Published online Dec 25, 2024. doi: 10.5527/wjn.v13.i4.99643
Revised: August 26, 2024
Accepted: September 19, 2024
Published online: December 25, 2024
Processing time: 103 Days and 17.1 Hours
Minimal change disease (MCD) is a significant cause of idiopathic nephrotic syndrome (INS) in adults, representing approximately 10%-15% of INS cases. The data is scanty on clinicopathological features, treatment responses, and long-term outcomes of MCD in adults.
To determine the clinicopathologic characteristics, treatment responses, and medium-term outcomes of adult patients with MCD in Pakistan.
This retrospective cohort study included all adult patients with biopsy-proven MCD treated at the adult nephrology clinic, Sindh institute of urology and transplantation, between January 2010 and December 2020. The data was retri
The study cohort included 23 adults [15 (65.2% males), mean age 26.34 ± 10.28 years]. Hypertension was found in 7 (30.4%) and microscopic hematuria in 10 (43.4%) of participants. Laboratory findings revealed a mean serum creatinine of 1.03 ± 1.00 mg/dL, mean serum albumin of 1.94 ± 0.90 g/dL and mean 24-hour urinary proteins of 4.53 ± 2.43 g. The mean follow-up time was 38.09 ± 22.3 months. Treatment with steroids was effective in 16/18 (88.8%) of patients, with 10/16 (62.5%) achieving CR and 6/16 (37.5%) achieving PR. Two patients were resistant to steroids and required second-line immunosuppressive therapy. Relapse occurred in 4/20 (19.04%) of patients, with a mean time to first relapse of 6.5 ± 3.31 months. At the last follow-up, 18/20 (85.7%) of patients were in remission, and 16/20 (76.1%) maintained normal renal function. No patients progressed to end-stage renal disease or died.
MCD in adults shows a favorable response to steroid therapy, with a majority achieving remission. However, relapses are common, necessitating second-line immunosuppressive treatments in some cases. The study highlights the need for standardized treatment guidelines for adult MCD to optimize outcomes.
Core Tip: Minimal change disease (MCD) constitutes the third most common cause of nephrotic syndrome in adults. The information is scanty on the clinicopathological features, treatment responses, and long-term outcomes of MCD in adults, particularly from developing countries. This study revealed that MCD in adults shows a favorable response to steroid therapy, with a majority achieving remission. However, relapses are common, necessitating second-line immunosuppressive treatments in some cases. The study highlights the need for standardized treatment guidelines for adult MCD to optimize outcomes.
- Citation: Shakeel S, Rashid R, Jafry NH, Mubarak M. Adult minimal change disease: Clinicopathologic characteristics, treatment response and outcome at a single center in Pakistan. World J Nephrol 2024; 13(4): 99643
- URL: https://www.wjgnet.com/2220-6124/full/v13/i4/99643.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i4.99643
Minimal change disease (MCD) is the predominant cause of primary or idiopathic nephrotic syndrome (INS) in the pediatric population worldwide. Approximately 70% to 90% of INS is caused by MCD in younger children below 10 years of age, while it accounts for 50% of INS in older children and the prevalence declines with an increase in age. MCD accounts for approximately 10% to 15% of INS in adults[1]. Compared to children, causes of MCD in adults are more varied and not only include primary (idiopathic) but many secondary causes like non-steroidal anti-inflammatory drugs (NSAIDs), lithium, viral infections, and lymphoproliferative disorders[2].
MCD is defined clinically by nephrotic syndrome (NS) along with no glomerular lesions or minimal mesangial prominence on light microscopy, completely negative panel or minimal staining for C3 and IgM on immunofluorescence microscopy, and foot process effacement on electron microscopy. No electron-dense deposits are visualized[3].
In adults, renal biopsy and histologic diagnosis are warranted for definitive diagnosis before starting steroid therapy. Adults generally show a slower response to steroids, commencing as late as 3-4 months after initiating the therapy; hence, the clinical course and response to treatment are unpredictable. Apart from steroids, second-line immunosuppressive agents may be required to achieve the ultimate goal of complete remission (CR)[4-6].
