | 0 | 1 (5.6) | 0 | 0 |
| and tubular atrophy | 1 | 10 (55.6) | 13 (56.5) | 0 |
| 2 | 5 (27.8) | 9 (39.1) | 1 (25) | |
| 3 | 2 (11.1) | 1 (4.3) | 3 (75) | |
| Interstitial inflammation | 0 | 3 (16.7) | 0 | 0 |
| 1 | 14 (77.8) | 22 (95.7) | 4 (100) | |
| 2 | 1 (5.6) | 1 (4.3) | 0 | |
| Arteriolar hyalinosis | 0 | 1 (5.6) | 1 (4.3) | 1 (25) |
| 1 | 3 (16.7) | 2 (8.7) | 0 | |
| 2 | 14 (77.8) | 20 (87) | 3 (75) | |
| Large vessel arteriosclerosis (yes) | 17 (94.4) | 19 (86.4) | 4 (100) | |
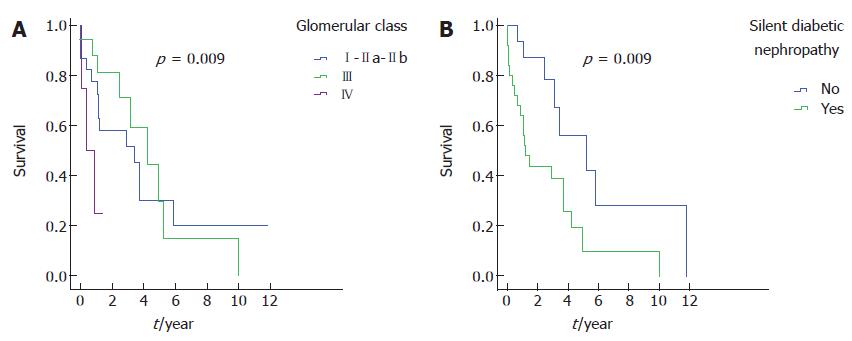
The patients who had advanced diabetic glomerulosclerosis were younger, had more cardiovascular diseases and retinopathy, and had worse renal function and lower figures of serum albumin than other histopathological types. Additionally, this group of patients showed a higher proportion and greater severity of interstitial fibrosis and tubular atrophy but no differences in vascular lesions or inflammation scores. Renal survival was variable in the different glomerular classes, not only comparing class IV with the other classes but also comparing class III to classes I and IIa-IIb (Figure 1A); the median in class I/IIa/IIb was 4.2 years (95%CI: 1.8-6.6), in class III, the median was 3.4 (95%CI: 0.6-6.2), and in class IV, it was 0.4 (95%CI: 0-1.2; P = 0.009).
Twenty-five patients were considered to have silent DN: thirteen patients with less than 5 years of duration of DM at biopsy, 4 of them diagnosed with diabetes at the same time as renal biopsy; 1 patient with RPKI and proteinuria < 0.5 g/d; and 14 patients who began RRT before 1.5 years after biopsy (3 with less than 5 years of duration of DM). As shown in Table 4, compared to the remainder of the patients, the silent DN subjects presented with a shorter evolution time of diabetes, had worse renal function at the time of biopsy, had a higher frequency of RPKI and less HbA1c, and had more advanced histopathological forms, and they presented more renal events. They frequently had more cardiovascular disease, although this difference was not statistically significant. To estimate the risk of silent DN, we adjusted a multivariate regression model including possible bias factors: age, eGFR, proteinuria, glomerular class, CVD and HbA1c. The final model (Table 5) estimated the risk for silent DN of 2.1 (95%CI: 0.8-5.1), adjusted for cardiovascular disease and HbA1c. The remainder of the factors were discarded as they were considered confounders. Figure 1B illustrates the renal survival curves in silent DN, compared to the other subjects.
| Non-silent DN (n = 20) | Silent DN (n = 25) | P value | |
| Age (yr) | 55.8 ± 12.2 | 60.3 ± 14.1 | |
| Sex (women) | 5 (25) | 8 (32) | |
| BMI (kg/m2) | 30.9 ± 6.3 | 28 ± 4.1 | |
| T2 DM | 17 (85) | 21 (84) | |
| Duration of diabetes (year) | 12.5 ± 5.3 | 7 ± 8.9 | 0.005 |
| Follow-up period (year) | 3.4 ± 3.5 | 3.5 ± 2.7 | |
| Smoking, active or past | 16 (80) | 18 (72) | |
| Retinopathy | 8 (40) | 10 (40) | |
| CVD | 6 (30) | 10 (40) | |
| HbA1c (%) | 7 ± 1.2 | 6.2 ± 1.5 | 0.03 |
| Serum creatine at biopsy (mg/dL) | 1.8 ± 1 | 2.7 ± 1.6 | 0.03 |
| eGFR at biopsy (mL/min per 1.73 m2) | 47 ± 22.5 | 32.9 ± 20.8 | 0.04 |
| Proteinuria (g/d) | 3.5 ± 2.6 | 5 ± 4.3 | |
| Hematuria | 8 (44.4) | 10 (40) | |
| RPKI | 3 (15) | 12 (48) | 0.03 |
| CKD progression | 3 (15) | 10 (50) | 0.04 |
| Renal events | 8 (40) | 20 (80) | 0.01 |
| Histopathological class AP III-IV | 9 (45) | 18 (72) | |
| Glomerular sclerosis percentage | 18.8 ± 14.3 | 27.2 ± 26.4 | |
| IFTA 0-1 | 12 (60) | 12 (48) | |
| IFTA 2 | 7 (35) | 8 (32) | |
| IFTA 3 | 1 (5) | 5 (20) | |
| Severe arteriolar hyalinosis | 15 (75) | 22 (88) | |
| Large vessel arteriosclerosis | 18 (94.7) | 22 (88) | |
| RAASI treatment | 18 (90) | 22 (88) | |
| Statins treatment | 15 (75) | 18 (72) |
| Variables in the equation | HR | 95%CI | |
| Lower | Upper | ||
| CVD | 3.943 | 1.649 | 9.429 |
| HbA1c% | 0.724 | 0.516 | 1.016 |
| Silent DN | 2.137 | 0.819 | 5.573 |
The results of univariate Cox proportional hazard analysis, according to clinical variables and histopathological variables, are shown in Tables 6 and 7, respectively. Clinical variables statistically significantly associated with renal end point were: baseline and renal biopsy eGFR and serum creatinine; BMI < 30 kg/m2; Hb A1 < 7%; RPKI; silent DN; and coexistence of cardiovascular disease. Of the histopathological variables, only glomerular class IV and percentage of global glomerulosclerosis were statistically significantly associated with the renal end point.
| HR | 95%CI | P value | ||
| Lower | Upper | |||
| Age (yr) | 1.00 | 0.97 | 1.03 | |
| Sex (men) | 1.19 | 0.50 | 2.85 | |
| Diabetes type (2/1) | 0.76 | 0.31 | 1.91 | |
| Diabetes duration (years) | 1.01 | 0.95 | 1.07 | |
| BMI < 30 (yes/no) | 2.94 | 1.09 | 7.69 | 0.03 |
| Smoker (yes/no) | 0.92 | 0.34 | 2.50 | |
| Hypertension (yes/no) | 1.01 | 0.13 | 7.69 | |
| CVD (yes/no) | 4.56 | 1.94 | 10.69 | 0.000 |
| Retinopathy (yes/no) | 1.24 | 0.56 | 2.75 | |
| Baseline Serum creatinine (mg/dL) | 2.18 | 1.14 | 4.16 | 0.02 |
| Baseline eGFR (mL/min per 1.73 m2) | 0.98 | 0.95 | 1.00 | |
| 1Baseline proteinuria (g/g) | 1.12 | 0.98 | 1.27 | |
| eGFR drop > 25% before biopsy | 3.96 | 1.54 | 10.18 | 0.004 |
| Serum creatinine (mg/dL) at biopsy | 2.97 | 1.91 | 4.61 | 0.000 |
| eGFR (mL/min per 1.73 m2) at biopsy | 0.94 | 0.92 | 0.97 | 0.000 |
| RPKI | 2.74 | 1.21 | 6.24 | 0.02 |
| 2Proteinuria at biopsy (g/d) | 1.09 | 0.97 | 1.23 | |
| Hematuria | 1.65 | 0.72 | 3.82 | |
| Serum albumin (g/dL) | 0.75 | 0.41 | 1.35 | |
| HbA1c % (< 7/≥ 7) | 3.37 | 1.23 | 9.25 | 0.02 |
| Total cholesterol (mg/dL) | 0.98 | 0.96 | 1.01 | |
| RAASI treatment (yes/no) | 0.59 | 0.22 | 1.59 | |
| Statin treatment | 0.66 | 0.27 | 1.62 | |
| Silent DN | 3.04 | 1.26 | 7.3 | 0.02 |
| HR | 95%CI | P value | |||
| Glomerular class | III/I-IIa-IIb | 1.2 | 0.5 | 2.9 | |
| IV/I-IIa-IIb | 5.6 | 1.6 | 19.7 | 0.007 | |
| IV/III | 4.6 | 1.4 | 15.1 | 0.01 | |
| % of global glomerulosclerosis | 1.0 | 1.0 | 1.0 | 0.01 | |
| IFTA | (> 25 ≤ 25%) | 1.2 | 0.5 | 2.5 | |
| Arteriolar hyalinosis | Severe/mild | 0.7 | 0.2 | 2.2 | |
| Large vessel arteriosclerosis (yes) | 1-2/0 | 1.2 | 0.4 | 4.1 | |
The results of multivariate Cox proportional hazard models are shown in Table 8. We found that eGFR, cardiovascular disease and HbA1c at the time of biopsy were risk factors for progression of DN (initiation of renal replacement therapy or decline ≥ 25% and change in CKD category), adjusted for age and sex. For every 10 mL/min per 1.73 m2 decrease in eGFR, we obtained a DN progression risk of HR = 2 (1.3-3.0) (P = 0.001). Patients with cardiovascular disease were at greater risk for DN progression (HR = 2.8, 1.1-7.1; P = 0.032). Although diabetic patients with CKD and HbA1c < 7% showed greater renal progression risk than patients with HbA1c ≥ 7%, with an HR of 2.9 (1.0-8.4), this effect was not statistically significant (P = 0.054). Harrel’s c index was 0.823, indicating acceptable predictive ability.
| HR | 95%CI for HR | P value | ||
| Lower | Upper | |||
| CVD | 2.75 | 1.07 | 7.11 | 0.036 |
| RPKI | 1.29 | 0.46 | 3.64 | 0.626 |
| eGFR (10 mL/min per 1.73 m2) | 1.96 | 1.28 | 3.00 | 0.001 |
| HbA1c% (< 7/≥ 7) | 2.88 | 0.98 | 8.44 | 0.054 |
The present study analyzed clinical and histopathological factors associated with worse renal prognosis in a cohort of patients with biopsy-proven diabetic nephropathy, mostly type 2 diabetics. Two thirds of the patients had an eGFR at the time of the biopsy < 45 mL/min per 1.73 m2, that is, irreversible damage to renal function, and half of the patients reached ESRD in a median period of 3.4 years.
In our series, eGFR at time of biopsy was a determinative factor for CKD progression, as is already well known. In contrast, proteinuria was not associated with worse renal prognosis, although the majority of patients showed proteinuria > 500 g/d, but most of patients without proteinuria also experienced renal events.
Although our study included selection bias, which was the clinical indication for renal biopsy, our series included only cases of DN in which other causes of renal damage had been excluded. Therefore, our findings, even if they cannot be extrapolated to all patients with DN, could increase understanding of why some patients with diabetes have atypical clinical courses and are diagnosed in advanced stages of renal disease, with minimal therapeutic possibilities.
Although the majority of patients had been medically followed up before biopsy, this fact did not prevent negative evolution or late diagnosis of the illness. The RPKI presentation form, predominant in 33% of the patients, was associated with a poor renal prognosis, although it behaved as a confounding factor and not as an independent risk factor.
It was shown[16] that, in DN, relatively small elevations in serum creatinine could significantly underestimate the degree of renal damage, and these elevations were unpredictable most of the time.
Without a doubt, this fact contributed to the large proportion of patients in our series that we classified with silent diabetic nephropathy, that is, cases that went unnoticed until advanced stages. These patients had shorter diabetes evolution times; they presented a higher frequency of RPKI, a major loss of renal function at the moment of the biopsy, and they had a higher proportion of renal events. Although they had more cardiovascular diseases compared to the remainder of the group, this difference was not statistically significant.
All these data support that serum creatinine is not a good parameter for monitoring renal function in diabetic patients and that even with normal serum creatinine levels, eGFR should be a routine test. It is probable that an eGFR at less than 90 mL/min per 1.73 m2, we should recommend several tests per year in these patients to detect CKD progression and optimize their treatment.
In a study of 22 biopsy-proven diabetic nephropathy cases[16], these authors found in the majority of cases evidence of acute kidney injury in their biopsies, including tubular necrosis and interstitial inflammation, although seven subjects had similar rates of progression and yet undetectable acute events. In our series, we excluded those patients in whom we suspected renal failure secondary to another etiology. However, it is possible that some cases of functional loss might have existed, especially in nephrotic patients, because in four patients, we observed an improvement in renal function during follow-up. Other authors found that interstitial lesions, but not glomerular class, was a significant predictor of renal prognosis in diabetic nephropathy in type 2 diabetes[20], but it was a small series of 69 type 2 diabetic patients, all with overt proteinuria.
The only histopathological finding of our series that proved to be a risk factor for renal progression was advanced diabetic glomerulosclerosis. It is possible that if our sample had been larger, we would have been able to demonstrate prognostic values of more benign histological types, as observed by the different lengths of renal survival seen in our series, which was worse for type III nodular sclerosis, compared to patients with types I and IIa-IIb.
Another risk factor associated with poor renal prognosis was a BMI < 30 kg/m2. Although obesity is a risk factor for CKD and ESRD[21], its effects have not been clear in patients with T2DM[22]. Although in our study, BMI seemed to have a paradoxical effect, similar to that described in the survival of patients with T2DM in TSR[23], it was not an independent risk factor for renal progression.
In the present study, HbA1c < 7% was correlated with worse renal prognosis in patients with established DN. Important large, randomized, controlled, multicenter trials have shown that intensive glycemic control in T2DM reduces the risk of albuminuria and proteinuria[24], but evidence has been lacking that intensive glycemic control reduces the risk of significant clinical renal outcomes, such as doubling of serum creatinine level, ESRD or death from renal disease[25]. In these trials, severe hypoglycemia was clearly increased among intensively treated patients[26]. In contrast, we know that the individuals with progressive renal dysfunction are at increased risk for hypoglycemia, which is multifactorial.
These trials have either failed to demonstrate a benefit of glucose lowering for CVD risk or have even suggested an increased CVD risk with very tight glycemic control, most likely explained by the adverse effects of hypoglycemia on the heart and blood vessels[27]. Acute hypoglycemia triggers a cascade of physiologic responses, including the activation of inflammatory pathways, release of counter-regulatory hormones, including epinephrine, and reduced blood flow to the myocardium.
In a recent prospective study in older adults with diabetes[28], an association between dementia and having presented hypoglycemic episodes during 12 years of follow-up was found. The authors indicated as possible etiopathogenic mechanisms hypoxia by vasoconstriction, neuronal loss, hyperinsulinemia, exacerbation of the oxidative stress and inflammatory mediators. Although this series was adjusted for cardiovascular events, small vessel vascular disease was not discharged, so it is possible that cerebral microinfarcts played some part in cerebral atrophy and cognitive deterioration.
In the present study, a past history of CVD was identified as an independent risk factor for CKD progression in DN, almost tripling the risk of progressive CKD. Although the effects that diabetic CKD has on CV risk are well known[29], the renal risk of CVD in DN has not been defined. Some authors have advocated that vascular disease of the kidney can explain nonalbuminuric progressive DN[30]. In our series, the prevalence of CVD was similar or even slightly lower than that reported by these authors (37% in patients with reduced eGFR), but we have already mentioned that this form of onset was very rare in our series. Similar degrees of intrarenal vascular disease, measured by the Doppler resistance index of the interlobar renal arteries, were found in diabetic patients with reduced GFR, regardless of their albuminuria status.
Our data sustain that regardless of albuminuric phenotype, past history of CVD is a risk factor for progressive renal function decline in DN, as other authors have found[31]. In support of this theory, a recent study[32] linked cerebral microinfarcts, diagnosed by magnetic resonance imaging, with low eGFR and worse renal prognosis in type 2 DM, regardless albuminuria. The risk of doubling of the serum creatinine concentration or the need for dialysis was significantly greater for patients with silent cerebral infarction (HR = 4.79, 95%CI: 2.72-8.46) than for patients without silent cerebral infarction. The authors believe that this association might have been due to the similarity between renal and cerebral vascular hemodynamic behaviors.
Therefore, it is interesting that in our series, we found that cardiovascular disease and tighter glycemic control were DN progression risk factors. Although our findings cannot be extrapolated to the totality of patients with diabetic nephropathy, we can speculate that at least in diabetic patients with vascular disease, the benefits of strict glycemic control do not improve renal prognosis when kidney failure has already been established. It is possible that on an already damaged renal parenchyma, hypoglycemia could induce the release of proinflammatory mediators by means of hypoxia, which could explain the accelerated evolution of renal failure in patients with an inflamed substrate prone to cardiovascular disease.
Some studies have revealed that serum levels of various proinflammatory cytokines, chemokines and adhesion molecules, particularly TNF-α and IP-10, were associated with the severity of DN and of atherosclerosis[33]. These molecules could be useful markers for the progression of DN and atherosclerosis.
In conclusion, in our study of a cohort of patients with biopsy-proven diabetic nephropathy and kidney failure, we found that a history of CVD was an independent progression factor for diabetic nephropathy and that levels of HbA1c less than 7% could favor renal progression, especially in cases with associated vascular disease. Whether this accelerated progression is due to renal vascular disease or to an underlying inflammatory state could not be clarified in this study.
It is necessary to diagnose diabetic patients at risk for cardiovascular disease and kidney disease progression before these lesions become irreversible. The biochemical parameters normally used in clinical settings are not good markers of renal progression. Prospective studies should be undertaken to evaluate the usefulness more refined parameters, such as cystatine C clearance and inflammatory and early vascular damage markers, in diabetic patients to detect and treat these patients earlier.
We thank Juan Orive and Kim Ball for their inestimable assistance in translating this article.
Diabetes mellitus (DM) is one of the leading causes of end-stage kidney disease. Different forms of presentation and progression of diabetic nephropathy have been described, both in DM1 and DM2.
The prognostic factors of diabetic nephropathy (DN) have not been well established, nor have been the indicators for identifying patients at greater risk for progression. Further translational studies should be performed to increase knowledge of the etiopathogenic mechanisms and treatment of this type of nephropathy.
This study supports that glomerular lesions were the basic substrates responsible for renal insufficiency in a subgroup of diabetic patients. DN sometimes presents with rapid progression despite proteinuria. It is probable that glomerular lesions and cardiovascular disease in diabetic patients share a common substrate that implies a worse prognosis for these patients. Further studies are needed to support the theory of a possible negative renal effect of strict metabolic control in patients with established diabetic nephropathy.
Serum creatinine and proteinuria are not early markers to detect the risk of progression in DN. The threshold of eGFR, less than which renal function must be monitored, should be much higher in diabetic patients than in other chronic kidney disease patients, especially if there are associated cardiovascular risk factors. Authors should be cautious in metabolic control of patients with cardiovascular disease and DN.
Diabetic nephropathy: Renal complications of diabetes; Histopathological diagnosis: The histopathological diagnosis of DN is based on light and electron microscopic glomerular lesions. Tubulointerstitial and vascular lesions often accompany glomerular changes, but they are not specific to diabetes; Silent disease: This term describes ischemic heart disease in diabetic patients who presents as myocardial ischemia without angina. In this study, the authors have extrapolated this term to nephropathy to refer to the way it presents, with hardly any clinical renal expression until advanced stages of illness.
This is an interesting observational study on the clinical course of DN, focusing in particular on a novel phenotype called silent DN.
P- Reviewers: Duan SB, Olowu WA, Kirmizis DA S- Editor: Song XX L- Editor: A E- Editor: Liu XM
| 1. | Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320:1161-1165. [PubMed] |
| 2. | Mooyaart AL, Valk EJ, van Es LA, Bruijn JA, de Heer E, Freedman BI, Dekkers OM, Baelde HJ. Genetic associations in diabetic nephropathy: a meta-analysis. Diabetologia. 2011;54:544-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171:1920-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Premaratne E, Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith T, Jerums G. Renal hyperfiltration in type 2 diabetes: effect of age-related decline in glomerular filtration rate. Diabetologia. 2005;48:2486-2493. [PubMed] |
| 5. | Doublier S, Lupia E, Catanuto P, Elliot SJ. Estrogens and progression of diabetic kidney damage. Curr Diabetes Rev. 2011;7:28-34. [PubMed] |
| 6. | Mogensen CE. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest. 1976;36:383-388. [PubMed] |
| 7. | Forsblom CM, Groop PH, Ekstrand A, Tötterman KJ, Sane T, Saloranta C, Groop L. Predictors of progression from normoalbuminuria to microalbuminuria in NIDDM. Diabetes Care. 1998;21:1932-1938. [PubMed] |
| 8. | Mogensen CE. Microalbuminuria and hypertension with focus on type 1 and type 2 diabetes. J Intern Med. 2003;254:45-66. [PubMed] |
| 9. | Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55:1832-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 696] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 10. | Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving HH. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004;66:1596-1605. [PubMed] |
| 11. | Moriya T, Tanaka S, Kawasaki R, Ohashi Y, Akanuma Y, Yamada N, Sone H, Yamashita H, Katayama S. Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan Diabetes Complications Study. Diabetes Care. 2013;36:2803-2809. [PubMed] |
| 12. | Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011;29:1802-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036-1040. [PubMed] |
| 15. | Onuigbo MA. Evidence of the syndrome of rapid onset end-stage renal disease (SORO-ESRD) in the acute kidney injury (AKI) literature - preventable causes of AKI and SORO-ESRD-a call for re-engineering of nephrology practice paradigms. Ren Fail. 2013;35:796-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol. 2010;32:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 1117] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 18. | Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825-830. [PubMed] |
| 19. | Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [PubMed] |
| 20. | Okada T, Nagao T, Matsumoto H, Nagaoka Y, Wada T, Nakao T. Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton). 2012;17:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Mohsen A, Brown R, Hoefield R, Kalra PA, O’Donoghue D, Middleton R, New D. Body mass index has no effect on rate of progression of chronic kidney disease in subjects with type 2 diabetes mellitus. J Nephrol. 2012;25:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695-1702. [PubMed] |
| 23. | Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, Nissenson AR, Krishnan M, Kopple JD, Mehrotra R. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Arch Intern Med. 2008;168:2440-2447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172:761-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 26. | Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34 Suppl 2:S132-S137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 27. | Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14 Suppl 1:S51-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Yaffe K, Falvey CM, Hamilton N, Harris TB, Simonsick EM, Strotmeyer ES, Shorr RI, Metti A, Schwartz AV. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 29. | Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 710] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 30. | MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, Matthews PG, Thomas MC, Power DA, Jerums G. Is nonalbuminuric renal insufficiency in type 2 diabetes related to an increase in intrarenal vascular disease? Diabetes Care. 2006;29:1560-1566. [PubMed] |
| 31. | Araki S, Nishio Y, Araki A, Umegaki H, Sakurai T, Iimuro S, Ohashi Y, Uzu T, Maegawa H, Kashiwagi A. Factors associated with progression of diabetic nephropathy in Japanese elderly patients with type 2 diabetes: sub-analysis of the Japanese Elderly Diabetes Intervention Trial. Geriatr Gerontol Int. 2012;12 Suppl 1:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Uzu T, Kida Y, Shirahashi N, Harada T, Yamauchi A, Nomura M, Isshiki K, Araki S, Sugimoto T, Koya D. Cerebral microvascular disease predicts renal failure in type 2 diabetes. J Am Soc Nephrol. 2010;21:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Kajitani N, Shikata K, Nakamura A, Nakatou T, Hiramatsu M, Makino H. Microinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;88:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |