Published online Mar 25, 2025. doi: 10.5527/wjn.v14.i1.101917
Revised: October 24, 2024
Accepted: January 3, 2025
Published online: March 25, 2025
Processing time: 111 Days and 16.7 Hours
When kidney function declines to a point where it can no longer maintain life and requires renal replacement therapy (i.e. renal transplant or dialysis), it is called end-stage renal disease (ESRD). Patients with ESRD often experience a range of gastrointestinal (GI) symptoms, with prevalence rates reported as high as 77%-79%. These symptoms and pathologies arise from various factors, including electrolyte imbalance, fluid imbalance, toxin buildup, uremia, medications, dietary and lifestyle restrictions, and the effects of dialysis. GI diseases in patients with renal failure can be further categorized into upper GI, small bowel, and lower GI issues. Common conditions include gastroesophageal reflux disease, nausea and vomiting, dysmotility within the esophagus and stomach, upper GI bleeding, peptic ulcer bleeding, angioectasia, irritable bowel syndrome, mesen
Core Tip: Due to electrolyte imbalance, fluid imbalance, toxin buildup, uremia, medications, dietary and lifestyle restrictions, and the effects of dialysis, patients with renal failure experience gastrointestinal complications with a prevalence of 77%-79%. Common conditions include gastroesophageal reflux disease, nausea and vomiting, dysmotility within the esophagus and stomach, upper gastrointestinal bleeding, peptic ulcer bleeding, angioectasia, irritable bowel syndrome, mesenteric ischemia, angiodysplasia, diverticular disease, constipation, pancreatitis, and diseases associated with peritoneal dialysis peritonitis and peritoneal stenosis.
- Citation: Khan A, Mushtaq M, Movva G, Sohal A, Yang J. Gastrointestinal disease in end-stage renal disease. World J Nephrol 2025; 14(1): 101917
- URL: https://www.wjgnet.com/2220-6124/full/v14/i1/101917.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i1.101917
Renal failure is a progressive disease caused by various pathophysiological mechanisms and disease processes, which leads to the progressive loss of nephrons with a decline in the ability of the body to maintain homeostasis, causing fluid retention, electrolyte imbalance, and pH disturbances[1]. When kidney function declines to a point where it can no longer maintain life and requires renal replacement therapy (i.e. renal transplant or dialysis), it is called end-stage renal disease (ESRD), which usually occurs when the glomerular filtration rate is below 15 mL per minute per 1.73 m2[2]. Disruption of homeostasis with an imbalance of electrolytes and build-up of various toxins leads to different clinical, metabolic, and biochemical disturbances in these patients.
Patients with ESRD have various gastrointestinal (GI) symptoms, with a prevalence as high as 77%-79%[3]. Underlying these symptoms is the broad spectrum of GI diseases affecting the entirety of the GI system. These symptoms and pathologies result from various factors, including electrolyte imbalance, fluid imbalance, toxin buildup, uremia, medications, dietary and lifestyle restrictions, and the effects of dialysis[4]. According to the United States Renal Data System, currently, there are approximately 815142 ESRD patients in the United States[5], which is expected to increase to one million by 2030[6]. As this population is expected to grow, understanding the impact of ESRD on the GI system is paramount.
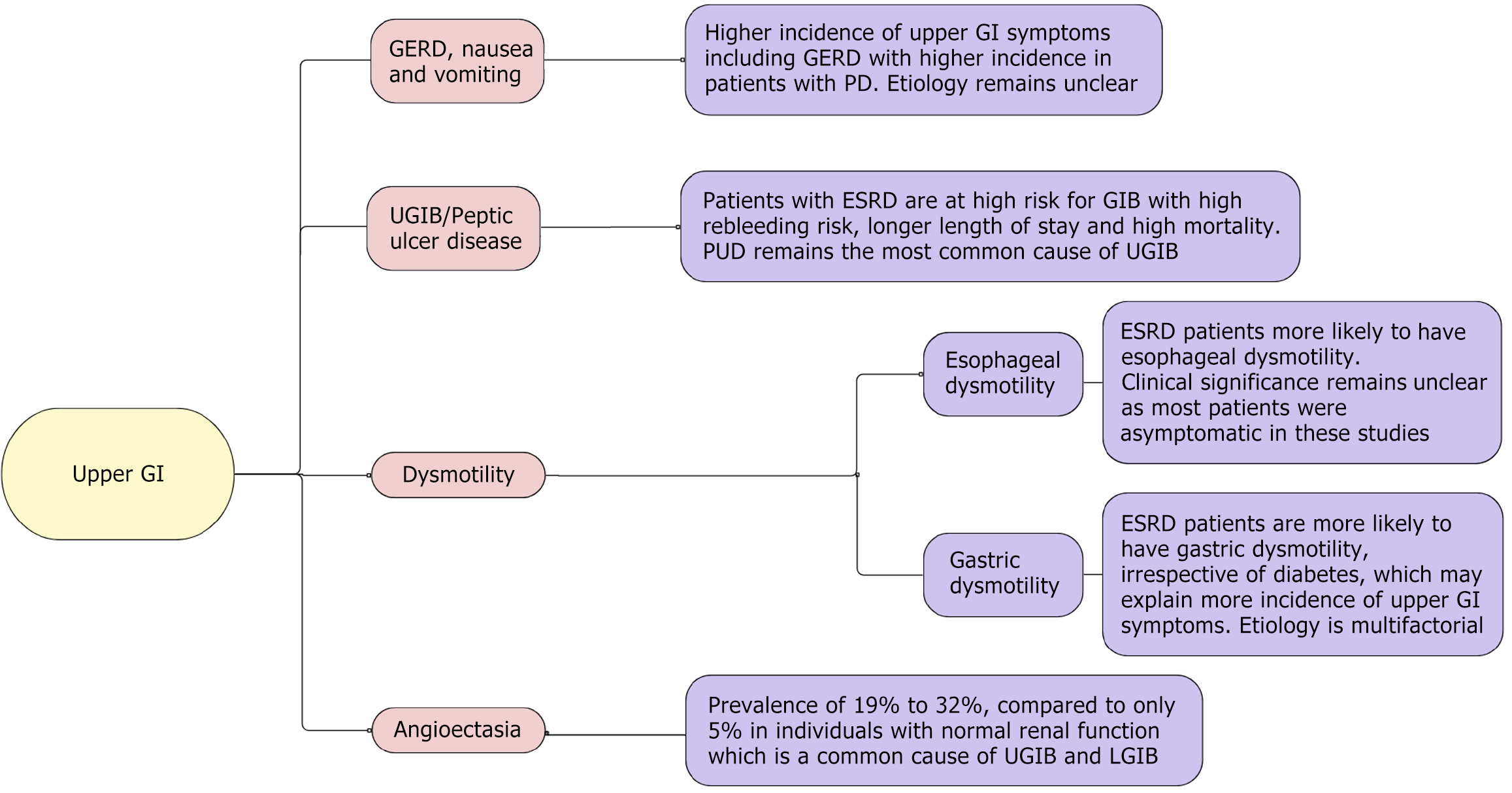
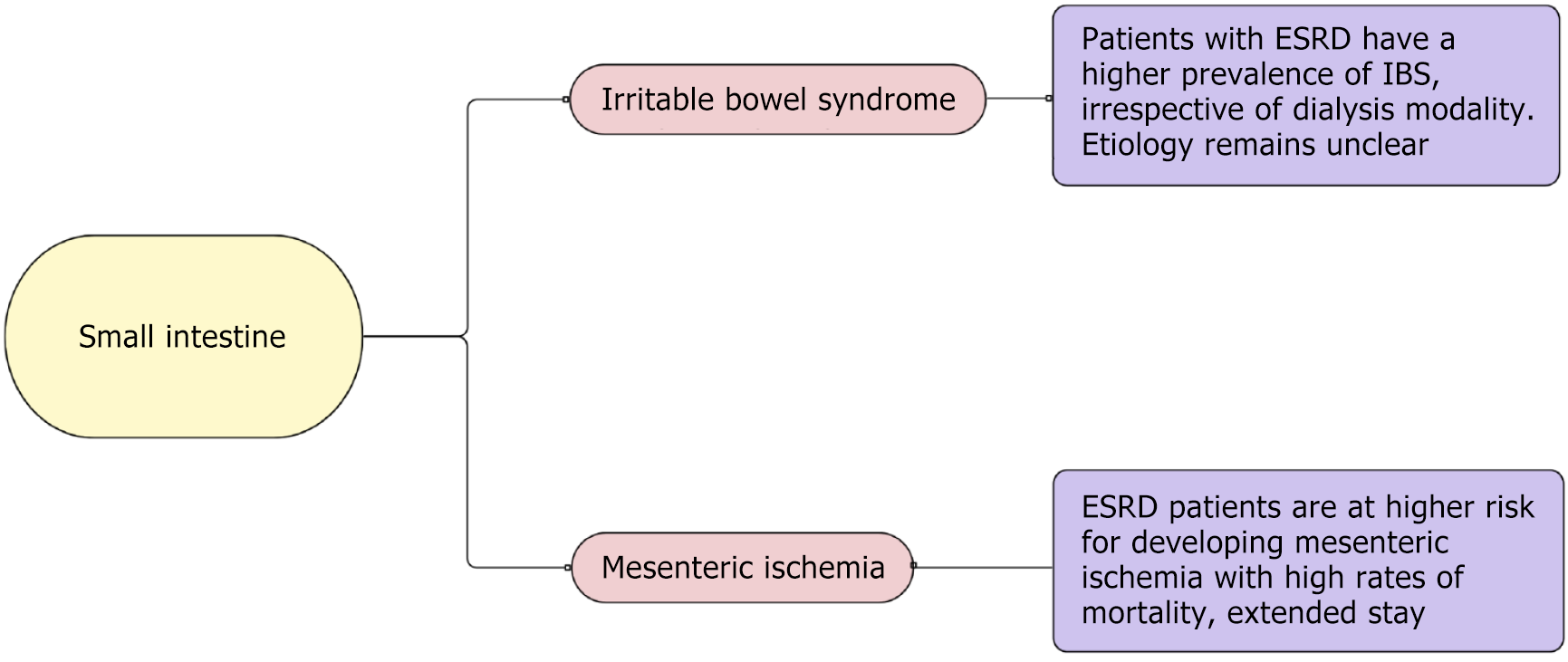
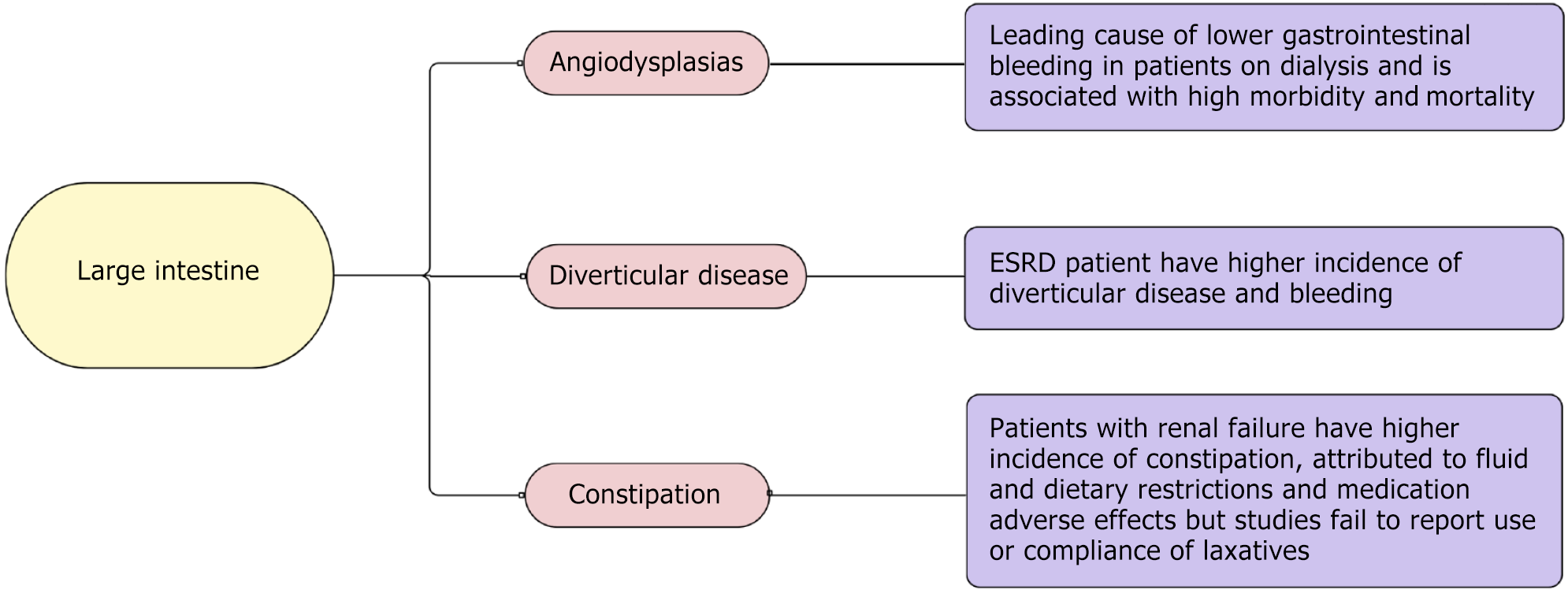
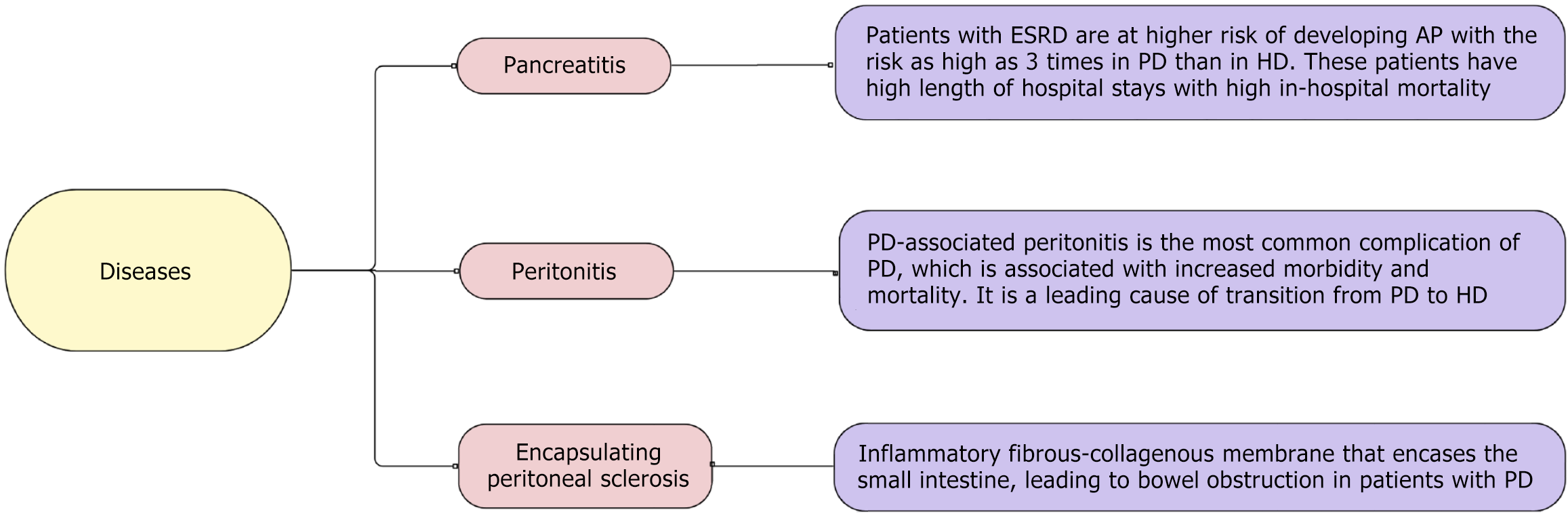
In this review, we highlight various GI pathologies encountered routinely in patients with ESRD in the light of current evidence, including upper GI diseases (Figure 1), small intestine diseases (Figure 2), and large intestine diseases (Figure 3) in ESRD based on anatomic location within the GI system, and other GI diseases in ESRD (Figure 4).
Patients with ESRD reportedly have a higher incidence of dyspepsia, gastroesophageal reflux disease (GERD), abdominal pain, nausea, vomiting, and eating disorders[3,7]. Using the Gastrointestinal Symptom Rating Scale, which assesses abdominal pain syndrome, constipation syndrome, reflux syndrome, indigestion syndrome, and diarrhea syndrome, along with evaluating for eating, Strid et al[8] showed that patients on dialysis had higher scores compared to controls. These upper GI symptoms were found to have a higher incidence in patients treated with peritoneal dialysis (PD) compared to hemodialysis (HD)[9].
The etiology remains unclear and is likely multifactorial. Given the higher incidence in PD, increased intraperitoneal pressure and decreased lower esophageal sphincter pressure are considered to cause GERD in the patient population. However, no association has been established between the level of intraperitoneal dialysate pressure and GERD[10]. Meanwhile, studies assessing decreased lower esophageal sphincter (LES) pressure and GERD with dialysate have also had mixed results[11,12]. While the symptoms are attributed to motility disorder (discussed later), the association has not been clear. Uremia and fluid overload can also be contributory; however, interestingly, Wang et al[13] showed that patients with late-stage chronic kidney disease (CKD), who would have higher uremia and fluid overload, did not have an increased incidence and risk of GERD compared with patients with early-stage CKD.
Patients with CKD experience a higher prevalence of upper GI symptoms, leading to increased use of acid-suppressive therapy. A single-center study from Korea demonstrated that low-dose proton pump inhibitors (PPIs) have a protective effect in preventing UGIB[14]. The incidence of upper GI bleeding (UGIB) was notably high in patients with ESRD, but the use of PPIs was significantly associated with a reduction in UGIB events[14]. Desbuissons and Mercadal[15] showed that using PPIs in patients with ESRD significantly improved quality of life and reduced bleeding but without overall survival benefit. Concerning the use of PPIs in CKD, several studies have shown that long-term PPI use is associated with the progression of CKD[16]. Rodríguez-Poncelas et al[17] found an association between high-dose (twice daily) PPI use and CKD, with a risk increase of up to 15% compared to once-daily use. This risk became more pronounced after 3 months of continuous use[18]. Other side effects with use of PPI include increased bone fragility, increased cardiovascular risk, and increased risk of death. Thus, PPI prescribing in ESRD should be weighed against the risk[15].
ESRD is associated with esophageal dysmotility. Francis first evaluated the motility disorder in 1984, when 5 patients on chronic PD were evaluated for motility with manometry and found to have esophageal dysmotility and achalasia. All of the patients had symptoms varying from nausea, vomiting, odynophagia, or dysphagia and none of the patients had diabetes or alcohol use[17]. Later, Siamopoulos et al[19] and Doğan et al[20] studied esophageal motility in asymptomatic patients using manometry. Siamopoulos et al[19] found that patients had tertiary contractions in the esophageal body with normal LES resting tone. Doğan et al[20] found higher contraction amplitudes with high mid-esophageal velocities with high resting LES tone. Kuwahara et al[21] used the high-viscosity liquid to assess esophageal motility with findings similar to Doğan et al[20]. Patients in all of these studies did not have diabetes, alcohol, or prokinetic drug use. Kayataş et al[22] studied a different approach and used radionuclide scintigraphy to measure esophagus transit time (ETT) in 26 patients with ESRD and 10 healthy patients to evaluate motility, but the study did not find a significant difference in ETT between groups. Interestingly, the ESRD group included 15 patients on dialysis and 11 patients on predialysis, and subanalyses of the group showed statistically significant differences in ETT between the groups with higher ETT in the predialysis group, highlighting the possible role of uremic neuropathy in motility disorder, which can be reversible with dialysis[22]. While motility disorder has been attributed to uremic neuropathy and axonal degeneration due to a chronic uremic state in patients with ESRD, other factors, i.e. electrolyte imbalance and high peptide plasma levels, have also been proposed to contribute to the dysmotility in these patients but has not been thoroughly studied[21]. While most studies are consistent with esophageal dysmotility, its clinical significance remains unclear as most patients were asymptomatic in these studies.
Patients with ESRD experience more early satiety, reflux, and eating dysfunction, which could be due to slow gastric motility. The pathogenesis of gut dysmotility in chronic renal failure (CRF) is multifactorial. It has been elucidated that mucosal edema, abnormal gastric myoelectrical activity, impaired autonomic function, the toxic effects of uremia, and accumulation of peptide hormones, including cholecystokinin, gastrin, secretin, and pancreatic polypeptide levels, may play a role in dysmotility[1,23]. Multiple studies from 1985 to 2019 have analyzed gastric motility in patients with CRF and ESRD, as highlighted in Table 1[23-34].
| Ref. | Study population | Results |
| Freeman et al[24], 1985 | Age: 29-67 years, 9 patients on pre-dialysis | No difference with liquid or solids between renal failure group and controls |
| Brown-Cartwright et al[25], 1988 | 10 patients with ESRD on CAPD, 15 controls | Delayed emptying in 5/10 patients with CAPD with dialysate, normal when dialysate removed. Excluded: Diabetics |
| Bird et al[26], 1994 | 20 CAPD (9/20 patients with diabetes), 8 controls | 9/20 (4 diabetic and 5 non-diabetic) delayed solid emptying, among which 4 also had delayed liquid emptying |
| Fernström et al[27], 1999 | 30 CAPD, 160 healthy controls | Half emptying time was prolonged. Retention at 90 and 120 minutes were higher, and gastric emptying rate was slower in male patients with CAPD compared to male controls. No significant differences were found in postprandial egg. |
| Lin et al[23], 1997 | 24 symptomatic patients with CRF (age 28-83 years; 15/24 patients with diabetes), 12 asymptomatic controls (median age: 36) | Patients with CRF showed a significantly lower percentage of normal 2-4 cpm waves in both fasting and fed states. The prevalence of an abnormal response of the egg to the test meal (no increase or decrease in egg dominant power after the test meal) was significantly higher in patients with CRF. There was no statistical difference between diabetic and non-diabetic patients with CRF. |
| Lee et al[28], 2000 | 41 patients: 22 HD, 19 CAPD | There was no difference in the normal slow-wave frequency between patients on HD and CAPD. There was a significant correlation between changes in tachygastria after CAPD and grade of early satiety in patients on CAPD with UGI symptoms. Dialysis modalities seem to affect gastric myoelectrical activity differently in patients with ESRD. Excluded: Diabetics |
| Stompór et al[29], 2002 | 20 CAPD (mean age: 501 ± 11 years), no control group | Gastric emptying is markedly impaired in patients on CAPD compared to healthy subjects. However, the presence of dialysate does not influence it significantly. |
| Strid et al[30], 2004 | 39 CRF, 131 controls | Delayed gastric emptying is common in patients with chronic renal failure, particularly in men. The delay was not associated with the presence of GI symptoms, underlying renal disease or H. pylori infection. However, the dialytic status might have an impact on gastric emptying in patients with CRF. Excluded: Diabetics |
| Hubalewska et al[31], 2004 | 20 patients on CAPD (mean age: 50 ± 11 years), 15 controls | Gastric emptying in subjects with chronic renal failure treated with CAPD is markedly delayed compared to healthy subjects. There was no significant effect of indwelling dialysate in the peritoneal cavity on gastric emptying rates found, based on the observation that its removal was not associated with any noticeable improvement of gastric emptying. The data strongly contraindicate the theory of peritoneal dialysate volume being the cause of this reversible disorder and indicate that the role of other possible factors leading to the development of gastropathy in those patients should be investigated. Excluded: Diabetics |
| Hirako et al[32], 2005 | 21 patients with ESRD just diagnosed before initiation of dialysis (7/21 were patients with diabetes), 21 controls | Low hypogastria post prandial in CRF, high bradygastria in both fasting and postprandial states. Low power ratio in CRF, no difference between diabetic and non-diabetic. Delayed gastric emptying in CRF, no difference between diabetic and non-diabetic. Inverse correlation between normogastria and emptying. A significant relation between bradygastria and GI symptoms but no other association suggesting delayed motility may not explain the symptoms. The patients with CRF showed gastric hypomotility, including impaired gastric myoelectrical activity and delayed gastric emptying. Gastric hypomotility appears to be an important factor in the generation of GI symptoms in patients with CRF |
| Adachi et al[33], 2007 | 19 patients with CRF on HD, 12 controls | Eleven patients had normal gastric motor function and eight showed abnormalities of either gastric myoelectrical activity or gastric emptying. More than half of the patients with CRF on HD demonstrated normal gastric motility, and no or slight GI symptoms |
| Broberg et al[34], 2019 | 24 patients with ESRD on chronic HD (further divided into 3 groups: Normal glucose tolerance, impaired glucose tolerance, diabetics), 8 controls | Altered gastrointestinal motility in dialysis patients, with higher gastric retention and prolonged gastric emptying, and higher total AUC of GIP and glucagon independent of the presence of diabetes or prediabetes |
With the exception of the initial study by Freeman et al[24], which showed no significant difference in gastric emptying times between patients with renal failure and healthy controls, all other studies in the table demonstrated impairment in gastric emptying among patients with dialysis compared to healthy subjects. However, the populations in these studies were relatively small. While most of the studies in the chart excluded patients with diabetes, studies conducted by Lin et al[23], Bird et al[26], and Hirako et al[32] found no statistically significant difference in gastric emptying between diabetic and non-diabetic patients with CRF. In a study by Lee et al[28], different dialysis modalities appeared to affect gastric myoelectrical activity differently in these patients.
Patients with ESRD are at high risk of GI bleeding (GIB), a risk that is five-fold higher than the general population[35,36]. Several different factors together play a role, including age, poor nutritional status, other medical comorbidities (diabetes, congestive heart failure, etc.), platelet dysfunction due to toxins, use of heparin products with HD, and use of antiplatelets in the patient population[37-40]. The incidence of bleeding has been on a steady rise over the years[38]. While the shift from PD to HD is thought to be a possible cause for the rise in GIB, given the exposure of blood to artificial dialysate membrane and use of heparin products with HD, however, when matched for dialysis type, it failed to explain the current rise in the incidence of GIB in this population[4]. Etiologies of UGIB in these patients are similar to the general population and include peptic ulcer disease (PUD), gastric erosions, vascular ectasia, esophagitis, Mallory-Weiss tears, and gastric cancer; however, PUD has been recorded to account for up to 60% of UGIB cases[37,41].
In early studies, CKD and UGIB were more linked to mucosal inflammatory changes causing gastritis and esophagitis, and ESRD was not considered to increase the risk of PUD[42-45]. Thus, the role of renal failure in PUD remained controversial. However, recent studies have found renal failure to be independently associated with high PUD. Sugimoto et al[46] showed that patients on dialysis had higher rates of gastritis (77.8% vs 46.8%), gastric ulcers (11.4% vs 4.1%), and duodenal ulcers (6.4% vs 3.3%). Huang et al[47] also showed that patients with CKD and ESRD receiving PD or HD were independently associated with PUD in a population-wide study. While both dialysis modalities carry a higher risk of PUD, the risk of PUD was higher in patients on HD compared to PD[35,47]. Several other factors associated with a high incidence of PUD in this population include age, diabetes, hypertension, cirrhosis, coronary artery disease, use of nonsteroidal anti-inflammatory drugs, and low albumin levels[47-49].
Patients with ESRD with PUD bleeding have a greater rebleeding risk, require more blood transfusions, longer length of stay, and have a high mortality, making them high risk[50,51]. The risk of rebleeding can be reduced with high-dose PPI and early endoscopic intervention to achieve homeostasis[52]. Their risk of re-bleeding is not just acutely high, but they are also predisposed to long-term risk of peptic ulcer rebleeding; the enhanced risk gradually decreases after the first year and stabilizes after 5 years[53]. The high risk of delayed ulcer rebleeding in these patients could be due to poor nutritional status, platelet dysfunction, and ischemic changes of the GI tract[54].
The most common causes of PUD include Helicobacter pylori and nonsteroidal anti-inflammatory drug (NSAID) use. Interestingly, the incidence of H. pylori is significantly lower in patients with ESRD compared to patients with normal kidney function, and the risk further decreases with time on dialysis[47]. However, early H. pylori eradication has been associated with a low incidence of PUD[55,56]. Some studies have shown that even after the eradication of H. pylori, these patients have a high recurrence of PUD, which highlights that there are likely more factors at play than H. pylori[57]. Meanwhile, the use of antiplatelets and anticoagulants due to comorbid disease puts the patient population at higher risk of PUD. CKD/ESRD alone does not indicate the use of prophylaxis for PUD, given the risks associated with the use of PPI long term.
Meanwhile, patients with age > 65, high-dose NSAIDs, history of previous uncomplicated peptic ulcer, and conco
Angiodysplasias has been identified as a common cause of UGIB in patients with ESRD and is a frequent cause of recurrent GIB in this population. The terms angiodysplasia, arteriovenous malformations, and vascular ectasia are used interchangeably. While discussed in the context of UGIB, these lesions are found throughout the digestive tract and thus can be a culprit lesion for UGIB and lower GIB (LGIB). The prevalence of angiodysplasia in patients with renal failure ranges from 19% to 32%, compared to only 5% in individuals with normal renal function[59].
Zuckerman et al[60] reported the incidence of angiodysplasia to be significantly higher in patients with renal disease compared to patients without renal disease (53% vs 11%) causing UGIB. Microscopically, angiodysplasias consist of thin-walled distorted vessels lined by endothelium, and infrequently, a thin smooth muscle layer[61]. Meanwhile, endoscopically, they appear as cherry red 2-10 mm flat or slightly raised lesions above the mucosal surface[60]. The etiology of angiodysplasia in renal disease is still unknown. Still, several factors have been attributed to the increased incidence in this population, including aging, uremic platelet dysfunction, defective calcium-phosphorus metabolism, use of antiplatelets and anticoagulants with aid from comorbid cardiovascular conditions, and diabetes[59,62].
Management of angiodysplasia can be challenging due to recurrent bleeding, given that patients usually have more than one lesion and sometimes in an entirely different portion of the GI tract. While initial assessment requires esophagogastroduodenoscopy or colonoscopy, if this fails to detect the lesion, other modalities are used including capsule endoscopy, push enteroscopy, and device-assisted enteroscopy (single or double balloon enteroscopy)[63]. Initial management includes endoscopic coagulation therapy with argon plasma coagulation. In patients with recurrent bleeding, besides endoscopic intervention, octreotide, estrogen, thalidomide, and desmopressin have been proposed[64-68]. Patients with life-threatening refractory bleeding may require surgical intervention.
While the mechanism is not fully understood, patients with ESRD have a higher prevalence of irritable bowel syndrome (IBS), irrespective of dialysis modality[3,69,70]. Several possible mechanisms have been proposed to explain the relationship between IBS and ESRD including uremia altering gut motility, the role of comorbid psychological conditions, or alteration of “kidney-colon-axis”[71]. Several studies have found the association between IBS and ESRD exists even after matching populations for psychological disorders[7,70], elucidating the direct relationship between kidney and gut function. Further studies are needed to investigate direct relationships.
Mesenteric ischemia is an uncommon disorder but has a very high mortality rate. Patients with ESRD are at higher risk of developing mesenteric ischemia, a risk as high as 44.1% compared to the general population[72]. Moreover, compared with patients with mesenteric ischemia without ESRD, patients with ESRD have higher rates of mortality, extended hospital stays, and healthcare costs[73]. Overlying symptoms between peritonitis (in patients on PD) and mesenteric ischemia can lead to a delay in diagnosis, and hence, higher mortality[4]. While the patient population is usually older and has comorbid extensive vascular disease with atherosclerosis, which puts them at higher risk of ischemia, intradialytic hypotension and instability contribute to a higher incidence of mesenteric ischemia[4,74]. Given the high mortality rate, a high index of suspicion is required to make a timely diagnosis.
Patients with ESRD on dialysis have a higher incidence of angiodysplasia compared to the general population and even patients with CKD[75]. Angiodysplasias are the leading cause of LGIB in patients on dialysis, with a rise in the number of hospitalizations[4,76] and high morbidity and mortality[77]. While it is localized mainly in the ascending colon and cecum, angiodysplasia is found throughout the GI tract[4]. Bleeding due to angiodysplasia is generally occult, presenting as iron deficiency anemia in the patient population, while massive bleeding is also present. While 90% of cases have spontaneous resolution, rebleeding occurs in 25%-47% of cases and can be challenging[4]. Several factors, including multiple angiodysplastic lesions, supratherapeutic anticoagulation, and high bleeding rate, have been associated with a high risk of rebleeding. The diagnosis and management of angiodysplasia were discussed earlier.
While age is a significant risk factor for diverticulosis, patients with ESRD have a higher incidence of diverticular disease, especially in patients with HD, as reported by Lee et al[78]. Meanwhile, given the risk of peritonitis due to diverticulosis, which remains controversial, diverticular disease was once considered a relative contraindication for PD as well[39] but later studies failed to show the association. Historically, patients with diverticulitis post-transplantation had a high mortality rate; thus, it was once considered a relative contraindication for transplantation[79]. However, later studies showed better outcomes.
Patients with ESRD are at high risk of diverticulitis, as highlighted by Chang et al[80], who demonstrated that patients with ESRD have an 11.2% higher risk of developing diverticulitis; however, the study was limited to the Asian population. While patients with uncomplicated diverticulitis had successful recovery with medical management, patients with complicated diverticulitis, especially patients who required surgery, had worse outcomes and high mortality, which was also highlighted by Moran-Atkin et al[81]. Thus, long-term outcomes are paramount for this population’s early detection and medical management. The incidence of LGIB due to diverticulosis in patients on dialysis is reportedly not different from the general population, but Lee et al[78] showed that this patient population has a higher incidence of diverticular disease and bleeding, especially patients on HD. Salamone[79] showed that patients with ESRD with diverticular bleeding are likely to require more blood transfusion.
Patients with renal failure requiring dialysis have a higher incidence of constipation[3,82-84], which in some instances, can lead to fecal impaction with stercoral ulceration causing LGIB[4,85]. The incidence of constipation is reportedly higher in patients on HD compared to PD[82-84]. However, this finding could be skewed, given that patients on PD dialysis are more often on laxatives to prevent enteric peritonitis. The high incidence has been attributed to fluid and dietary restrictions and medication adverse effects such as exchange resins. Patients on dialysis usually have very low fiber intake[84]. Medications such as phosphate binders are also known to contribute to constipation. Objectively, patients on HD have a high colonic transit time compared to patients with PD[86]. However, most studies have failed to report the use or compliance of laxatives, which can be studied in the future.
Patients with ESRD on chronic dialysis are at higher risk of developing acute pancreatitis (AP)[87-89]. A longitudinal study by Chen et al[90] showed an incidence rate of 5.11 per 1000 person-years in patients on HD and 5.86 per 1000 person-years in PD patients. The risk of AP is up to 3-fold higher in PD than in HD[91]. These patients are also at high risk of developing severe pancreatitis compared to the general population[90]. They have increased in-hospital mortality, length of hospital stays, and hospitalization costs and charges[90-92].
Etiology for AP differs in patients with ESRD as they tend to have more idiopathic AP or hypercalcemia-related AP[87,89,92]. Besides general risk factors for pancreatitis, patients with ESRD have additional risk factors related to renal insufficiency (i.e. uremia, secondary hyperparathyroidism with hypercalcemia, decreased renal clearance of GI hormones, etc.), as well as aspects related to dialysis (i.e. ischemia related to dialysis, dialysate irritation etc.), which place them at higher risk of AP[93]. Elderly age, female sex, elderly age, severe pancreatitis, diabetes mellitus, and liver disease are associated with higher mortality rates with AP in the HD population[90,94]. Diagnosis can be challenging, given overlaps of clinical manifestation with peritonitis as well as altered lipase and amylase levels due to renal failure and dialysis[93]. While ruling out peritonitis is paramount, assessing markers other than lipase and amylase has been proposed, including combined assays of elastase 1 and lipase in HD and pancreatic-type amylase and ratio of P-type isoamylase to creatinine clearance[93]. Management is similar, which includes pain control, fluids with electrolytes, and metabolic corrections. Further research is needed to elucidate the biochemical association between ESRD and increased risk of PD.
PD is consistent with utilizing the peritoneal cavity and membrane to remove solutes, fluids, and toxins. The process involves filling the peritoneal cavity with sterile solutions of different osmolality via a permanent indwelling catheter in a process called exchange, which employs pores within the peritoneal cavity[95]. Dialysate solutions are primarily glucose-based and used in varying concentrations to promote exchange. They are now buffered with lactate or bicarbonate to reduce glucose degradation product generation during the sterilization process and advanced glycosylation products within the body. Solution buffered with lactate has lower pH (5.2), while bicarbonate-buffered solutions are neutral (7.2). A recent meta-analysis showed that neutral pH/low growth and differentiation factor is associated with preserving residual kidney function[96]. In clinical practice, neutral pH/low growth and differentiation factor is usually preserved for patients with infusion-related pain[95]. The most common complication associated with PD includes peritonitis, while encapsulating peritoneal sclerosis is a rare complication.
PD-associated peritonitis is the most common complication of PD, which is associated with increased morbidity and mortality, which can be > 15% with long-term effects on peritoneal membrane filtration capacity, becoming the leading cause of transitioning to HD[97,98]. Per the International Society for Peritoneal Dialysis, peritonitis is defined when two of the following are present: Positive effluent culture, clinical features consistent with peritonitis (e.g., abdominal pain, cloudy dialysis effluent), or dialysis effluent with white cell count > 100/μL or > 0.1 × 109/L, with > 50% polymorphonuclear leukocytes[98]. Etiologies of peritonitis can be divided into two broad categories: Culture-positive peritonitis, where the organism is identified, meanwhile culture-negative peritonitis can still be infectious (i.e. patients with recent antibiotics, poor sample), but other causes can include eosinophilic or chemical peritonitis due to dialysate[1,99]. The portal of entry for organisms is usually intraluminal (catheter) and periluminal (tunnel infection), followed by hematogenous, vaginal and intestinal translocation[1]. Patients with peritonitis should be started on empirical antibiotic therapy, preferably intraperitoneally, initiated as soon as possible covering Gram-positive (first-generation cephalosporins or vancomycin) and negatives (third-generation cephalosporins or aminoglycosides)[99].
Most recent guidelines also recommend draining dialysate and prophylactic antibiotics before gynecological and endoscopic procedures, given high rates of peritonitis when compared between groups with and without antibiotics (3.4% vs 8.5% for colonoscopy and 1.2% vs 3.2% for gastroscopy), respectively[100-103]. The most common organism isolated in this population was Escherichia coli. Currently, there are no specific recommendations for the choice of antibiotic, but any antibiotics targeted to cover E. coli can be used.
Encapsulating peritoneal sclerosis is a rare clinical syndrome caused by an inflammatory fibrous-collagenous membrane that encases the small intestine, leading to bowel obstruction in patients on PD[104]. The pathophysiology includes predisposing non-inflammatory sclerosis (due to repeated dialysis) and proinflammatory insult (medications, infection, other chronic inflammatory disease), leading to fibrogenesis with increased extracellular matrix production, ultimately leading to a fibro-collagenous peritoneal membrane[105]. Diagnosis is usually made clinically, while radiological evidence (abdominal X-ray, small bowel series, computer tomography) can assist with diagnosis[105]. Once the diagnosis is made, treatment includes stopping the offending agent (including stopping PD) and using corticosteroids +/- tamoxifen, wherever surgical intervention is reserved for cases that fail to improve with medical management[105].
Patients with ESRD have various GI symptoms that can be divided into various anatomic locations within the GI system. This review article highlights some of the common diseases encountered in patients with ESRD.
| 1. | Etemad B. Gastrointestinal complications of renal failure. Gastroenterol Clin North Am. 1998;27:875-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Wouk N. End-Stage Renal Disease: Medical Management. Am Fam Physician. 2021;104:493-499. [PubMed] |
| 3. | Cano AE, Neil AK, Kang JY, Barnabas A, Eastwood JB, Nelson SR, Hartley I, Maxwell D. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 2007;102:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Kızıltaş Ş, Şahin S, Şahin G. Lower Gastrointestinal Disorders Among Dialysis Patients. Turk Neph Dial Transpl. 2018;27:119-126. [DOI] [Full Text] |
| 5. | National Institute of Diabetes and Digestive and Kidney Diseases. ESRD Quarterly Update. [cited 3 July 2024]. Available from: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds/esrd-quarterly-update. |
| 6. | McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD Incidence and Prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 7. | Kahvecioglu S, Akdag I, Kiyici M, Gullulu M, Yavuz M, Ersoy A, Dilek K, Yurtkuran M. High prevalence of irritable bowel syndrome and upper gastrointestinal symptoms in patients with chronic renal failure. J Nephrol. 2005;18:61-66. [PubMed] |
| 8. | Strid H, Simrén M, Johansson AC, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Salamon K, Woods J, Paul E, Huggins C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J Ren Nutr. 2013;23:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Dejardin A, Robert A, Goffin E. Intraperitoneal pressure in PD patients: relationship to intraperitoneal volume, body size and PD-related complications. Nephrol Dial Transplant. 2007;22:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Kim MJ, Kwon KH, Lee SW. Gastroesophageal reflux disease in CAPD patients. Adv Perit Dial. 1998;14:98-101. [PubMed] |
| 12. | Hylander BI, Dalton CB, Castell DO, Burkart J, Rössner S. Effect of intraperitoneal fluid volume changes on esophageal pressures: studies in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1991;17:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Wang X, Wright Z, Patton-Tackett ED, Song G. The Relationship between Gastroesophageal Reflux Disease and Chronic Kidney Disease. J Pers Med. 2023;13:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Song YR, Kim HJ, Kim JK, Kim SG, Kim SE. Proton-pump inhibitors for prevention of upper gastrointestinal bleeding in patients undergoing dialysis. World J Gastroenterol. 2015;21:4919-4924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Desbuissons G, Mercadal L. Use of proton pump inhibitors in dialysis patients: a double-edged sword? J Nephrol. 2021;34:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Ang SP, Chia JE, Valladares C, Patel S, Gewirtz D, Iglesias J. Association between Proton Pump Inhibitor Use and Risk of Incident Chronic Kidney Disease: Systematic Review and Meta-Analysis. Biomedicines. 2024;12:1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Rodríguez-Poncelas A, Barceló MA, Saez M, Coll-de-Tuero G. Duration and dosing of Proton Pump Inhibitors associated with high incidence of chronic kidney disease in population-based cohort. PLoS One. 2018;13:e0204231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Francos GC, Besarab A, Joseph RE. Disorders of oesophageal motility in chronic haemodialysis patients. Lancet. 1984;1:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Siamopoulos KC, Tsianos EV, Dardamanis M, Berecos C. Esophageal dysfunction in chronic hemodialysis patients. Nephron. 1990;55:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Doğan I, Unal S, Sindel S, Tunçer C, Arinsoy T, Bali M, Kandilci U, Hasanoğlu E. Esophageal motor dysfunction in chronic renal failure. Nephron. 1996;72:346-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Kuwahara CM, Rosa-E-Silva L, Mocelin AJ, Zebian M, Pontes RM, Dantas RO. Esophageal Dysmotility in Chronic Hemodialysis Patients After Ingestion of Liquids With Different Viscosities. Gastroenterology Res. 2011;4:51-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Kayataş M, Ustundag Y, Okudan B, Gülçelik N, Köseoglu T. Evaluation of esophageal [correction of osephageal] motor function in chronic renal failure and the role of hemodialysis treatment. Nephron. 2002;91:534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Lin X, Mellow MH, Southmayd L 3rd, Pan J, Chen JD. Impaired gastric myoelectrical activity in patients with chronic renal failure. Dig Dis Sci. 1997;42:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Freeman JG, Cobden I, Heaton A, Keir M. Gastric emptying in chronic renal failure. Br Med J (Clin Res Ed). 1985;291:1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Brown-Cartwright D, Smith HJ, Feldman M. Gastric emptying of an indigestible solid in patients with end-stage renal disease on continuous ambulatory peritoneal dialysis. Gastroenterology. 1988;95:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Bird NJ, Streather CP, O'Doherty MJ, Barton IK, Gaunt JI, Nunan TO. Gastric emptying in patients with chronic renal failure on continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 1994;9:287-290. [PubMed] |
| 27. | Fernström A, Hylander B, Grybäck P, Jacobsson H, Hellström PM. Gastric emptying and electrogastrography in patients on CAPD. Perit Dial Int. 1999;19:429-437. [PubMed] |
| 28. | Lee SW, Song JH, Kim GA, Yang HJ, Lee KJ, Kim MJ. Effect of dialysis modalities on gastric myoelectrical activity in end-stage renal disease patients. Am J Kidney Dis. 2000;36:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Stompór T, Hubalewska-Hola A, Staszczak A, Sulowicz W, Huszno B, Szybinski Z. Association between gastric emptying rate and nutritional status in patients treated with continuous ambulatory peritoneal dialysis. Perit Dial Int. 2002;22:500-505. [PubMed] |
| 30. | Strid H, Simrén M, Stotzer PO, Abrahamsson H, Björnsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Hubalewska A, Stompór T, Płaczkiewicz E, Staszczak A, Huszno B, Sułowicz W, Szybiński Z. Evaluation of gastric emptying in patients with chronic renal failure on continuous ambulatory peritoneal dialysis using 99mTc-solid meal. Nucl Med Rev Cent East Eur. 2004;7:27-30. [PubMed] |
| 32. | Hirako M, Kamiya T, Misu N, Kobayashi Y, Adachi H, Shikano M, Matsuhisa E, Kimura G. Impaired gastric motility and its relationship to gastrointestinal symptoms in patients with chronic renal failure. J Gastroenterol. 2005;40:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Adachi H, Kamiya T, Hirako M, Misu N, Kobayashi Y, Shikano M, Matsuhisa E, Kataoka H, Sasaki M, Ohara H, Nakao H, Orito E, Joh T. Improvement of gastric motility by hemodialysis in patients with chronic renal failure. J Smooth Muscle Res. 2007;43:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Broberg B, Madsen JL, Fuglsang S, Holst JJ, Christensen KB, Rydahl C, Idorn T, Feldt-Rasmussen B, Hornum M. Gastrointestinal motility in patients with end-stage renal disease on chronic hemodialysis. Neurogastroenterol Motil. 2019;31:e13554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Luo JC, Leu HB, Huang KW, Huang CC, Hou MC, Lin HC, Lee FY, Lee SD. Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. CMAJ. 2011;183:E1345-E1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Niikura R, Aoki T, Kojima T, Kawahara T, Yamada A, Nakamura H, Inoue K, Morikoshi E, Migita R, Shimizu T, Kojima T, Koike K. Natural history of upper and lower gastrointestinal bleeding in hemodialysis patients: A dual-center long-term cohort study. J Gastroenterol Hepatol. 2021;36:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Toke AB. GI bleeding risk in patients undergoing dialysis. Gastrointest Endosc. 2010;71:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Trivedi H, Yang J, Szabo A. Gastrointestinal bleeding in patients on long-term dialysis. J Nephrol. 2015;28:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Kosmadakis G, Albaret J, da Costa Correia E, Somda F, Aguilera D. Gastrointestinal Disorders in Peritoneal Dialysis Patients. Am J Nephrol. 2018;48:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Lin Y, Li C, Waters D, Kwok CS. Gastrointestinal bleeding in chronic kidney disease patients: a systematic review and meta-analysis. Ren Fail. 2023;45:2276908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. 1996;91:2329-2332. [PubMed] |
| 42. | Prakash J, Agrawal BK. Upper gastrointestinal mucosal lesions in chronic renal failure. Indian J Gastroenterol. 1991;10:131-132. [PubMed] |
| 43. | Margolis DM, Saylor JL, Geisse G, DeSchryver-Kecskemeti K, Harter HR, Zuckerman GR. Upper gastrointestinal disease in chronic renal failure. A prospective evaluation. Arch Intern Med. 1978;138:1214-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Goenka MK, Kochhar R, Mehta SK, Nagi B, Malik AK, Chugh KS. Upper gastro-intestinal mucosal changes in patients with chronic renal failure. J Assoc Physicians India. 1989;37:564-566. [PubMed] |
| 45. | Kang JY, Wu AY, Sutherland IH, Vathsala A. Prevalence of peptic ulcer in patients undergoing maintenance hemodialysis. Dig Dis Sci. 1988;33:774-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Huang KW, Leu HB, Luo JC, Chan WL, Hou MC, Lin HC, Lee FY, Kuan YC. Different peptic ulcer bleeding risk in chronic kidney disease and end-stage renal disease patients receiving different dialysis. Dig Dis Sci. 2014;59:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Chen YT, Yang WC, Lin CC, Ng YY, Chen JY, Li SY. Comparison of peptic ulcer disease risk between peritoneal and hemodialysis patients. Am J Nephrol. 2010;32:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Kim M, Kim CS, Bae EH, Ma SK, Kim SW. Risk factors for peptic ulcer disease in patients with end-stage renal disease receiving dialysis. Kidney Res Clin Pract. 2019;38:81-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Cheung J, Yu A, LaBossiere J, Zhu Q, Fedorak RN. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Parasa S, Navaneethan U, Sridhar AR, Venkatesh PG, Olden K. End-stage renal disease is associated with worse outcomes in hospitalized patients with peptic ulcer bleeding. Gastrointest Endosc. 2013;77:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Lin SC, Wu KL, Chiu KW, Lee CT, Chiu YC, Chou YP, Hu ML, Tai WC, Chiou SS, Hu TH, Changchien CS, Chuah SK. Risk factors influencing the outcome of peptic ulcer bleeding in end stage renal diseases after initial endoscopic haemostasis. Int J Clin Pract. 2012;66:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Long-term peptic ulcer rebleeding risk estimation in patients undergoing haemodialysis: a 10-year nationwide cohort study. Gut. 2011;60:1038-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Tseng GY, Lin HJ. Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc. 2010;71:1333-4; author reply 1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Chang SS, Hu HY. Association between early Helicobacter pylori eradication and a lower risk of recurrent complicated peptic ulcers in end-stage renal disease patients. Medicine (Baltimore). 2015;94:e370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Hsu CH, Hu HY, Huang N, Chang SS. Early eradication has a lower risk of peptic ulcer bleeding in Helicobacter pylori-infected chronic kidney disease patients. Eur J Intern Med. 2016;33:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Tseng GY, Lin HJ, Fang CT, Yang HB, Tseng GC, Wang PC, Hung TL, Deng YC, Cheng YT, Huang CH. Recurrence of peptic ulcer in uraemic and non-uraemic patients after Helicobacter pylori eradication: a 2-year study. Aliment Pharmacol Ther. 2007;26:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 420] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 59. | Galanopoulos G. Angiodysplastic lesions as a cause of colonic bleeding in patients with chronic renal disease: is there an association? Saudi J Kidney Dis Transpl. 2012;23:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Zuckerman GR, Cornette GL, Clouse RE, Harter HR. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med. 1985;102:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 119] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Kaaroud H, Fatma LB, Beji S, Boubaker K, Hedri H, Hamida FB, El Younsi F, Abdallah TB, Maiz HB, Kheder A. Gastrointestinal angiodysplasia in chronic renal failure. Saudi J Kidney Dis Transpl. 2008;19:809-812. [PubMed] |
| 62. | Matesanz R, Teruel JL, Liaño F, Ortuño J. Angiodysplasia of the colon and chronic renal failure. Nephron. 1987;46:223-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | Verma S, Attallah MA, Jarrin Jara MD, Gautam AS, Khan S. Angiodysplasia in Renal Disease Patients: Analysis of Risk Factors and Approach to Manage Such Patients. Cureus. 2020;12:e9784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 64. | Chen H, Wu S, Tang M, Zhao R, Zhang Q, Dai Z, Gao Y, Yang S, Li Z, Du Y, Yang A, Zhong L, Lu L, Xu L, Shen X, Liu S, Zhong J, Li X, Lu H, Xiong H, Shen Y, Chen H, Gong S, Xue H, Ge Z. Thalidomide for Recurrent Bleeding Due to Small-Intestinal Angiodysplasia. N Engl J Med. 2023;389:1649-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 65. | Ge ZZ, Chen HM, Gao YJ, Liu WZ, Xu CH, Tan HH, Chen HY, Wei W, Fang JY, Xiao SD. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology. 2011;141:1629-37.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 66. | Goltstein LCMJ, Grooteman KV, Bernts LHP, Scheffer RCH, Laheij RJF, Gilissen LPL, Schrauwen RWM, Talstra NC, Zuur AT, Braat H, Hadithi M, Brouwer JT, Nagengast WB, Oort FA, Tenthof van Noorden J, Kievit W, van Geenen EJM, Drenth JPH. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024;166:690-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 67. | Livio M, Mannucci PM, Viganò G, Mingardi G, Lombardi R, Mecca G, Remuzzi G. Conjugated estrogens for the management of bleeding associated with renal failure. N Engl J Med. 1986;315:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 133] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Malyszko J, Pietraszek M, Buczko W, Mysliwiec M. Study on mechanisms of a haemostatic effect of 1 deamino-8-D-arginine vasopressin (desmopressin) in uraemic patients. Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:319-324. [PubMed] |
| 69. | Mortazavi M, Adibi P, Hassanzadeh Keshteli A, Feizi A, JameShorani M, Soodavi M, Jafari M. Comparison of Gastrointestinal Symptoms between Patients Undergoing Hemodialysis and Healthy Population. Middle East J Dig Dis. 2022;14:310-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 70. | Zuvela J, Trimingham C, Le Leu R, Faull R, Clayton P, Jesudason S, Meade A. Gastrointestinal symptoms in patients receiving dialysis: A systematic review. Nephrology (Carlton). 2018;23:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 71. | Costa-Moreira P, Vilas-Boas F, Teixeira Fraga A, Macedo G. Particular aspects of gastroenterological disorders in chronic kidney disease and end-stage renal disease patients: a clinically focused review. Scand J Gastroenterol. 2020;55:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Li SY, Chen YT, Chen TJ, Tsai LW, Yang WC, Chen TW. Mesenteric ischemia in patients with end-stage renal disease: a nationwide longitudinal study. Am J Nephrol. 2012;35:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Kumar V, Gala D, Green M, Shah M, Moparty H, Gayam VR, Bandaru P, Gokturk S, Reddy M, Gadaputi V. Outcomes of Acute Mesenteric Ischemia in End-Stage Renal Disease and Predictors of Mortality: A Nationwide Assessment. Cureus. 2023;15:e37657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Seong EY, Zheng Y, Winkelmayer WC, Montez-Rath ME, Chang TI. The Relationship between Intradialytic Hypotension and Hospitalized Mesenteric Ischemia: A Case-Control Study. Clin J Am Soc Nephrol. 2018;13:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Tsai TJ, Chen WC, Huang YT, Yang YH, Feng IC, Wu WC, Hu HM, Wu DC, Hsu PI. Hemodialysis Increases the Risk of Lower Gastrointestinal Bleeding and Angiodysplasia Bleeding: A Nationwide Population Study. Gastroenterol Res Pract. 2020;2020:7206171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Tariq T, Karabon P, Irfan FB, Goyal S, Mayeda MM, Parsons A, Judd S, Ehrinpreis M. Secondary angiodysplasia-associated gastrointestinal bleeding in end-stage renal disease: Results from the nationwide inpatient sample. World J Gastrointest Endosc. 2019;11:504-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Garlapati P, Gajjar B, Then EO, Gayam V. End-stage renal disease and lower gastrointestinal bleeding-A propensity-matched analysis of nationwide inpatient sample. Int J Clin Pract. 2021;75:e13633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 78. | Lee YC, Hung SY, Wang HH, Wang HK, Lin CW, Chang MY, Ho LC, Chen YT, Wu CF, Chen HC, Wang WM, Sung JM, Chiou YY, Lin SH. Different Risk of Common Gastrointestinal Disease Between Groups Undergoing Hemodialysis or Peritoneal Dialysis or With Non-End Stage Renal Disease: A Nationwide Population-Based Cohort Study. Medicine (Baltimore). 2015;94:e1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Salamone L. Outcomes in patients with end-stage renal disease and colonic diverticular disease. [cited 3 July 2024]. Available from: https://elischolar.library.yale.edu/ymtdl/454/. |
| 80. | Chang SS, Huang N, Hu HY. Patients with end-stage renal disease were at an increased risk of hospitalization for acute diverticulitis. Medicine (Baltimore). 2016;95:e4881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Moran-Atkin E, Stem M, Lidor AO. Surgery for diverticulitis is associated with high risk of in-hospital mortality and morbidity in older patients with end-stage renal disease. Surgery. 2014;156:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Zhang J, Huang C, Li Y, Chen J, Shen F, Yao Q, Qian J, Bao B, Yao X. Health-related quality of life in dialysis patients with constipation: a cross-sectional study. Patient Prefer Adherence. 2013;7:589-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Dong R, Guo ZY, Ding JR, Zhou YY, Wu H. Gastrointestinal symptoms: a comparison between patients undergoing peritoneal dialysis and hemodialysis. World J Gastroenterol. 2014;20:11370-11375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, Tochikubo O. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis. 2002;39:1292-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Saeed F, Kalra A, Kousar N, Pace LA, Holley JL. Stercoral ulcer as a cause of lower gastrointestinal (LGI) bleeding in chronic hemodialysis patients. Clin Nephrol. 2012;77:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, Shu KH, Tang MJ. Colonic transit time in long-term dialysis patients. Am J Kidney Dis. 2004;44:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Hou SW, Lee YK, Hsu CY, Lee CC, Su YC. Increased risk of acute pancreatitis in patients with chronic hemodialysis: a 4-year follow-up study. PLoS One. 2013;8:e71801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Bruno MJ, van Westerloo DJ, van Dorp WT, Dekker W, Ferwerda J, Tytgat GN, Schut NH. Acute pancreatitis in peritoneal dialysis and haemodialysis: risk, clinical course, outcome, and possible aetiology. Gut. 2000;46:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Cheungpasitporn W, Thongprayoon C, Ungprasert P, Wijarnpreecha K, Raimondo M, Kroner PT. Acute pancreatitis in end-stage renal disease patients in the USA: a nationwide, propensity score-matched analysis. Eur J Gastroenterol Hepatol. 2019;31:968-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Chen HJ, Wang JJ, Tsay WI, Her SH, Lin CH, Chien CC. Epidemiology and outcome of acute pancreatitis in end-stage renal disease dialysis patients: a 10-year national cohort study. Nephrol Dial Transplant. 2017;32:1731-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Padilla B, Pollak VE, Pesce A, Kant KS, Gilinsky NH, Deddens JA. Pancreatitis in patients with end-stage renal disease. Medicine (Baltimore). 1994;73:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Kroner PT, Mareth K, Raimondo M, Lee DD, Alsaad A, Aslam N, Abader P, Wadei HM. Acute Pancreatitis in Advanced Chronic Kidney Disease and Kidney Transplant Recipients: Results of a US Nationwide Analysis. Mayo Clin Proc Innov Qual Outcomes. 2019;3:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Barbara M, Tsen A, Rosenkranz L. Acute Pancreatitis in Chronic Dialysis Patients. Pancreas. 2018;47:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Wang H, Rong J, Song C, Zhao Q, Zhao R, Xie Y, Xiong H. Hemodialysis and risk of acute pancreatitis: A systematic review and meta-analysis. Pancreatology. 2021;21:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Auguste BL, Bargman JM. Peritoneal Dialysis Prescription and Adequacy in Clinical Practice: Core Curriculum 2023. Am J Kidney Dis. 2023;81:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 96. | Yohanna S, Alkatheeri AM, Brimble SK, McCormick B, Iansavitchous A, Blake PG, Jain AK. Effect of Neutral-pH, Low-Glucose Degradation Product Peritoneal Dialysis Solutions on Residual Renal Function, Urine Volume, and Ultrafiltration: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2015;10:1380-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 97. | Ling CW, Sud K, Peterson G, Fethney J, Van C, Patel R, Zaidi STR, Castelino R. Characteristics and outcomes of hospital-acquired and community-acquired peritonitis in patients on peritoneal dialysis: a retrospective cohort study. J Nephrol. 2023;36:1877-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 98. | Khan SF. Updates on Infectious and Other Complications in Peritoneal Dialysis: Core Curriculum 2023. Am J Kidney Dis. 2023;82:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 99. | Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, Kanjanabuch T, Kim YL, Madero M, Malyszko J, Mehrotra R, Okpechi IG, Perl J, Piraino B, Runnegar N, Teitelbaum I, Wong JK, Yu X, Johnson DW. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 313] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 100. | Chan GC, Wong SH, Ng JK, Li PK, Szeto CC, Chow KM. Risk of peritonitis after gastroscopy in peritoneal dialysis patients. Perit Dial Int. 2022;42:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Wu HH, Li IJ, Weng CH, Lee CC, Chen YC, Chang MY, Fang JT, Hung CC, Yang CW, Tian YC. Prophylactic antibiotics for endoscopy-associated peritonitis in peritoneal dialysis patients. PLoS One. 2013;8:e71532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Al-Hwiesh AK, Abdul-Rahman IS, Hussameldeen MA, Al-Audah N, Abdelrahman A, Moaigel HM, El-Salamony T, Noor AS, Al-Osail A, Al-Sayel D, Al-Dossari N. Colonoscopy in automated peritoneal dialysis patients: value of prophylactic antibiotics: a prospective study on a single antibiotic. Int J Artif Organs. 2017;40:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Gweon TG, Jung SH, Kim SW, Lee KM, Cheung DY, Lee BI, Choi H. Risk factors for peritonitis in patients on continuous ambulatory peritoneal dialysis who undergo colonoscopy: a retrospective multicentre study. BMC Gastroenterol. 2019;19:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20 Suppl 4:S43-S55. [PubMed] |
| 105. | Danford CJ, Lin SC, Smith MP, Wolf JL. Encapsulating peritoneal sclerosis. World J Gastroenterol. 2018;24:3101-3111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (5)] |