In recent reports from different parts of the world, MCD is the third leading pathologic lesion presenting with NS in adults[7-10]. We have also earlier observed the same frequency of MCD among 316 adult nephrotic patients over 10 years, where it accounted for 15.82% of NS cases[11].
There is currently no information on detailed clinicopathologic characteristics of MCD in adults from Pakistan. This study will help in better understanding of clinical and pathologic aspects, treatment response, and outcome of MCD in adults, which has a relatively less predictable clinical course, response to treatment, and outcome in adults. The study aimed to determine the clinicopathological course, treatment responses, and long-term renal outcome of MCD in adults at a single center.
This retrospective observational study included adults with biopsy-proven MCD who presented to the Nephrology outpatient department, Sindh Institute of Urology and Transplantation from January 2010 to December 2020. Their renal biopsy request forms were retrieved from the archives of the Histopathology database. All clinical characteristics including, demographic features, signs and symptoms including edema, duration of symptoms, presence or absence of acute kidney injury, hypertension, presence of any cause of secondary MCD like a history of drug use, etc., baseline laboratory findings, including serum creatinine, serum albumin, 24-hour urinary proteins, blood or proteins on the dipstick, treatment given (drug, dosage and duration), response to treatment, frequency and time of relapse, intervention done at the time of relapse and final outcome till last follow-up were recorded from renal biopsy request forms and case files. None of the patients had repeat renal biopsy in this study period.
Treatment protocol followed at our institute, comprised of initiation of oral prednisolone at a dosage of 1 mg/kg for 6 weeks followed by 0.75 mg/kg for the next 6 weeks. If remission was not achieved by the end of 12 weeks, prednisolone was tapered over the next 4 weeks, and stopped, or a low dose of steroids was continued with the addition of second-line immunosuppression. If remission occurred at any time during treatment, the same dose of steroids was continued for 2 weeks followed by slow tapering of steroids over 3 months. Second-line immunosuppression [Cyclosporine (CyA)/Tacrolimus], when required. CyA was given at a dose of 4 mg/kg/day. Once a complete or partial response occurred, the same drug was continued for at least 12 months with monitoring of serum creatinine and albumin levels. The drug was stopped if no response was observed by the end of 8 weeks.
CR was defined as proteinuria < 0.3 g/24-hour or when the urine dipstick was negative for protein or only trace proteins were detected. Partial remission (PR) was defined as proteinuria between 0.3 g-2.0 g/24-hour with a minimum of 50% reduction in proteinuria from baseline or albumin detected on dipstick (+ 4 to + 1). Relapse was defined as proteinuria > 3 g/24-hour after the initial reduction of proteinuria to < 2 g/24-hour or albumin detected in dipstick (+ 1 to + 3 or + 4). Time to relapse was calculated from the time when remission was achieved to the time of onset of proteinuria. Frequent relapse was defined as four or more relapses in one year. Steroid resistance was defined as the persistence of proteinuria despite high-dose prednisolone requiring the managing clinician to start an additional immunosuppressive agent. Steroid dependence was defined as relapse on tapering steroid therapy or commencement of proteinuria within two weeks of completing steroid therapy. Hypertension was defined as patients already receiving antihypertensive drugs or with systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg. Proteinuria was measured initially from 24-hour urine collection and on follow up it was measured with urine dipstick to determine the remission or relapse of proteinuria. Haematuria was defined as > 5 red blood cells per high power field. Acute kidney Injury was defined as a rise in serum creatinine to > 50% above baseline presumed to have occurred in the preceding seven days [as per the kidney disease improving global outcomes acute kidney injury (AKI) guidelines][12].
Statistical analyses were performed using statistical product and service solutions statistics, Version 22 (international business machines corporation, version 22, United States). Categorical variables were expressed as numbers and percentages, and continuous variables were expressed as mean ± SD or median with interquartile range. Differences between continuous variables were tested for statistical significance using Student’s t-test and for categorical variables, by Mann-Whitney U test. Due to the small number of patients, Kaplan-Meier or Cox regression analysis to assess the associations between baseline characteristics and time to CR or time to relapse could not be performed. Statistical comparison was done by log-rank test. Statistical significance was taken to be a P value < 0.05.
A total of 23 adult patients with biopsy-proven MCD were identified during the study period of 10 years (January 2010 to December 2020). Main demographic data, and clinical and laboratory parameters at the time of presentation are summarized in Table 1. The majority of the patients were males, 15 (65.2%) with the male to female ratio of 1.9:1. Mean age was 26.34 ± 10.28 years (range: 18-60 years). Hypertension was found in 7 (30.4%) and microscopic hematuria in 10 (43.4 %) patients. Mean serum creatinine was 1.03 ± 1.00 mg/dL and mean serum albumin was 1.94 ± 0.90. Mean duration of symptoms was 5.23 ± 8.64 months (range: 1-30 months). Mean 24-hour urinary proteins were 4.53 ± 2.43 g/24-hour (range: 3-11 g/24-hour). AKI was found in only one patient (4.3%) at the time of presentation but unfortunately, his case file along with one more patient’s file was not retrieved hence these two patients were censored from further analysis. A comparison of presenting features in this cohort with other studies reported from different parts of the world is shown in Table 2. The mean duration of follow-up of 21 patients was 38.09 ± 22.3 months (range: 4-84 months).
| Characteristics | Value |
| Age (years) | 26.34 ± 10.28 (18-60) |
| Gender [Male/Female, n (%)] | 15/8 (65.2/34.8) |
| Duration of symptoms (months) | 5.23 ± 8.64 (1-30) |
| Serum creatinine (mg/dL) | 1.03 ± 1.00 (0.50-5.50) |
| Serum albumin (g/dL) | 1.94 ± 0.90 (1.0-4.0) |
| Systolic blood pressure (mmHg) | 121.21 ± 16.76 (100-150) |
| Diastolic blood pressure (mmHg) | 79.47 ± 12.92 (58-110) |
| Urinary proteins (g/day) | 4.53 ± 2.43 (3-11) |
| Hematuria, n (%) | 2 (8.7) |
| Hypertension, n (%) | 7 (30.47) |
| AKI at presentation, n (%) | 1 (4.3) |
| Characteristics | Present study | Korbet et al[13] | Nolasco et al[14] | Mak et al[15] | Fujimoto et al[16] | Keskar et al[17] |
| Number of patients | 23 | 40 | 89 | 51 | 33 | 61 |
| Age (years) (mean ± SD) | 26.34 ± 10.28 | 41 ± 20 | 42 ± 19 | 37 ± 11 | 28 ± 11 | 30.46 ± 13.43 |
| Males (%) | 65.2 | 48 | 56 | 70 | 67 | 54 |
| Urinary proteins (g/day) (mean ± SD) | 4.53 ± 2.43 | 8.7 ± 5.7 | 10.2 | 16.4 | 12.4 | 5.33 ± 3.75 |
| Serum Albumin (g/dL) (mean ± SD) | 1.94 ± 0.90 | 2.13 ± 0.8 | 1.9 | 1.7 | 1.8 | 2.0 ± 0.71 |
| Hypertension (%) | 30.47 | 21 | 30 | 55 | 9 | 6.55 |
| Hematuria (%) | 8.7 | 21 | 28 | 33 | 15 | 13.11 |
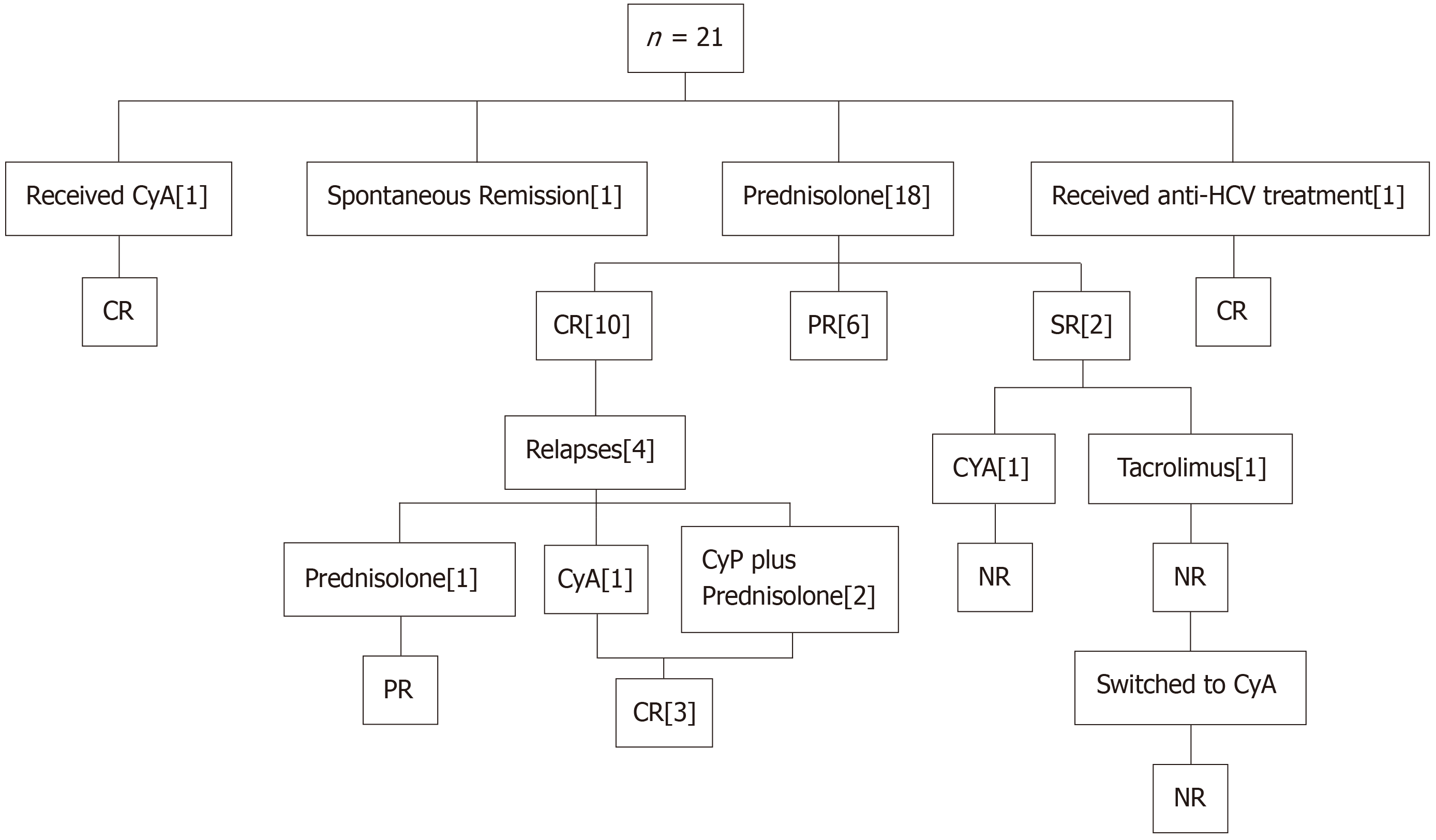
Four (17.4%) patients were suspected of having secondary MCD with the underlying cause being pulmonary tuberculosis (TB) in one patient, hepatitis C virus, (HCV) in one patient, combined HCV and pulmonary TB in one patient, and one patient had drug history of NSAIDs, who was later on censored due to unavailability of clinical record. One out of 21 patients (4.76%) entered spontaneous remission before induction of any immunosuppression. This patient had a history of donor nephrectomy two years ago and had no symptoms but had incidental findings of nephrotic-range proteinuria on donor follow-up. Among the rest of the 20 patients, the majority (n = 18, 90%), received daily oral prednisolone (1 mg/kg/ day) as first-line immunosuppression, one received CyA (4 mg/kg/day) initially due to mild renal dysfunction at the time of presentation (5%) and one patient of secondary MCD due to HCV infection (5%) was treated with Interferon once weekly and Ribavarin once daily for total duration of 24 months. Mean duration of treatment was 30.0 ± 52.7 months (range: 8.0-240 months).
The response to immunosuppression in 20 patients is shown in Figure 1. A total of 16 out of 18 (88.88%) patients were steroid-responsive. Among these, 10 (62.5%) patients achieved CR, while 6 (37.5%) achieved PR. One patient who was treated with CyA also attained CR, while one patient who was treated for primary disease (HCV infection) attained PR at 4 weeks and he was continued on the same treatment and ultimately achieved CR after 3 weeks. The mean time to achieve CR was 3.18 ± 0.40 weeks (range: 3-4 weeks). Two steroid-resistant patients underwent induction with second-line immunosuppression. CyA was given to one patient in the above-mentioned dosage but the patient was not responding to it and the proteinuria persisted till the last follow-up. Another steroid-resistant patient received Tacrolimus (5 mg once daily) for 12 weeks, but no response was observed. Tacrolimus was switched to CyA due to a rise in serum creatinine (from 0.8 mg/dL to 1.0 mg/dL) for 9 months but still had proteinuria so CyA was also tapered and stopped. He was kept on anti-proteinuric drugs only.
Four (19.04%) patients had relapsed, among these 2 patients had one episode of relapse while 2 patients had 2 episodes of relapse. The mean time to first relapse was 6.5 ± 3.31 months (range: 3-11 months) and the mean time to 2nd relapse was 24 ± 16.9 months (range: 12-36 months). Interestingly, all four patients were steroid responders who achieved CR on initial treatment. Of these, one was put back on high-dose steroids and attained re-remission but had a second relapse after 36 months. Again, he was started on steroids and he achieved PR till the last follow-up. Cyclophosphamide (CyP) was added as a second-line immunosuppression in two patients at a dosage of 2 mg/kg in addition to steroids (1 mg/kg in one patient and 0.75 mg/kg in the second patient for 8 weeks and 12 weeks, respectively) and they both achieved CR. The fourth patient received CyA, 125 mg, BD for 16 weeks, and attained CR. Renal functions were normal in all four patients till the last follow-up. One patient who achieved spontaneous remission never relapsed. No significant difference among baseline characteristics was noted between relapsers and non-relapsers except for systolic and diastolic blood pressures (P = 0.037 and 0.043, respectively). No significant difference was seen in the total duration of treatment, initial serum creatinine, and last serum creatinine (P = 0.53, 0.18, and 0.37, respectively) as shown in Table 3.
| Characteristics | Patients with relapse (n = 4) | Patients without relapse (n = 17) | P value |
| Age (years) | 21.0 ± 2.94 | 26.76 ± 10.65 | 0.30 |
| Males, (%) | 100 | 58.8 | 0.16 |
| Systolic BP (mmHg) | 105.5 ± 9.71 | 123.8 ± 15.4 | 0.03 |
| Diastolic BP (mmHg) | 67.0 ± 6.0 | 81.76 ± 13.09 | 0.04 |
| Serum Albumin (g/dL) | 1.25 ± 0.25 | 2.10 ± 0.96 | 0.05 |
| Serum Cr (mg/dL) | 0.68 ± 0.12 | 0.86 ± 0.25 | 0.18 |
| Urinary proteins (g/day) | 4.52 ± 2.81 | 4.21 ± 2.07 | 0.80 |
| Duration of follow up (months) | 36.5 ± 19.6 | 38.47 ± 23.4 | 0.87 |
| Serum Cr at last follow up (mg/dL) | 0.71 ± 0.55 | 0.83 ± 0.26 | 0.37 |
| Remission at last follow up (%) | 75 | 70.6 | 0.57 |
| Normal renal function at last follow up (%) | 75 | 76.5 | 0.69 |
Two out of 18 (11.1%) patients who were treated with steroids, developed therapy-related side effects. One patient developed acne and tremor, hence steroids were stopped and the patient was put on CyA, while the other patient developed weakness and muscle cramps. Low-dose or alternate-day steroids were not given to any of these patients as per institute policy. None of the patients developed any infection, septicemia, or any thromboembolic event during follow-up. No frequent relapsers were found in this cohort. The mean serum creatinine at the last follow-up was 0.81 ± 0.23 mg/dL (range: 0.5-1.7 mg/dL). Normal renal functions were observed in two third of this cohort; 76.1%, while five patients were lost to follow-up (23.8%). Fortunately, none of the patients progressed to end-stage renal failure and there were no deaths till the last available follow-up. Table 4 shows a comparison of the final outcome of adult-onset MCD reported from different studies.
| Characteristics | Present study | Korbet et al[13] | Nolasco et al[14] | Mak et al[15] | Fujimoto et al[16] | Keskar et al[17] |
| Follow up (months) (mean ± SD) | 38.09 ± 22.3 | 53.5 ± 47.1 | 91 ± 63 | 38.93 | 46.2 ± 29 | 34.4 |
| Remission (%) | 85.7 | 80 | 77 | 94 | 94 | 87.5 |
| Complete (%) | 71.4 | 73 | 66 | 90 | 94 | 61.29 |
| Partial (%) | 14.2 | 7 | 11 | 4 | - | 26.22 |
| Nephrotic at last follow-up (%) | 14.2 | 20 | 6 | 2 | 3 | |
| ESRD (%) | - | 5 | 1 | 2 | - | 1.6 |
| Died (%) | - | 8 | 17 | 2 | 3 | - |
To the best of our knowledge, this is the first study on detailed clinical features, treatment response, and outcomes in adults with MCD from this country. A few studies have been published in international literature but there is still a paucity of data on the management of this disease in adults and it remains challenging for practicing nephrologists. No consensus-based guidelines are available for the treatment of MCD in adults. We retrospectively reviewed data from 21 adults who were diagnosed and managed at our institute over 10 years.
MCD is one of the three most common causes of NS in adults. Although the majority of patients respond to initial therapy with corticosteroids, some authors have observed variable response patterns, clinical course of disease, and long-term outcomes. Despite attempts to identify the clinicopathologic features that can help predict the course of disease, it poses significant challenges to practicing nephrologists to select the optimal type and duration of immunosuppression[1-5]. In children, MCD is mainly idiopathic, while in adults, secondary causes of MCD are seen in approximately 13% of cases, which have peculiar therapeutic implications as underlying causes may vary from viral and bacterial infections, and malignancies, to numerous medications[3]. The findings from this study in this context are similar to those reported previously and we had three patients with secondary MCD accounting for 13.04%. The gender distribution of this cohort is also more like that of the pediatric population rather than the adult counterparts. These differences may be because of the cohort’s younger average age.
The presenting clinical features in adults with MCD are a little different from their children counterparts as apart from the nephrotic-range proteinuria, adults are more likely to have hematuria, hypertension, and AKI at the time of presentation[1,5]. In this cohort, hypertension was found in 30.4%. Similar findings have been observed by Korbet et al[13] and Nolasco et al[14], while Waldman et al[1] and Mak et al[15] have reported a slightly higher frequency of hypertension accounting for 42.9 % and 55%, respectively. On the other hand, Fujimoto et al[16] and Keskar et al[17] have reported lower frequency of hypertension at 9% and 6.5%, respectively. We observed a lower frequency of microscopic hematuria, i.e. 8.7%. This finding is different from those reported by other studies, as the majority of them reported a higher frequency of hematuria varying from 13% to 33%[13,15,18]. AKI was found in two patients in this cohort; one of these was excluded due to a lack of clinical details and the remaining one out of 21 patients (4.3%) had renal insufficiency (serum creatinine: > 1.3 mg/dL). This observation is also not in agreement with those reported from other parts of the world. The reason behind this discordance may be that the majority of patients in this cohort were younger (< 40 years of age) and only three patients were above 40 years and a higher frequency of AKI at presentation is reported in older patients with severe hypoalbuminemia[5]. In addition, very stringent criteria were used by our pathologists for the diagnosis of MCD in adults, so that no false positive diagnosis of MCD could be made. It is challenging to differentiate it from early focal segmental glomerulosclerosis (FSGS) on renal biopsies and the dividing line between the diseases is very fine and tenuous. Often times, the entire biopsy material was cut and examined to rule out the possibility of FSGS. This may be one of the reasons for very high response rates to corticosteroids in the present study and varying clinical presentations in the present study and some other studies. In addition, categorization of MCD into primary and secondary forms is also challenging and sometimes, it may be misclassified, leading to changes in response rates. A careful selection of the cases for biopsy by the clinicians might have contributed to the lower incidence of secondary causes in this cohort. A higher mean age has been reported in almost all previously published studies compared to the present study. In this study, the mean age was 26 years, which might be one of the contributing factors to benign presentation in this group of patients[13-15]. We hypothesize that MCD in young adults in the carefully selected groups of patients behaves more like pediatric MCD.
Steroids remain the cornerstone as a first-line therapy in MCD both in children as well as in adults with excellent response rates. Favorable response occurred in 88.88% of adults within 8 weeks in this study and these results are in agreement with those reported from other Asian countries, which have reported remission rates ranging from 80%-90% by 8 weeks[1]. This is one of the most significant novel findings from this study, closely simulating the response rates and durations in children. Historically, a slower response to steroid therapy has been described in adults as compared to children with a response rate of 75% by 13 weeks[1,13]. The reason behind this difference in response rate might be explained by ethnic differences among different study populations. Some authors have also documented almost similar response rates to alternate-day steroid therapy and they advocate the efficacy of this regimen almost equivalent to the daily high-dose oral steroids with fewer potential adverse effects[3,18]. Imbasciati et al[18] have reported a marginally higher remission rate (almost 100%) with conventional high-dose daily oral steroids as compared to three pulse doses of methylprednisolone followed by low-dose steroids. Perhaps, a similar relapse rate was observed in both groups, suggesting the utility of low-dose therapy in patients who are at higher risk for steroid toxicity[18]. Despite these observations, Colattur and Kobert[3] have concluded that adults require a more prolonged duration of high-dose steroid therapy to attain excellent remission rates comparable to children. None of the patients at our center was treated with an alternate-day steroid therapy protocol. Two (11.1%) patients were steroid-resistant in this cohort, which is comparatively slightly higher than that reported from other studies ranging from 3%-9%[3]. Interestingly, these two patients did not respond to second-line immunosuppression as well and they remained persistently proteinuric till the last follow-up. These differences in steroid response rates and patterns call for broader regional collaborative studies to investigate the possible ethnic underpinnings of these findings and formulate regional guidelines and management strategies for optimal dosing and duration of steroid therapy in adult MCD.
Six patients in this cohort (30%) received second-line immunosuppression therapy. Of these, two patients who were steroid dependent received CyP in addition to steroids and both of them underwent remission by four weeks but both of them also relapsed, one at 3 months, while another one had two relapses at 6 months and 12 months. Two more steroid-dependent patients required second-line therapy and they were kept on CyA in standard dose and duration. One of them attained CR by 6 weeks but relapsed at 6 months and the other attained PR by 3 weeks and never relapsed. These findings are similar to those reported in other studies as calcineurin inhibitors were the most commonly administered second-line drug followed by CyP, Mycophenolate mofetil, and Rituximab with remission rates ranging from 84% to 100% but the relapse rates were significantly higher in these patients[1,3]. Apart from a higher relapse rate, these drugs have well-documented significant adverse effects after prolonged use. We did not observe adverse effects to any of these drugs in our cohort. Although data on tacrolimus treatment in MCD is limited, it has been reported to have a potential advantage over CyA in terms of cosmetic effects as well as a higher risk of dyslipidemia and hypertension[1].
The mean duration of follow-up in this cohort was 38.09 ± 22.3 months. Almost two-thirds of the patients maintained normal renal function till the last follow-up and 71.4% of patients were in CR at the last follow-up. These findings are similar to those reported by Korbet et al[13] and Nolasco et al[14] while Mak et al[15] and Fujimoto et al[16] have reported higher remission rates in their cohorts. Idelson et al[19] have reported that even adults with PR in MCD had preserved renal function over long-term follow-up. Furthermore, they observed that a significant number of non-steroid responders progressed to end-stage renal disease (ESRD) within 3 years. Waldman et al[1] have also documented more favorable outcomes in steroid responders as compared to non-responders. Szeto et al[20] have reported a higher frequency of progression to ESRD, accounting for 9.41% and 62 (18.2%) patients who died due to varying causes. There were only two non-responders in this study but both of them had normal renal functions till the last follow-up of 20 months and 72 months, respectively. It might be possible that repeat renal biopsy in these two patients may show disease progression to FSGS which should be searched for in patients who do not respond to steroids.
This is the first study from Pakistan on adults with MCD who were diagnosed and treated at our institute, one of the largest nephrology centers in this region. However, the number of patients in this cohort is small and this is one of the main limitations of this study. Another limitation is retrospective collection of data from clinical records and review of original biopsy request forms which is dependent on the completeness of clinical charts. This may affect the robustness of the results. As only 21 patients were diagnosed with MCD over 10 years, it would be very difficult to collect prospective data in this particular population. These limitations may impact the generalizability of the findings and can be mitigated by large-scale, regional, and multicenter collaborative studies to validate the possible ethnic and environmental implications of the phenotypic differences of MCD in adults in this region. The findings from these studies can provide evidence for formulating regional or national guidelines for managing this challenging disease in adults. Future research should be directed to elucidate the underlying etiopathogenesis of the condition in the context of different ethnic and environmental cohorts, which could pave the path to tailored treatment.
In conclusion, MCD is a highly steroid-responsive disease in adults with an overall good prognosis and outcome as the majority achieve remission with oral high-dose steroids alone but relapses are common. Steroid-dependent patients require second-line immunosuppression to attain remission. There is a lack of prospective, large observational studies and randomized controlled trials in adults with MCD and there is a dire need to develop management guidelines, especially for steroid-resistant and relapsing cases.
| 1. | Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D'Agati V, Appel G. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol. 2017;12:332-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 3. | Colattur SN, Korbet SM. Long-term Outcome of Adult Onset Idiopathic Minimal Change Disease. Saudi J Kidney Dis Transpl. 2000;11:334-344. [PubMed] |
| 4. | Hogan J, Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol. 2013;24:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Fenton A, Smith SW, Hewins P. Adult minimal-change disease: observational data from a UK centre on patient characteristics, therapies, and outcomes. BMC Nephrol. 2018;19:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Lee H, Yoo KD, Oh YK, Kim DK, Oh KH, Joo KW, Kim YS, Ahn C, Han JS, Lim CS. Predictors of Relapse in Adult-Onset Nephrotic Minimal Change Disease. Medicine (Baltimore). 2016;95:e3179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Golay V, Trivedi M, Kurien AA, Sarkar D, Roychowdhary A, Pandey R. Spectrum of nephrotic syndrome in adults: clinicopathological study from a single center in India. Ren Fail. 2013;35:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, Kang SW, Choi KH, Han DS, Jeong HJ, Lee HY. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Heaf J, Løkkegaard H, Larsen S. The epidemiology and prognosis of glomerulonephritis in Denmark 1985-1997. Nephrol Dial Transplant. 1999;14:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zhou FD, Shen HY, Chen M, Liu G, Zou WZ, Zhao MH, Wang HY. The renal histopathological spectrum of patients with nephrotic syndrome: an analysis of 1523 patients in a single Chinese centre. Nephrol Dial Transplant. 2011;26:3993-3997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clin Exp Nephrol. 2009;13:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3349] [Article Influence: 257.6] [Reference Citation Analysis (0)] |
| 13. | Korbet SM, Schwartz MM, Lewis EJ. Minimal-change glomerulopathy of adulthood. Am J Nephrol. 1988;8:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG. Adult-onset minimal change nephrotic syndrome: a long-term follow-up. Kidney Int. 1986;29:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 141] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Mak SK, Short CD, Mallick NP. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant. 1996;11:2192-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Fujimoto S, Yamamoto Y, Hisanaga S, Morita S, Eto T, Tanaka K. Minimal change nephrotic syndrome in adults: response to corticosteroid therapy and frequency of relapse. Am J Kidney Dis. 1991;17:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Keskar V, Jamale TE, Kulkarni MJ, Kiggal Jagadish P, Fernandes G, Hase N. Minimal-change disease in adolescents and adults: epidemiology and therapeutic response. Clin Kidney J. 2013;6:469-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, Della Volpe M, Perfumo F, Petrone P, Picca M. Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. Br Med J (Clin Res Ed). 1985;291:1305-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Idelson BA, Smithline N, Smith GW, Harrington JT. Prognosis in steroid-treated idiopathic nephrotic syndrome in adults. Analysis of major predictive factors after ten-year follow-up. Arch Intern Med. 1977;137:891-896. [PubMed] [DOI] [Full Text] |
| 20. | Szeto CC, Lai FM, Chow KM, Kwan BC, Kwong VW, Leung CB, Li PK. Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis. 2015;65:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |